IRB Protocol
HPHC2 FDA IRB Protocol_CLEAN_9.11.20.docx
Experimental Study on Measuring Consumer Comprehension of Displays of Harmful and Potentially Harmful Constituents (HPHCs) in Tobacco Products and Tobacco Smoke
IRB Protocol
OMB: 0910-0880
D EPARTMENT
OF HEALTH AND HUMAN SERVICES
EPARTMENT
OF HEALTH AND HUMAN SERVICES
_________________________________________________________________________________________________________
Food and Drug Administration
Center for Tobacco Products
10903 New Hampshire Ave
Silver Spring, MD 20903
I. Protocol Title: Measuring Consumer Comprehension of Displays of Harmful and Potentially Harmful Constituents (HPHCs) in Tobacco Products: Pilot Cognitive Testing and Experimental/Quantitative Study
II. Protocol Version or Date: September 11, 2020
III. Study Mechanism, Research Team, and Credentials of Investigators
A. Identify the mechanism for conducting the study (e.g., contract, IAA, MOU, etc.), its ID number, and attach any associated instrument: Contract: HHSF223201110005B
B. Contractor Info:
RTI International
3040 E. Cornwallis
Road
Research Triangle Park, NC 27709
Telephone:
919-541-6453
Point of Contact:
Ashley Feld, [email protected]
C. FDA Sponsor: Katherine Margolis, PhD and Jennifer Bernat, PhD
D. Principal Investigator: Jessica Pepper, PhD (RTI)
Additional
Study Staff: Ashley Feld, MPH (RTI)
E. Overseeing IRBs: Dual IRB review; RTI and FDA
IV. Abstract
The U.S. Food and Drug Administration’s (FDA’s) Center for Tobacco Products (CTP) is seeking to conduct an experimental/quantitative study (target sample of 4,500 online surveys) consisting of adult and youth (13 to 17 years old) participants to evaluate the best way to convey information about harmful and potentially harmful constituents (HPHCs) in tobacco products and smoke by brand and by quantity in each brand and subbrand in a format that is understandable and not misleading to a layperson. The purpose of the research is to gain insight on consumer comprehension of and preferences regarding presentation of information about HPHCs in tobacco products and tobacco smoke, which will inform the Agency’s efforts to implement the mandatory publicly available list of HPHCs required by the Family Smoking Prevention and Tobacco Control Act (the Tobacco Control Act). Experimental/quantitative study participants will view sample formats of HPHC information and will complete an online survey that will include questions regarding the understanding and misleadingness of the HPHC information. This study was informed by nine in-person cognitive interviews that helped FDA pilot the survey questionnaire and online platform. The protocol for the cognitive interviews was approved on November 26, 2018 by FDA IRB and December 3, 2018 by RTI IRB, and the interviews were conducted in January 2019. Information about the cognitive interviews has been removed from this modified protocol, which exclusively addresses updates to the online survey component.
V. Regulatory and Scientific Significance of the Research
A. No IND, IDE, or ITP application is needed for the study.
B. Describe the regulatory background and significance of the research.
On June 22, 2009, President Obama signed the Family Smoking Prevention and Tobacco Control Act (Tobacco Control Act) into law. The Tobacco Control Act amended the Federal Food, Drug, and Cosmetic (FD&C) Act by adding a new chapter granting the FDA important new authority to regulate the manufacture, marketing, and distribution of tobacco products to protect the public health generally and to reduce tobacco use by minors. Section 904(e) of the FD&C Act requires FDA to establish, and periodically revise as appropriate, ‘‘a list of harmful and potentially harmful constituents (HPHCs), including smoke constituents, to health in each tobacco product by brand and by quantity in each brand and subbrand.’’ Section 904(d)(1) of the Act further requires that the list be published in a format that is understandable and not misleading to laypersons.
To fulfil this mandate, FDA’s CTP requires a randomized, experimental/quantitative study that builds on prior research to gain insight on consumer comprehension of information about HPHCs in tobacco products and tobacco smoke, which are chemicals or chemical compounds in a tobacco product or tobacco smoke that cause, or could cause, harm. Examples of HPHCs include toxicants, carcinogens, and addictive chemicals and chemical compounds.
CTP will conduct an experiment using an Internet panel to gather information about different ways of presenting HPHC information in a format that is understandable and not misleading to a layperson.
C. Identify the CTP research priorities addressed
i. The research in the proposed study will inform regulatory activities defined by the Tobacco Control Act. Specifically, the research addresses the following priority: Effective communication strategies regarding harmful and potentially harmful constituents and risks of tobacco products.
VI. Objectives and Hypotheses
The goal of this study is to test the effectiveness of sample formats to communicate HPHC information. Results will be used to inform strategies to effectively communicate about HPHC information that is understandable and not misleading to the layperson.
Survey questions will be designed to answer the following research questions:
After viewing the sample format, what do adults and youth know about HPHCs in tobacco products?
After viewing the sample format, what do adults and youth perceive about HPHCs in tobacco products?
After viewing the sample format, what formats do adults and youth prefer to receive information regarding HPHCs?
After viewing the sample format, is the information presented misleading?
Are participants able to understand the information presented about HPHCs?
VII. Background
Section 904(d)(1) of the Tobacco Control Act states “the Secretary shall publish in a format that is understandable and not misleading to a lay person, and place on public display (in a manner determined by the Secretary) the list [of harmful and potentially harmful constituents] established under subsection (e).”
