SS Part B (DPRP)_11-30-20_FINAL
SS Part B (DPRP)_11-30-20_FINAL.docx
CDC Diabetes Prevention Recognition Program (DPRP)
OMB: 0920-0909
Information Collection Request
CDC’s National Diabetes Prevention Program (National DPP) Diabetes Prevention Recognition Program (DPRP) Standards
Revision
OMB No. 0920-0909
Supporting Statement: Part B
Program official/project officer: Stephanie Gruss, PhD, MSW
Centers for Disease Control and Prevention
National Diabetes Prevention Program
Atlanta, Georgia
Telephone number: 770-488-8173
Fax number: 770-488-4639
Email address: [email protected]
November 19, 2020
TABLE OF CONTENTS
B. COLLECTION OF INFORMATION EMPLOYING STATISTICAL METHODS
B-1 Respondent Universe and Sampling Methods
B-2 Procedures for the Collection of Information
B-3 Methods to Maximize Response Rates and Deal With No Response
B-4 Tests of Procedures or Methods to Be Undertaken
B-5 Individuals Consulted on Statistical Aspects and Individuals Collecting and/or
Analyzing Data
REFERENCES IN SSA
ATTACHMENT
1 Authorizing Legislation PHSA
2A 60-Day Federal Register Notice
2B Summary of Public Comments (Placeholder)
3 DPRP Standards 2021
4A DPRP Application Form and Instructions (screenshot)
4B DPRP Evaluation Data Collection Form (screenshot)
5 Table of Changes
6 Human Subjects Research Determination
Section B. Collections of Information Employing Statistical Methods
Statistical methods are not used to select respondents. Respondents (organizational entities offering the National DPP lifestyle change program [LCP] and seeking CDC recognition through the DPRP) self-select by applying for recognition.
1. Respondent Universe and Sampling Methods
The potential respondent universe is any organizational entity in the United States, its territories, or military Army Post Office (APO) sites desiring CDC recognition of its type 2 diabetes prevention lifestyle change program. We anticipate 300 applicant organizations per year and 2,100 CDC-recognized organizations (annualized) submitting evaluation data during the 3-year OMB approval period (March 1, 2021 through February 28, 2024).
Calculating the Burden Hours for Submitting Process/Evaluation Data
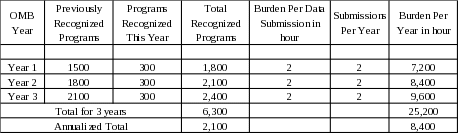
2. Procedures for the Collection of Information
For organizations applying on or after March 1, 2021 (pending OMB approval)
The DPRP administers the CDC DPRP Standards and Operating Procedures (DPRP Standards) (Attachment 3). Any organization with the capacity to deliver the National DPP LCP may apply for CDC recognition. Organizations seeking recognition must complete and submit an online application form (Attachment 4A) which includes organization-level information such as address, contacts, curriculum, and delivery mode. The DPRP application form is located on the National DPP web site (https://nccd.cdc.gov/DDT_DPRP/ApplicationForm.aspx ) and may be submitted at any time.
After submitting the application form, the organization sees a confirmation web page and receives a confirmation e-mail. When the applicant indicates that it is using a CDC-approved curriculum, DPRP staff will notify the applicant by e-mail of the results of the CDC review within 15 working days. When the applicant indicates that it is using an alternate curriculum, DPRP staff will review the alternate curriculum along with the application. In this case, DPRP staff will notify the applicant by e-mail of the results of the CDC review within 4-6 weeks. When an organization’s application has been reviewed and approved, the DPRP will send an e-mail to the organization’s Program Coordinator indicating that the organization has achieved pending recognition status. This e-mail will include the unique organization code assigned by the DPRP, the organization’s effective date (the first day of the month immediately following CDC approval of its application), the organization’s data submission timeline, and instructions for data submission. At the same time, the organization will be listed in the DPRP Registry on the CDC website or in a National DPP program locator application. This entire process takes approximately 15 working days.
When an application for recognition is approved, the organization will have pending recognition status and may begin offering classes on or after the application approval date. An organization is required to start offering classes no later than six months after its effective date.
