Non substantive change request
0014 Change Request to FDA 1571 March 2021.docx
Investigational New Drug Regulations
Non substantive change request
OMB: 0910-0014
UNITED STATES FOOD & DRUG ADMINISTRATION
Applications for FDA Approval to Market New Drug
OMB Control Number 0910-0014
Non-substantive Change Request:
I. Background:
This information collection supports FDA regulations in 21 CFR Part 312, which govern the use of investigational new drugs, including procedures and requirements for the submission of Form FDA 1571 entitled, “Investigational New Drug Application (IND).” Our primary objective in reviewing an IND is to help protect the rights and safety of subjects and to help ensure that the quality of the clinical trial is adequate to evaluate a drug’s effectiveness and safety for the studied indication. To supplement the Form FDA 1571 instructions, we issued a draft guidance in May of 2015 entitled “Investigational New Drug Applications Prepared and Submitted by Sponsor Investigators” available at https://www.fda.gov/files/drugs/published/Investigational-New-Drug-Applications-Prepared-and-Submitted-by-Sponsor-Investigators.pdf. Form FDA 1571 is available as a fillable PDF document and accompanying instructions may be found on our website at https://www.fda.gov/media/116608/download.
II. Proposed change:
We have developed an additional collection method for data elements in Form FDA 1571 for research INDs (often/sometimes referred to as “non-commercial” INDs), however the scope of data being collected will remain unchanged. Also, the new collection method will only apply to research INDs. We generally consider a research IND to be one for which the sponsor (typically an individual investigator, academic institution or non-profit entity) does not intend to later commercialize the