To fulfil this mandate, CTP requires a study that builds on prior research to gain insight on consumer comprehension of information about HPHCs in tobacco products and tobacco smoke, which are chemicals or chemical compounds in a tobacco product or tobacco smoke that cause, or could cause, harm. Examples of HPHCs include toxicants, carcinogens, and addictive chemicals and chemical compounds.
VIII. Study Design and Methods
A. Inclusion criteria for subjects, samples, or data, as applicable
Young Adult Cigarette Smokers (18 to 24 years)
Older Adult Cigarette Smokers (25+ years)
Young Adult and Older Adult Smokeless Tobacco Users (18+ years)
Youth Cigarette Smokers (13 to 17 years)
Youth Susceptible to Smoking Cigarettes (13 to 17 years)
Youth Smokeless Tobacco Users (13 to 17 years)
Young Adult and Older Adult Nontobacco Users (18+ years)
B. Exclusion criteria for subjects, samples, or data, as applicable
Younger than 13 years of age
Does not match tobacco use or susceptibility criteria
Youth without parental permission to participate
We will also ensure a sufficient number of low socioeconomic status (income of less than $25,000/year) adult participants (at least 20% of adults) and a sufficient number of adults without a high school diploma or GED (at least 20% of adults) are included in the sample. These groups are not mutually exclusive and may overlap.
Otherwise eligible respondents may not be invited to participate based on the following selection criteria:
Maximum number of participants in a given group (based on age and tobacco use) has already been achieved
Minimum percentage of adult participants with low socioeconomic status has not been met
Minimum percentage of adult participants with low education has not been met
C. The number of subjects to be studied, per arm and total, if applicable, along with the rationale for the sample size(s).
i. Experimental/Quantitative
Study:
The target sample size is 4,500 participants: 3,000
adults (including 500 smokeless tobacco users, 2,000 cigarette
smokers, and 500 non-tobacco users) and 1,500 youth (including at
least 100 smokeless tobacco users, at least 150 cigarette smokers,
and the remainder susceptible to smoking). To achieve this target
sample size of 4,500, up to 3,300 adults and 1,650 youth will
complete the survey. The overage accounts for the possibility that
recruitment is not closed as soon as the target sample size has been
obtained.
We conducted power calculations to estimate power to detect statistically significant changes in Understanding, Misleadingness, and Harm Perception domain scores after exposure to each HPHC display format relative to baseline scores, given a sample size of 750 subjects per display format.
Power was estimated based on the assumption that items under each domain are independent and identically distributed and that no correlation exists between pre- and post-exposure responses for each subject. In practice, responses within individuals may be correlated. Alternative methods for simulating data incorporating within-subject correlations may be applied. Wilcoxon signed rank tests were used in the Monte Carlo simulations. Although the central limit theorem provides a basis for using t tests to test whether the mean differences in domain scores are zero in large samples, because of the bounded nature of the outcomes, the nonparametric rank test, which does not assume normality, may be more appropriate. Power to detect significant differences in scores between pre- and post-exposures are displayed by format in Table 1. Overall, power to detect changes for primary outcomes Understanding and Misleadingness scores is > 99.9% for all display formats. The powers to detect changes in Harm Perception scores in formats 1 through 6 are 40%, 86%, 90%, 97%, 75%, and 5%, respectively.
Table 1. Power to Detect Median Change in Pre- and Post-exposure Domain Scores at α = .05 by Format
HPHC Formats |
Understanding |
Misleadingness |
Harm Perceptions |
Format 1 |
>99.9% |
>99.9% |
40% |
Format 2 |
>99.9% |
>99.9% |
86% |
Format 3 |
>99.9% |
>99.9% |
90% |
Format 4 |
>99.9% |
>99.9% |
97% |
Format 5 |
>99.9% |
>99.9% |
75% |
Format 6 |
>99.9% |
>99.9% |
Based on the results presented in Table 1 and given sample sizes of 750 subjects per display format, there is sufficient power at α = .05 to detect significant differences between pre- and post-exposure for primary outcomes Understanding and Misleadingness domain scores.
D. Study design, including the controls, blinding, or randomization to be used, if any, and the methods used to minimize bias on the part of subjects, investigators, and analysis
Experimental/Quantitative
Study:
This study is an online survey, and participants will
be randomized to view one of six sample formats. Questions will be
asked before viewing the experimental HPHC display format and after
viewing the format. Sample formats are included below in Section XX
and also appear in Appendix L.
E. Describe all study procedures in the order in which they will be performed or affect subjects
Experimental/Quantitative Study:
1. Participants will be recruited via a vendor database maintained by Lightspeed Research LLC. In order to be a member of this database, participants have viewed and consented to a privacy policy which is available in Appendix M. Lightspeed may partner with other survey vendors who maintain online survey panels. These partner vendors will only participate in recruitment; they will not be involved in survey programming, survey hosting, data collection, or data storage. Lightspeed and any partners will recruit participants from their databases by emailing adult panel members. Youth participants will be recruited by emailing adult panel members whose profiles indicate they have a child in the relevant age range. Sample recruitment e-mails are provided in Appendices N and O.
2. If necessary to achieve the target sample size, Lightspeed and its partners will send up to 2 reminder emails. Reminders will only be sent to non-responders. Sample reminder emails are provided in Appendices P and Q.
3. Informed consent will be obtained for adult participants; youth assent and parental consent will be obtained for youth participants (refer to Appendixes G, H, and I for web-based adult consent, parental permission, and youth assent, respectively) prior to participants completing a screener. Potential adult participants will be routed out of the study if they do not provide consent; potential youth participants will be routed out of the study if their parents do not permission or the youth does not provide assent.