Evaluation data are transmitted to the DPRP by the organization every 6 months in accordance with the DPRP Standards (Attachment 3). To minimize the burden on organizations and to ensure the quality and utility of the data, each evaluation data transmission consists of a single CSV data file submitted via the DPRP Data Submission Portal (Attachment 4B). Detailed specifications for the evaluation data file are contained in the DPRP Standards Data Dictionary: Evaluation Data Elements (Attachment 3).
As with the 2018 DPRP Standards, the revised 2021 DPRP Standards require organizations to submit evaluation data to the DPRP every 6 months from the organizations’ effective dates (the first day of the month following the approval of an organization’s application). There must be at least one session record per participant in the organization’s submission at six months post effective date and at least six months of participant data in the organization’s submission at 12 months post effective date. Each data submission must include one record per participant for each session attended during the preceding 6 months. The first data submission (6 months post-effective date) must also include records for any sessions attended between the application approval date and the effective date. This will allow for timely data analysis and provide opportunities for the organization to receive interim feedback on its progress in meeting recognition requirements. Data may be submitted at any time during the submission due month. Organizations failing to submit complete and acceptable data in the month in which it is due or failing to report attendance in a 6-month period could lose recognition and face a 6 month wait before re-applying. CDC will send an email notice of loss of recognition only after organizations have not responded to multiple e-mail reminders and technical assistance offers.
Subsequent data submissions should not include participant data previously submitted. After the first 6 month data submission, CDC will provide the organization with an interim Progress Report (PR). When an organization has submitted a full 12 months of complete program data, CDC will prepare the first Evaluation Report (ER) to assess whether the organization has met the requirements for preliminary or full recognition. An organization can progress directly to full recognition and bypass preliminary if all requirements for full recognition are met. Either preliminary or full recognition status will permit an organization to apply to become an Medicare Diabetes Prevention Program (MDPP) supplier. The evaluation will be based on data from participants who attended their first session at least one year but not more than 18 months before the submission due date.
The DPRP sends two data submission reminders to an organization’s contacts. The first is approximately one month prior to the data submission due month and the second is approximately 2 weeks after the data submission due month begins. If CDC does not receive the data submission after the multiple e-mail reminders and offers of technical assistance by CDC, the organization will lose recognition and will be removed from the DPRP Registry. CDC will grant extensions and exceptions to data submission requirements on a case-by-case basis due to extenuating circumstances including, but not limited to, natural disasters, public health emergencies, or unexpected staff losses. However, organizations must communicate these occurrences to CDC as soon as possible so that proper guidance can be given.
For organizations approved prior to March 1, 2021 (pending OMB approval)
Organizations recognized prior to the implementation date of the 2021 DPRP Standards will be sent instructions in advance of March 1, 2021, with instructions on how to make their first data submission under these new Standards.
Organizational evaluation for CDC recognition
An organization can be evaluated at the appropriate time for two different types of CDC recognition as described below—preliminary and full. Organizations will be evaluated for preliminary and full recognition only at the time of required data submissions. To be eligible for an evaluation, organizations must have submitted data on at least one completed cohort. A cohort is considered complete when 365 days have lapsed since the first session of that cohort was held. Organizations will have the option of defining cohorts or allowing each participant to serve as its own cohort. An organization can have multiple cohorts running at the same time. Evaluations are always based on data from participants enrolled in cohorts that held their first session at least one year but not more than 18 months before the submission due date (referred to as evaluation cohorts). This means that multiple participant cohorts can be part of a single 6-month evaluation cohort (which could comprise multiple participant cohorts as a result of multiple classes held). If a new organization wants to be eligible for an evaluation at its first 12-month data submission, it will need to begin offering sessions immediately after approval of its application and before the effective date. This is the only way that the organization will have the full 12 months of data required for an evaluation. Organizations that are not eligible for an evaluation at 12 months will need to wait until their next required data submission at 18 months to determine whether they have achieved preliminary or full recognition.
Pending Recognition
To begin the recognition process, an organization submits an application to the DPRP for each delivery mode it plans to offer. An organization achieves pending recognition when the DPRP determines that the organization has met requirements 1-4 in the DPRP Standards (successful application submission, use of a CDC-approved LCP curriculum, agreement to provide a yearlong LCP, and agreement to provide the stated LCP intensity).
An organization with pending recognition is required to make its first data submission 6 months after its effective date. An organization may remain in pending recognition indefinitely if it continues to submit the required data every 6 months.
Preliminary Recognition
Organizations must meet the requirements for pending recognition.