product. These studies are strictly for research, are usually shorter in duration than INDs for commercial development, and may result in publications in peer-reviewed journals. For sponsors (including sponsor/investigators) interested in filing or updating a research IND, a new web-based interface will be available on a mobile device or desktop to help sponsors fill out Form FDA 1571. The web-based interface will also allow sponsors to electronically submit the completed Form FDA 1571 and associated files.
The information being collected is described in 21 CFR 312.23 (a) through (f) and on Form FDA 1571, and will be submitted to FDA through a new, secure web-based interface. Use of this web-based submission is voluntary, and respondents may still elect to use a fillable PDF version of Form FDA 1571, submitted via mail, email, or fax. We believe the web-based collection method will enhance user experience with data submission to FDA and will help improve agency efficiencies. At the same time, we have made no adjustment to the currently estimated burden for the information collection at this time. Upon later evaluation we will modify our estimate as appropriate. Screenshots for the interface are included below.
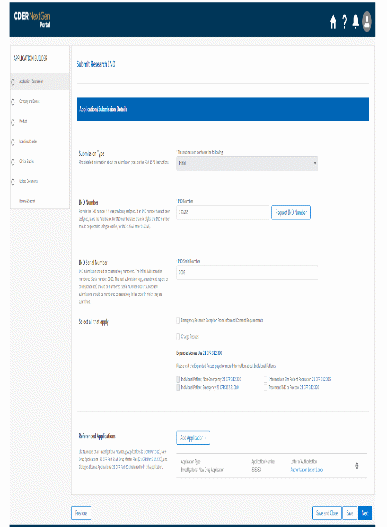
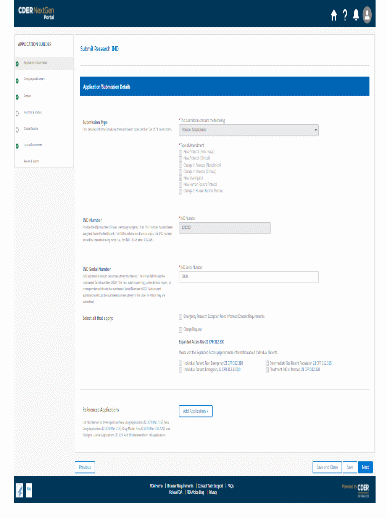
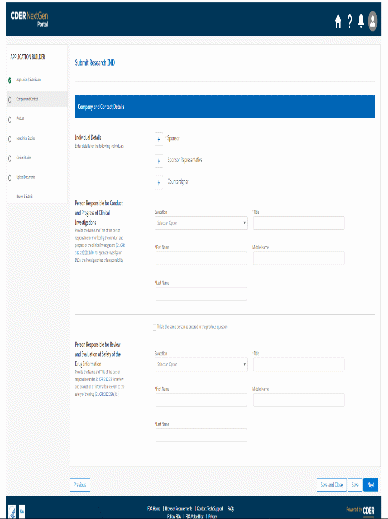
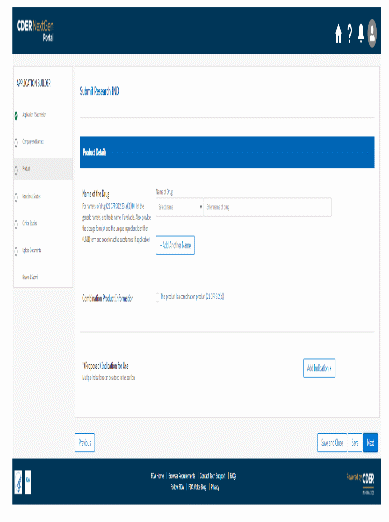
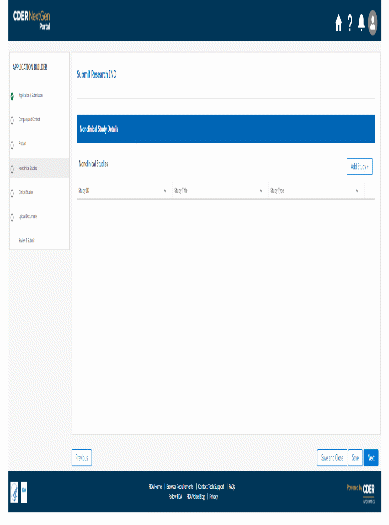
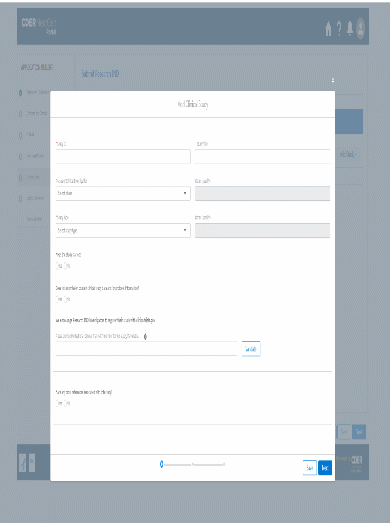
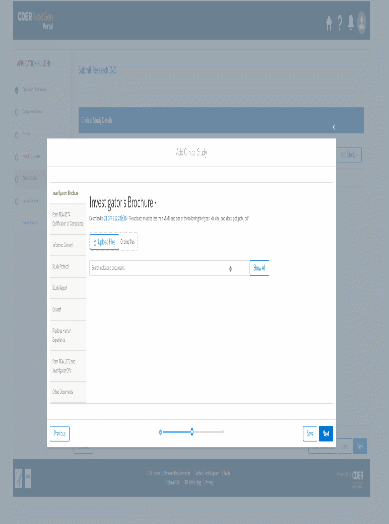
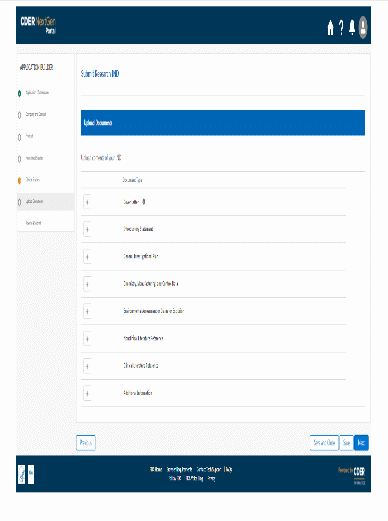
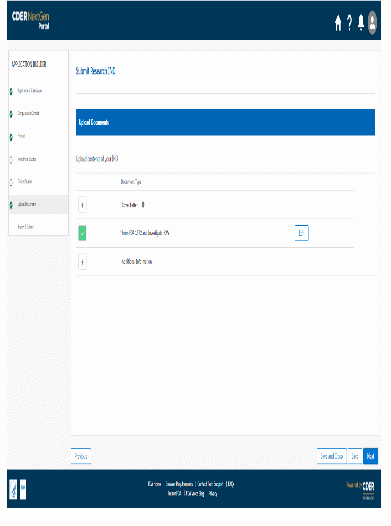
We also included the screenshots of the current FDA form 1571.



| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| File Modified | 0000-00-00 |
| File Created | 2021-03-15 |
© 2026 OMB.report | Privacy Policy