4. Participants will complete a screener that collects information on their age, sex, tobacco use, education (among adults only), income (among adults only), race/ethnicity, and participation in tobacco-related surveys in the past 6 months(refer to Appendix J for the web-based adult screener and youth screener). Participants are required to answer all questions on the screener; however, the items on sex, race, and ethnicity offer a response option of “I do not wish to answer.” Eligibility is based on tobacco use and age. For adults, there are also quotas based on education and income to ensure representation of individuals with low education and low income.
5. The survey will be conducted online and will last approximately 20 minutes (refer to Appendix K for the web-based survey). Participants will be instructed to thoroughly view the stimulus because they will be answering questions about it.
6. Participants will complete pre-exposure questions.
7. Participants will view the stimulus and answer post-exposure questions. The stimulus will present during the post-exposure period.
8. Each participant will only see one stimulus to avoid any learning effects.
9. At the end of the survey, participants will be thanked and debriefed (i.e., notified that the brand of cigarettes described in the study is a fictitious brand).
10. Adult participants and parents of youth participants will be provided incentives in accordance with the established incentive procedures that have been developed for the Internet panel from which they were recruited (e.g., gift points). Parents of youth participants will receive incentives even if the youth do not qualify or complete the survey; in this instance, it may take up to 90 days for the parent to receive the incentive.
F. Describe the tobacco cessation message and optional cessation intervention or referral you will employ for tobacco users, including its content, format, and timing. Alternatively, justify not including such a message.
At the conclusion of the survey, all participants will see the following message:
“Thank you for participating in this study. The purpose of this study is to understand how people think about the chemicals in tobacco products and smoke. “Durham,” the brand of cigarettes mentioned in this study, is a fictitious brand and is not a product currently for sale. This research was sponsored by the U.S. Food and Drug Administration also known as the FDA. FDA would like to thank you for sharing your opinions as they will be very useful in helping to understand people’s reactions and thoughts to tobacco product information. If you are a tobacco user, or have a friend or family member who is a tobacco user, and you would like information on how to quit, please visit https://smokefree.gov/.”
IX. Product Information
Participants will not use any tobacco products during the course of this study.
X. Duration of the Study:
The period of performance for the task order under which this study is being conducted is scheduled to end on February 28, 2021. Online data collection should take approximately 1-2 months to complete. Each online survey will take approximately 20 minutes. Each participant will complete one survey and no follow-up will occur. Recruitment will start after approvals are received from OMB, FDA’s Institutional Review Board (IRB), and RTI’s IRB.
XI. Location
Experimental/quantitative data collection will occur over the Internet. Lightspeed will host the survey. Lightspeed may use other online market research panels as partners for recruiting, as is standard practice. If using partners, those partners will be recruiting using links that go directly to the Lightspeed-based survey. The survey will not be programmed or administered by the partner.
XII. Survey Questions
See Appendix K for the online survey.
XIII. Analysis of the Study
Data Analysis and Reporting
The methodology and findings report provided by the study contractor will include monitoring information, sample selection procedures, response rates, data collection procedures, data preparation and processing information, complete code book, and any common themes and problems encountered during the execution of the study. The report will contain no information by which participants can be identified. The contractor will also provide FDA with raw data in an SPSS-compatible data file. The raw data will contain no information by which participants can be identified. Survey data will be analyzed with statistical methods (e.g., Wilcoxson Signed Rank Test, ANOVA, regression).
XIV. Subject Selection and Recruitment
A. Experimental/Quantitative Study:
Lightspeed will sample from U.S. participants in its market research panel. Lightspeed’s panel provides a cross-section of the U.S. population. Lightspeed may also partner with other market research panels for recruitment. Participants recruited through partners would be directed to a Lightspeed link, just as those recruited through Lightspeed. The composition of the Lightspeed panel and partner panels does not necessarily match the U.S. population or a specific target population. We ensure a reasonable degree of diversity in terms of age based on eligibility criteria and adults’ income, and adults’ education based on quotas.
Lightspeed and their partners send adult panel members a study participation invitation via email. For potential youth participants, Lightspeed and their partners send emails to adult panel members who have previously indicated that they have a child in the sample age range. The email instructs them to click the unique link to access the survey. Sample recruitment e-mails are provided in Appendices N and O.
If recruitment targets have not been met within 48 hours of survey launch, Lightspeed and any partners will send a reminder email. If recruitment targets still have not been met, Lightspeed and any partners will send a second reminder email 48 hours after the first reminder. There will be no more than two reminder emails sent. Reminders will only be sent to non-responders. Sample reminder emails are provided in Appendices P and Q.
C. Provide the rationale for research subject selection based on the gender/ethnicity/race/socioeconomic categories at risk for the condition under study.
All genders, ethnicities, races, and socioeconomic categories can participate in the web-based survey if they meet the inclusion criteria and the quota for their recruitment group has not been met.
D. Provide the rationale for subgroupings of research subjects, if applicable.
Participants will not be subgrouped, but certain groups that are typically difficult to engage in online research (i.e., individuals with less than a high school education, individuals with low income) will be recruited based on quota minimums to ensure representation.
XV. Risk-to-Benefit Ratio
A. Describe any potential risks, whether physical, psychological, social, economic, legal, or other as a result of participation in your research study.