Organizations must meet this additional requirement--Requirement 5: Organizations must retain at least 5 completers in the evaluation cohort (eligible participants in the evaluation cohort who attended at least 8 sessions in months 1-6 and whose time from the first session held by the cohort to the last session attended by the participant is at least 9 months).
Once an organization meets the requirements for preliminary, the organization may remain in preliminary recognition indefinitely if it continues to submit the required data every 6 months and is able to meet the requirements for preliminary within 3 years of first achieving it, and then at least every 3 years thereafter.
Temporary Preliminary Recognition
If an organization has preliminary or full recognition for one delivery mode and subsequently applies to deliver the National DPP LCP through an additional delivery mode, the DPRP will convey temporary preliminary recognition to the new delivery mode. This is a special designation that will only last until the organization has its first evaluation for the new delivery mode. At that time, the organization will achieve recognition based only on data submitted for the new delivery mode. If the organization is unable to meet the requirements for preliminary or full recognition based on that evaluation, the DPRP will place it in pending recognition status.
Full Recognition
Organizations may receive full recognition for a period of either three years or five years. See additional requirements below for an organization to qualify for full recognition for five years. An organization may remain in full recognition for 3 years if it continues to submit the required data every 6 months. Those organizations still active and submitting data that do not re-achieve full recognition status after 36 months will return to preliminary. MDPP suppliers that lose full recognition will be able to continue as an MDPP supplier with preliminary recognition. Once an organization meets the requirements for full recognition, it will be allowed to remain in full for 3 years despite not meeting the requirements, as long as it continues to make data submissions in every submission due month. Organizations will achieve full recognition when they meet the following criteria:
The requirements for pending recognition.
The requirement for preliminary recognition.
Requirement 6: Organizations must show that there has been a reduction of risk of developing type 2 diabetes among completers in the evaluation cohort by showing that at least 60% of all completers achieved at least one of the following outcomes:
at least 5% weight loss 12 months after the cohort began or
at least 4% weight loss and at least 150 minutes/week on average of physical activity 12 months after the cohort began or
at least a 0.2% reduction in HbA1C
Requirement 7: Organizations must show that at least 35% of completers in the evaluation cohort are eligible for the yearlong National DPP LCP based on either a blood test indicating prediabetes or a history of GDM.
Organizations will be granted an additional 2 years of full recognition (for a total of 5 years) if, at the time full recognition is achieved, the following retention criterion is met:
Eligible participants in the evaluation cohort must have been retained at the following percentages:
A minimum of 50% at the beginning of the fourth month since the cohorts held their first sessions.
A minimum of 40% at the beginning of the seventh month since the cohorts held their first sessions.
A minimum of 30% at the beginning of the tenth month since the cohorts held their first sessions.
3. Methods to Maximize Response Rates and Address Non Responses
CDC designed this information collection to minimize the burden to respondents and to the government, to maximize convenience and flexibility, and to ensure the quality and utility of the information collected. All information submitted to the DPRP is submitted electronically, as specified in the DPRP Standards.
CDC provides regular training to organizations that have questions or may be encountering challenges in providing the required information within the required timeframes. Training includes monthly webinars to review the Standards and data submission requirements and resources on the National DPP Customer Service Center (CSC) site: https://nationaldppcsc.cdc.gov/s/. The DPRP also offers tailored technical assistance calls to organizations to help them achieve preliminary or full recognition. The frequency and nature of technical assistance efforts are based on demand, CDC staff resources, and emerging issues that are shared by multiple organizations. Concurrent with the release of the 2021 Standards, the DPRP will post quick, self-paced webinars on the National DPP CSC website. These will include, but are not limited to, an updated Welcome Kit, helpful hints for Lifestyle Coaches and organizations, and data submission assistance.
The online application form (Attachment 4A), the evaluation data elements (Attachment 4B), and the instructions for their transmission (Attachment 3) are posted on the National DPP website https://www.cdc.gov/diabetes/prevention/pdf/dprp-standards.pdf. Potential applicants are encouraged to read and carefully review this information before applying for recognition. They are also strongly encouraged to complete the Organizational Capacity Assessment found in the Standards (Attachment 3) as Appendix A.
