0277ss21
0277ss21.docx
Pesticide Product Performance Data Requirements for Products Claiming Efficacy Against Certain Invertebrate Pests (Proposed Rule)
OMB: 2070-0060
January 26, 2021
SUPPORTING STATEMENT
FOR AN INFORMATION COLLECTION REQUEST (ICR)
1. IDENTIFICATION OF THE INFORMATION COLLECTION
1(a) Title of the Information Collection
TITLE: Proposed Rule-related
ICR Amendment for
Pesticide Product Performance Data
Requirements for Products Claiming Efficacy Against Certain
Invertebrate Pests (RIN 2070-AJ49)
ICR
Numbers: EPA ICR No.:
0277.21; OMB Control No.: 2070-0060
Docket
ID No.:
EPA-HQ-OPP-2020-0124
1(b) Short Characterization/Abstract
This information collection request (ICR) addresses the information collection activities in the proposed rule entitled “Pesticide Product Performance Data Requirements for Products Claiming Efficacy Against Certain Invertebrate Pests” (RIN 2070-AJ49). EPA is proposing to codify product performance data requirements to support registration of pesticidal products claiming efficacy against three categories of invertebrate pests: Those identified to be of significant public health importance (e.g., ticks, mosquitoes, cockroaches, etc.), wood-destroying insects (e.g., termites), and certain invasive invertebrate species (e.g., Asian longhorned beetle). The two latter categories are non-agricultural pests considered to be of significant economic or ecological importance. Product performance data (efficacy studies) document how well the pesticide performs the intended function (such as killing or repelling) against an invertebrate pest.
This ICR is designed to serve as an amendment to the existing ICR entitled “Application for New and Amended Pesticide Registration,” identified as EPA ICR No. 0277.21 and approved under OMB Control No.: 2070-0060. The existing ICR addresses the information collection activities associated with the registration of a pesticide product under Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA) Section 3. FIFRA provides EPA with the authority to regulate the distribution, sale and use of pesticides in the United States and ensure that pesticides will not pose unreasonable adverse effects to human health and the environment.
This information collection is designed to provide the EPA with the necessary information to evaluate an application for the registration of a pesticide product, as required under Section 3 of the FIFRA and section 408 of the Federal Food, Drug, and Cosmetic Act (FFDCA) (see Attachment A). Under FIFRA, EPA must evaluate pesticides comprehensively before they can be marketed and used in the United States to ensure that they will not pose unreasonable adverse effects to human health and the environment. This registration evaluation includes determining whether the composition of the pesticide warrants the proposed claims for it, the pesticide's labeling complies with all applicable requirements (e.g., is not false or misleading), and the pesticide will perform its intended function without unreasonable adverse effects on the environment. See FIFRA sec. 3(c)(5). Pesticides that meet this test are granted a license or "registration" which permits their distribution, sale and use according to requirements set by EPA to protect human health and the environment.
Product performance data (efficacy studies) document how well the product performs the intended function (such as killing or repelling) against an invertebrate pest. This proposed rule would change the regulatory mechanism by which this information would be collected, but for the most part, the data requirements that EPA is proposing for codification are consistent with EPA’s current practices in data supporting applications for registration of a pesticide product that bears a pesticidal claim against one or more of these pests. Additionally, the Pesticide Registration Improvement Extension Act of 2018 (PRIA 4) requires EPA to finalize product performance data requirements by September 30, 2021. Specifically, the Act states that, “The Administrator shall, not later than September 30, 2021, issue regulations prescribing product performance data requirements for any pesticide intended for preventing, destroying, repelling, or mitigating any invertebrate pest of significant public health or economic importance specified in clauses (i) through (iv) of subparagraph (B) [bed bugs; premise (including crawling insects, flying insects, and baits), pests of pets (including pet pests controlled by spot-ons, collars, shampoos, powders, or dips), and fire ants].”
2. NEED FOR AND USE OF THE COLLECTION
2(a) Need/Authority for the Collection
This action is issued under the authority of sections 3, 5, 10, 12, and 25 of FIFRA (7 U.S.C. 136–136y), as amended. Under FIFRA section 3(c)(2)(A), EPA is required to specify “the kinds of information which will be required to support the registration of a pesticide and shall revise such guidelines from time to time.” EPA’s codification of these data requirements is in 40 CFR part 158.
2(b) Practical Utility/Users of the Data
EPA requires the submittal of the product performance information to review the efficacy studies and other information to make determinations under FIFRA.
3. NON-DUPLICATION, CONSULTATIONS, AND OTHER PRA REQUIREMENTS
3(a) Non-duplication
Duplication should not occur in this program, as EPA has the sole authority to regulate pesticides in the United States and establish efficacy data requirements under 40 CFR part 158. Therefore, this information is not requested by other agencies. EPA support of data sharing (subject to data compensation as directed by FIFRA) to reduce the potential for duplicative studies being submitted to EPA.
3(b) Public Notice Required Prior to ICR Submission to OMB
Pursuant to 5 CFR 1320.11(b), the proposed rule will announce the availability of this ICR and provide a 60-day public comment opportunity. Comments received on the proposed rule and this ICR will be addressed in the final rule. At which time EPA will provide an ICR for the final rule and complete the amendment to the existing ICR.