There are minimal psychological, legal, or social risks to participating in this study. Participants will be asked to share their attitudes, opinions, and behaviors. Participants may not feel comfortable answering some of the questions in the survey, such as those about tobacco use. Some questions about tobacco use may be more sensitive because tobacco use among adolescents under age 18 is illegal in a few states, and sales to individuals under 21 are illegal nationwide. Participation is voluntary, and participants can choose not to answer any of the questions in the survey. Some questions in the screener are required in order to determine eligibility and whether quotas have been met; however, the potential participant can exit the survey by closing their browser if they do not want to answer these items. Participants are notified of this as part of the consent process.
B. Assess the likelihood and seriousness of the potential risks.
Any serious risks are extremely unlikely. Participants may be uncomfortable reporting behavior like their tobacco use but can choose to not answer any questions in the survey. Some questions in the screener are required in order to determine eligibility and whether quotas have been met; however, the potential participant can exit the survey if they do not want to answer these items.
C. Describe procedures you will use to minimize any risks and their likely effectiveness.
We will use multiple procedures to minimize risks:
No personally identifiable information (PII) will be collected as part of this project, nor will Lightspeed combine the data collected from this project with other data (PII or non-PII) they already have. The study data sent from Lightspeed to RTI will identify participants only through study-specific ID numbers. Any effort to link these ID numbers to individuals’ PII would require a multi-step process. Those steps include: requesting that Lightspeed provide PII that is linked to responses, a review of that request by Lightspeed’s Privacy Office, approval of that request by Lightspeed’s Privacy Office, and signing non-disclosure agreements. Partner companies that assist with recruitment will never have access to study data, so they cannot link responses to responses to respondents’ PII.
Lightspeed will not use the data it collects through the screener or survey to update panelists’ profiles. Because Lightspeed will not share the data it collects from the screener or survey with the companies they partner with for recruitment, it will not be possible for the partners to use this data to update profiles of their own panel members.
Participants can terminate the survey or refuse to answer any survey question at any time, although some screener items are required, as described above.
We advise individuals to complete the screener and survey in a private setting where no one can observe their answers. In addition, respondents cannot back up in the survey to view previous responses. For example, if a youth were to exit the survey, the parent could not view previously entered responses.
The FDA study team has completed a Privacy Impact Assessment in consultation with FDA’s Privacy Office to identify and mitigate privacy risks related to this study.
This study is covered by a Certificate of Confidentiality from FDA.
All project files, including data, are maintained on secure share drives on the RTI network. Permission must be granted by the project director or project manager before anyone can gain access to these files. Only project staff can have access.
All RTI project staff handling human subjects data must use password-protected devices for any project-related work.
All data transmission from Lightspeed to RTI will be encrypted.
Lightspeed will invite adolescent children of adult panel participants to complete the survey through an email invitation to their parent asking for their consent to have their child’s opinions, which is fully compliant with the Children’s Online Privacy Protection Act’s revised standards.
Providing compensation to parents regardless of whether the youth is eligible or completes the survey mitigates the possibility of coercion of youth.
Adult respondents and parents of youth respondents access the survey through a unique link provided by Lightspeed. The link cannot be shared for others to use because it is unique.
Finally, participants will be provided with a specific toll-free phone number and email address for the RTI Office of Research Protection to contact in case they have a question or concern.
D. Describe potential benefits.
There are no direct benefits from participating in this study. Participants’ opinions will help improve FDA’s understanding of how people think about and use tobacco products.
E. Discuss the circumstances and procedures for terminating subject participation, under both voluntary and involuntary circumstances, including who may make the decision and who communicates with the subject about this issue.
Participants complete the study on their own computers and may terminate the survey at any time. If participants exit the survey, they have the option to return to the survey using the same link (although they cannot back up to previous answers).
Lightspeed examines data from completed surveys to determine whether respondents were speeding (defined as taking in the survey in less than two-fifths of the median time) or “straight-lining” (i.e., repeatedly selecting the same response across all questions). If Lightspeed determines that respondents provided poor quality data because of speeding or straight-lining, they will flag these cases and not send their data to RTI as part of the final dataset. However, individuals flagged for providing poor quality data will still receive the incentive.
F. Make the case (summative statement) for study approval based on the justified risks to benefits within the context of the knowledge to be gained.
There is little to no risk involved with this study.
XVI. Screening Tests Prior to Subject Enrollment
See Appendix J
XVII. Compensation, Incentives, and Rewards for Participation
A. Describe the type(s), amount(s), and schedule of incentive(s) or reward(s).
Adult participants recruited through Lightspeed receive points from Lightspeed that accrue over time. Adult participants must complete the survey in order to receive points. For youth participants, these points are awarded to the parent if youth initiate the screener, regardless of whether they qualify or complete the survey. The approximate value of points for participating in this study is $1.00-1.25. Panelists may redeem their accumulated points for online gift certificates, merchandise, and PayPal cash deposits.
For respondents who are recruited from partner survey vendors, the partner company will provide equivalent compensation in the form typically used by that company with its panelists. As is done by Lightspeed, partner companies will compensate adult participants only if they complete the survey and parents of youth participants if the youth initiates the screener. Lightspeed will provide the unique, alphanumeric survey IDs of individuals who should be compensated to the partner company.
XVIII. Informed Consent
A. Experimental/Quantitative Study:
The informed consent process will occur before participants are screened into the study. Adult participants will view an online informed consent explaining the potential risks and benefits of participating in the study and provide online consent by selecting “Yes” to the question “Do you agree to participate in the screening survey to determine your eligibility and (if you are eligible) to participate in the longer survey?” For youth participants, parents will view an informed consent and provide online permission for their child to participate by selecting “Yes” to the question “Do you give permission for your child to participate in the screening survey to determine your child’s eligibility and (if they are eligible) do you also give permission for your child to participate in the longer survey?” The child will view an informed consent and provide online assent by selecting “Yes” to the question “Do you agree to:
Take a short screening survey to find out if you are eligible for the study, and
If you are eligible, take part in the study by answering questions in the longer survey?”