Participation by organizations is strictly voluntary. Organizations may withdraw from the DPRP at any time. In addition, an organization’s CDC-recognition may be revoked for not submitting evaluation data to the DPRP; but, every effort will be made by CDC to work with organizations prior to revocation of recogntion. Regardless of the circumstances of the withdrawal, once withdrawn, these organizations must wait 6 months prior to reapplying for CDC recognition.
4. Tests of Procedures or Methods to be Undertaken
Prior to receiving initial OMB approval in 2011, a DPRP workgroup comprised of internal and external stakeholders, including representatives from academic institutions and other organizations, met several times over the course of one year. During this time, the workgroup provided input on required data elements, on the frequency of data transmission to CDC, and on the application form and instructions. A hard copy of the DPRP application form was pilot tested with seven individuals (three CDC employees and four staff of potential applicant organizations).
During subsequent revisions (2015, 2018, and 2021), CDC continued to gather feedback through listening sessions with key National DPP stakeholders on the effectiveness and relevance of the Standards. Stakeholders included: state health departments; federal partners such as CMS, national organizations such as the American Medical Association and the American Diabetes Association, and a sample of currently recognized organizations based on size, ownership, and delivery mode. Recommendations from stakeholder listening sessions directly informed previous revisions and will inform this one. In addition, CDC has conducted updated DPRP data analyses on organizational performance trends, including organizations serving as MDPP suppliers.
Feedback was reviewed by National DPP staff and other CDC leaders in type 2 diabetes prevention. The DPRP Standards (Attachment 3), the online application form (Attachment 4A), and the evaluation data elements (Attachment 4B) were revised as deemed appropriate.
The MDPP Expanded Model was announced in early 2016, when the Secretary of Health and Human Services determined that the National DPP LCP met the statutory criteria for inclusion in Medicare’s expanded list of healthcare services for beneficiaries (https://innovation.cms.gov/initiatives/medicare-diabetes-prevention-program/). This is the first time a preventive service model from the CMS Innovation Center has been expanded. After extensive testing of the DPP model in 17 sites across the U.S. in 2014-2016, CMS implemented the MDPP in Sections 1102 and 1871 of the Social Security Act (42 U.S.C. 1302 and 1395hh §424.59), authorizing CDC-recognized organizations to enroll as MDPP suppliers in order to bill CMS for these services. The enrollment process began in April 2018. Only organizations in good standing with the DPRP are eligible to become MDPP suppliers. MDPP reimbursement continues to be dependent on organizations achieving either preliminary or full recognition status from the DPRP.
The intent of the current Standards revision is to continue to align with the CMS MDPP rule that was finalized in 2017 and went into effect on January 1, 2018, and to account for new evidence in the type 2 diabetes prevention literature.
5. Individuals Consulted on Statistical Aspects and Individuals Collecting and/or Analyzing Data
Staff in CDC’s Division of Diabetes Translation (DDT) (CDC personnel and contractors) have been consulted on the development and maintenance of the DPRP data collection processes and automated platforms, including the online application form, the online registry, and the web application [program] for data submission. All data management, analysis, and reporting are performed at CDC by DDT staff and on-site contractors. The individuals listed below were consulted on all statistical aspects of the DPRP. DDT staff will be responsible for DPRP data collection and data analysis.
Ann Albright, PhD, RD
Director
Division of Diabetes Translation
National Center for Chronic Disease Prevention and Health Promotion
Centers for Disease Control and Prevention
Phone: 770-488-5000
Stephanie Gruss, PhD, MSW
Program Manager, Diabetes Prevention Recognition Program
Division of Diabetes Translation
National Center for Chronic Disease Prevention and Health Promotion
Centers for Disease Control and Prevention
Phone: 770-488-8173
E-mail: [email protected]
Elizabeth Ely, MS
Mathematical Statistician
Division of Diabetes Translation
National Center for Chronic Disease Prevention and Health Promotion
Centers for Disease Control and Prevention
Phone: 770 488-8086
Email: [email protected]
Kunthea Nhim, DrPH, MPH
Epidemiologist
Division of Diabetes Translation
National Center for Chronic Disease Prevention and Health Promotion
Centers for Disease Control and Prevention
Phone: 770-488-0912
E-mail: [email protected]
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| File Title | OMB Supporting Statement B |
| Author | arp5 |
| File Modified | 0000-00-00 |
| File Created | 2021-02-02 |
© 2026 OMB.report | Privacy Policy