If a potential adult participant, parent of a youth participant, or youth participant does not select “Yes,” they will be routed out of the survey.
After adults provide consent or parents and youth provide permission and assent, respondents begin the screener. The first item on the screener asks for the respondent’s age. Survey programming logic will check their response to this item against how the individual entered the survey. Those responding 18 and older must have entered the survey through the consent process for adults. Those responding 13-17 must have entered the survey through the parent permission and youth assent process. Those responding 12 and under will be routed out of the survey due to ineligibility.
C. Data and Safety Monitoring [Adverse Events, Unanticipated Problems, Protocol Deviations, Violations, and Study Monitoring] Detail your plan for monitoring individual research subjects for protocol compliance and adverse health effects.
There are no anticipated adverse events, serious adverse events, or unanticipated problems. Participants may end involvement at any time by exiting the survey.
The study documents list the RTI Principal Investigator (PI), Jessica Pepper, as the contact for questions about the study and the RTI Office of Research Protection (i.e., IRB) as the contact for questions about their rights as study participants. For any participant complaint received by Dr. Pepper (or received by RTI IRB and forwarded to Dr. Pepper) or study staff observation of a protocol deviation, the complaint and/or study staff observation will be assessed against the following RTI IRB threshold for sharing with the RTI IRB via a Report of New Information (RNI):
A situation that may indicate an unanticipated risk to a participant or others,
An interaction occurring in the course of the research that resulted in intervention by or a request for intervention by child welfare, law enforcement, or emergency personnel
An interaction occurring in the course of the research that resulted in physical injury or other harm to any party including study staff, or any other unanticipated problems.
Non-compliance: Non-compliance with the federal regulations governing human research or with the requirements or determinations of the IRB, or an allegation of such non-compliance.
Audit: Audit, inspection, or inquiry by a federal agency.
Report: Written reports of study monitors. (Not applicable for this study)
Researcher error: Failure to follow the protocol due to the action or inaction of the investigator or research staff.
Confidentiality: Breach or unintended disclosures of confidentiality or loss of control of assets containing human subject data.
Unreviewed change: Change to the protocol taken without prior IRB review to eliminate an apparent immediate hazard to a subject.
Complaint: Complaint of a subject that cannot be resolved by the research team.
Suspension: Premature suspension or termination of the research by the sponsor, investigator, or institution.
For any complaint received by Dr. Pepper (or received by RTI IRB and forwarded to Dr. Pepper) or study staff observation that does not meet the threshold for an RNI, the RTI technical staff (Dr. Pepper and/or Ashley Feld, Project Manager)) will inform the FDA technical staff (Katherine Margolis and Jennifer Bernat).
For any complaint or study staff observation that does meet the threshold for an RNI, Dr. Pepper will inform the RTI IRB via an RNI. If the RTI IRB independently receives a complaint that rises to the threshold of an RNI, the RTI IRB will inform Dr. Pepper and instruct her to submit an RNI.
For any submitted RNI, the RTI technical staff will inform the FDA technical staff within 2 business days of submission. FDA technical staff will then inform FDA IRB of the pending RNI. Once the RTI IRB reviews the RNI, the resulting determination will be shared with the RTI technical staff. The RTI technical staff will inform the FDA technical staff of the RNI determination, and FDA technical staff will then inform FDA IRB of the RNI determination. Similarly, if the FDA IRB makes a determination about the information contained in an RNI, the FDA technical staff will inform the RTI technical staff such that the resulting determination can be shared with the RTI IRB. Lastly, the RTI IRB reserves the right to communicate directly with the FDA IRB about an RNI if such communication is warranted.
The FDA technical staff contacts include:
Katherine Margolis
Jennifer Bernat
The FDA IRB contacts include:
FDA IRB: [email protected]
XIX. Data Management, Subject Privacy, and Data Confidentiality (Ref: 45 CFR §46.111(a)(7))
A. Discuss your data collection and management procedures
Lightspeed and any partner survey panel providers will have participants’ names and contact information as part of panelists’ profiles in their databases. However, this information will not be paired with responses to the screener or survey. RTI and FDA will neither receive nor request this information. Lightspeed (the only survey company with access to the study data) will not use that study data to update respondents’ profiles.
Affirmative consent will be obtained online from adult participants and permission from parents of participating youth. Affirmative assent will be obtained online from youth participants.
The following is documentation of RTI’s confidentiality procedures:
In this section, we describe RTI’s approach to maintaining the confidentiality of study data from Lightspeed. These data include information provided directly by respondents to Lightspeed as part of the screener and survey. Partners of Lightspeed will never obtain this information because the survey is programmed, administered, and hosted by Lightspeed; partners merely provide a link to access the survey. Lightspeed will not combine information obtained about respondents from other sources (e.g., panelists’ profiles) with data from the study. We also describe the legal foundation for confidentiality and the confidentiality procedures we follow at RTI.
i. Confidentiality
Lightspeed LLC and any partners maintain an online opt-in panel to collect participant information. Partners might be used to assist with recruiting; however, they only provide links to the Lightspeed survey. Lightspeed Research collects survey data via their platform (surveys.globaltestmarket.com) which has a Domain Validation certificate issued by Let's Encrypt. Lightspeed Research does not store personally identifiable data in the cloud, but deidentified survey data from this study may be stored using cloud-based services. Lightspeed was approved by RTI’s Cloud Technology Approval Committee (CTAC), which assesses the security and confidentiality of cloud computing providers, on January 30, 2020. Lightspeed is not FedRAMP certified. A full copy of Lightspeed LLC’s privacy policy can be found in Appendix M. Lightspeed will store data for 1 year before deletion.
The survey will not include any personally identifying information (PII), nor will any PII be requested. Though Lightspeed and its recruitment partners maintain databases of names and email addresses of potential participants as part of their normal operations, this information will not be paired with responses and neither FDA nor RTI will receive this information or any other PII from Lightspeed as part of the data set. Each respondent will be known to FDA and RTI only by a unique alphanumeric variable provided by Lightspeed. The data will be stored by RTI and FDA on a restricted-access folder on a shared network drive. To ensure compliance with all applicable information security laws, statutes, and agency directives, RTI has implemented the IT security guidelines and principles published by the National Institute of Standards and Technology (NIST). RTI’s network meets all NIST confidentiality, integrity, and availability security standards for both low and moderate risk. RTI’s security practices include the use of a virtual private network (VPN) and SSL and IPsec for encryption of data in transit when required based on project needs. RTI will send the deidentified dataset to FDA using a secure and password-protected online portal (e.g., ftp site). RTI will store data for 5 years before deletion.
ii. RTI’s Confidentiality Statement
Employing appropriate confidentiality procedures and presenting them to potential study respondents are essential to the success of RTI research studies. Our confidentiality procedures are designed to implement safeguards to ensure potential study respondents that their data are protected should they decide to participate in the research.
As part of the informed consent process for every RTI study, potential study participants are informed about the
purpose of the data collection,
types of information to be obtained,
persons or entities who will have access to their information, and
fact that all information provided by the respondent will be kept confidential to the fullest extent of the law, including that this study is covered by a Certificate of Confidentiality.
iii. Legal Foundation for Confidentiality
Government at all levels has acted to ensure research subjects’ rights to privacy and confidentiality, and these rights are protected by numerous laws and regulations. RTI is committed to complying with the statutes and regulations relating to data collection that safeguard the confidentiality of responses, as demonstrated by the following:
RTI researchers conform to all requirements of the Privacy Act of 1974.
Under sections 45 CFR 46 and 21 CFR 50 and 56 of the Code of Federal Regulations, the RTI IRB evaluates research projects to ensure that human subjects are protected and that confidentiality procedures are adequate.
Under 42 USC section 241(d), RTI will seek authorization to protect the privacy of study subjects by withholding their names and other identifying characteristics from all unauthorized persons.
In addition to the legal requirements listed above, we take other steps to secure the confidentiality of study data. We promise respondents that their data will be treated as confidential and released to the public only in the form of aggregate statistics that cannot be associated with any individual or household. Because this project holds a Certificate of Confidentiality, members of the research team cannot be forced to disclose or provide any private identifiable information, in any Federal, State, or local civil, criminal, administrative, legislative, or other proceeding without permission. Disclosure of research information may only occur in limited instances, such as requests from FDA or situations involving imminent danger.
iv. RTI’s Confidentiality Procedures
RTI is dedicated to maintaining the confidentiality of all information on human subjects, particularly sensitive or identifiable data. We manage information about human subjects in ways that prevent unauthorized access to study data at any time during the study. In this study, RTI will neither request nor receive PII. Respondents must be fully informed about how and to whom their information is released. Each project staff member is required to uphold our confidentiality guidelines and procedures. The primary responsibility for protecting the confidentiality of subjects rests with the project director, who oversees the proper implementation of the procedures and who institutes any changes necessary to ensure confidentiality. All RTI project staff have completed Collaborative Institutional Training Initiative (CITI) training.
The confidentiality procedures that we routinely implement at RTI to maintain the confidentiality of study data are listed below.
The project director must describe for the IRB the confidentiality and data security measures to be used in the study as part of the IRB approval process.
The confidentiality protections must be described in the informed consent form that participants sign.
The principal investigator provides the necessary instruction on confidentiality requirements and procedures to all project staff.
Each staff member involved in any phase of handling sensitive personal information is required to sign a legally binding confidentiality agreement. (Both existing staff and newly hired personnel working on the contract must sign the agreement.)
Staff members are informed that they must not discuss the study with anyone who is not directly involved in the project.
Access to data is restricted to authorized staff members.
Electronic data are stored in a location within the RTI network that provides the appropriate level of security based on the sensitivity or identifiability of the data.
XX. Sample Formats
The formats below, which will be tested in the online survey, have been developed based on previous rounds of research.
Figure 1. Bar Graphs
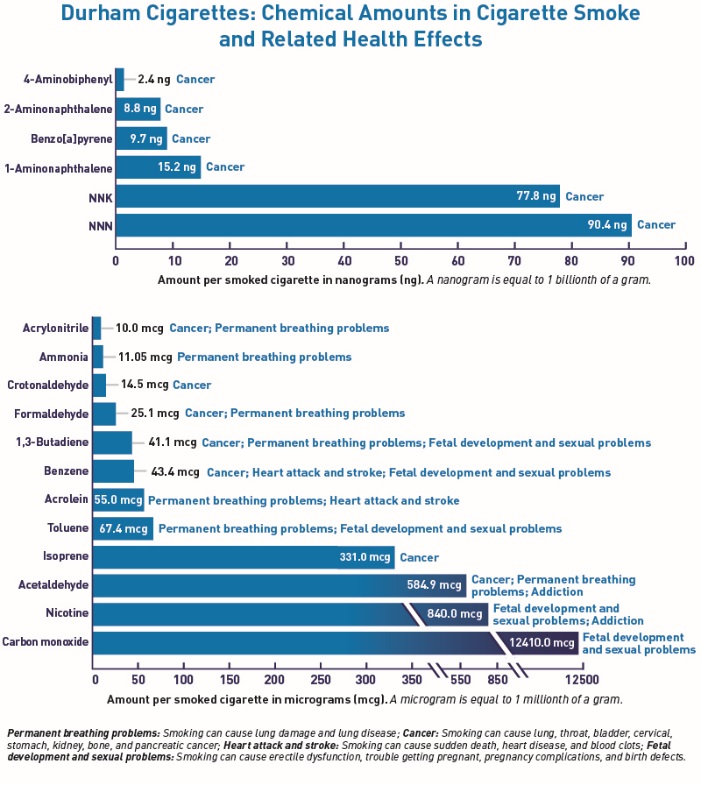
Figure 2. Connecting Lines
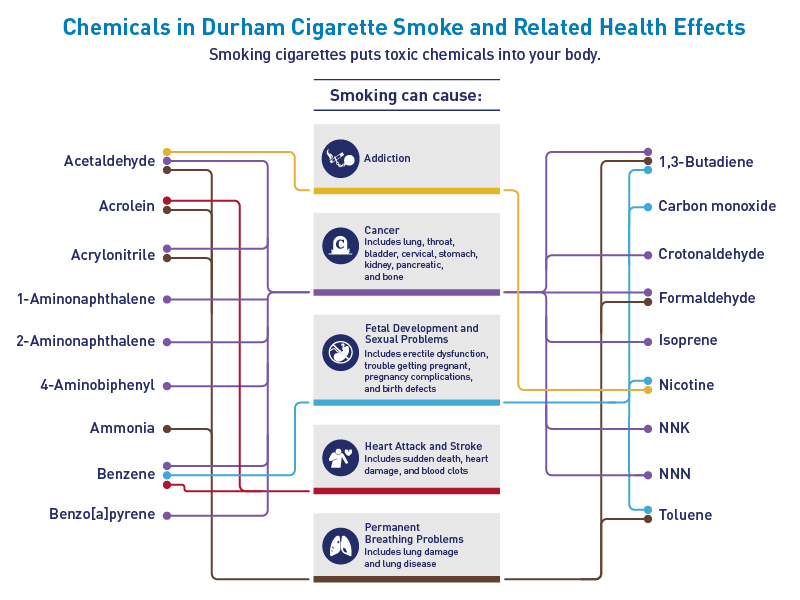
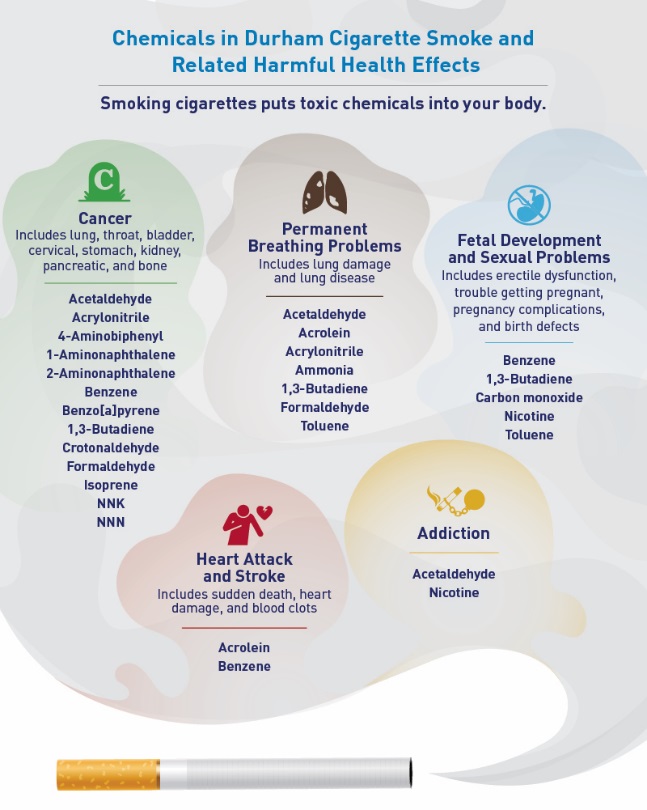
Figure 3. Smoke Cloud of Health Problems
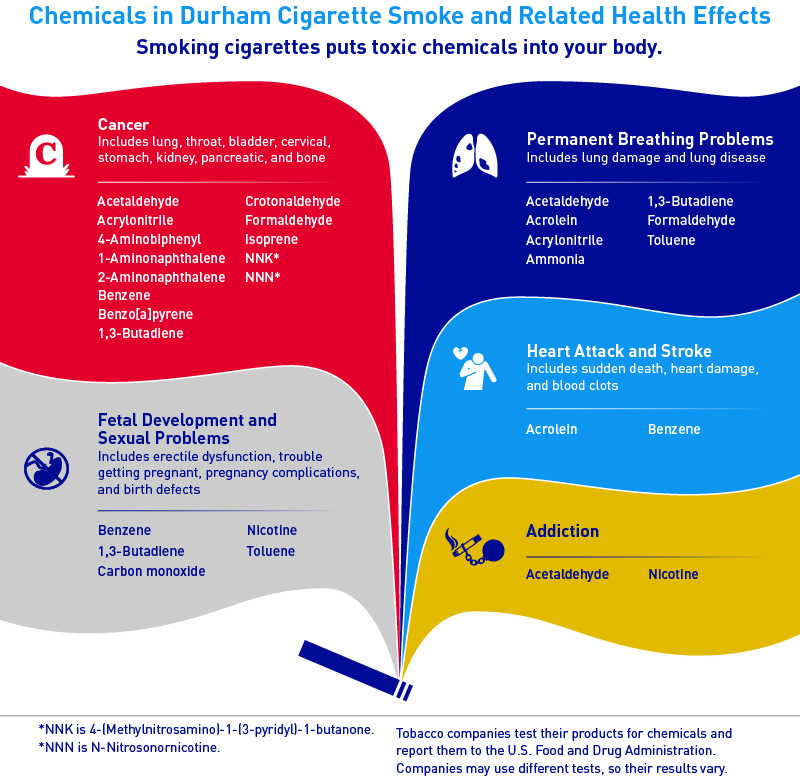
Figure 4. Revised FDA Chart
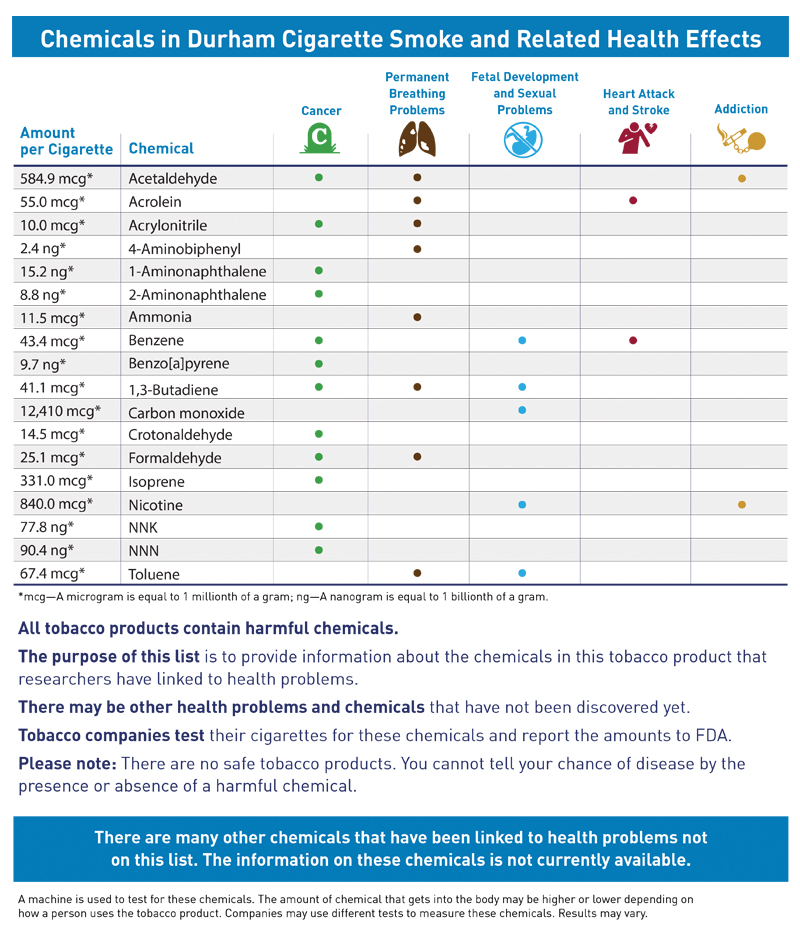
Figure 5. Infographic
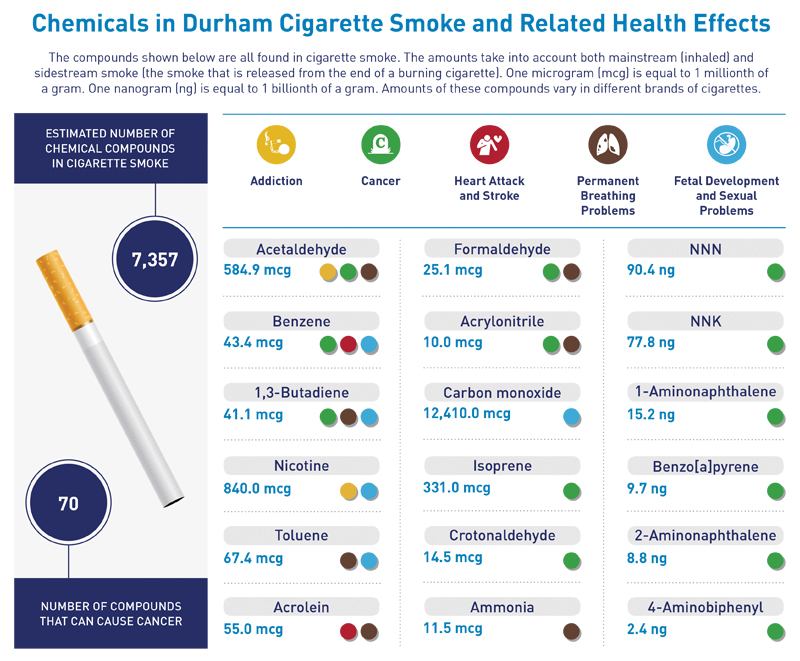
Figure 6. Wildcard with Smoke Clouds
OMB 0910-0880 Expires 11/30/2022
FDA Protocol 18-038
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| File Title | DHHS Letterhead |
| Subject | Letterhead - To Customize |
| Author | JBowers |
| File Modified | 0000-00-00 |
| File Created | 2021-01-13 |
© 2026 OMB.report | Privacy Policy