3(c) Consultations
Under 5 CFR 1320.8(d)(1), agencies are required to consult with respondents about the information collections before submitting approval requests to OMB. In accordance with this regulation, EPA staff will contact representatives from a cross section of stakeholders to seek feedback on the burden estimates in this ICR, and on the clarity of the information collection process.
3(d) Effects of Less Frequent Collection
The burden assumes a minimum of one submittal per product registration which is the least possible collection that allows the Agency to conduct the statutorily required review.
3(e) General Guidelines
Since the proposed rule involves a submission (i.e., the notification) and recordkeeping, the following discussion is a summary of a more detailed discussion of applicable PRA guidelines that is contained in the existing ICR.
(i) Recordkeeping.
The recordkeeping activities applicable to pesticide registrants exceed OMB’s guideline that agencies not require that records be retained for more than three years (5 CFR 1320.5(d)(2)(iv)). As authorized under FIFRA Section 8, EPA regulations under 40 CFR 169.2(k) require that registrants retain records containing research data relating to a registered pesticide, including all data submitted to EPA in support of a registration, for as long as the registration is valid, and the producer is in business. However, the burden related to the recordkeeping requirements is covered under another ICR (see OMB Control No. 2070-0028, Recordkeeping Requirements for Producers of Pesticides under Section 8 of FIFRA).
(ii) Electronic submissions.
EPA intends to offer the use of the Pesticide Submission Portal (PSP), a fully electronic alternative as an option for submitting registration forms electronically. The PSP leverages the Agency’s existing Central Data Exchange (CDX) to provide a secure method of submitting information within a secure online environment. CDX requires initial user registration for which the paperwork burden estimate is covered under “Cross-Media Electronic Reporting Rule” ICR, OMB No. 2025-0003.
EPA will continue to accept paper applications but encourages applicants to take advantage of this new, more efficient option and forego the courier costs to send to EPA. For electronic submissions, applicants do not need to submit multiple copies of any pieces of their application, the requirement for multiple copies of data and five copies draft labeling only applies to paper submissions. Extensive guidance regarding available electronic submission options is available to registrants via the Office of Pesticide Program’s (OPP) website at http://www2.epa.gov/pesticide-registration/electronic-submissions-pesticide-applications.
3(f) Confidentiality
In accordance with FIFRA Section 10 and 40 CFR Part 2, Subpart B, EPA will protect from disclosure all data and/or information brought to the Agency in conjunction with this information collection that may be claimed as trade secret, commercial, or financial information.
3(g) Sensitive Questions
No information of a sensitive or private nature is requested in conjunction with this collection activity. In addition, this information collection activity complies with the provisions of the Privacy Act of 1974 and OMB circular A-108.
4. THE RESPONDENTS AND THE INFORMATION REQUESTED
4(a) Respondents - NAICS Codes
The proposed notification addressed in this ICR affects individuals or entities engaged in activities related to the development and submission of product performance data for the specified invertebrate pests and are described in more detail in the cost analysis prepared for the proposed rule, which is also available in the docket for the proposed rule. EPA has determined that these entities can be identified by the following North American Industrial Classification System (NAICS) codes:
• Chemical Producers (NAICS 32532), e.g., pesticide manufacturers or formulators of pesticide products, pesticide importers or any person or company who seeks to register a pesticide.
• Research and Development in the Physical, Engineering, and Life Sciences (NAICS code 541712), e.g., research and development laboratories or services that perform efficacy testing for invertebrate pests.
• Colleges, universities, and professional schools (NAICS code 611310), e.g., establishments of higher learning which are engaged in development and marketing of products for invertebrate pest control.
4(b) Information Collection Activities
Currently, submission of an application for a registration requires the following application materials:
Forms for pesticide registration applications.
Supporting data may be required as part of the submission.
Draft labeling that meets the regulatory requirements set out in 40 CFR 152.50.
This action codifies the product performance data requirement for products claiming efficacy for certain invertebrate pests (pests of public health significance, pests of economic significance, and certain invasive exotic species). These activities and associated burdens are already approved under OMB Control No. 2070-0060 (EPA ICR No. 0277.21). As detailed in the existing ICR, the estimated annual burden per respondent ranges from 14 to 646 hours for registration application activities, and from 527 to 46,000 hours for data generation related to registration of a new active ingredient, with the variation related to the type of registration involved.
EPA estimates that this proposed rule would result in $1 million annual savings to registrants. The savings would be entirely attributable to reduced uncertainty from codification of requirements. It would include $900,000 annual savings from registrants avoiding conducting unnecessary efficacy tests (e.g., conducting tests on four tick species when only three are needed to receive a tick claim), and $100,000 in savings in the processing of applying for label claims. EPA estimates that registrants submit 60 data packages to the Agency annually for efficacy review, and thus this impact is equivalent to about $17,000 in cost savings per data package submitted. Of this $900,000, $330,000 is savings attributable to reduced paperwork burden - $15,000 from reduced submission paperwork burden, and $315,000 from reduced data generation paperwork burden.
(i) Data items - Proposed reporting & recordkeeping requirements.
The proposed data requirements are presented, as appropriate, in table formats, with the needed data specified according to the claim on the label, the species to be tested, and the performance standards to be met. More detailed information on the proposed data requirement tables can be found in the docket for this action: EPA-HQ-OPP-2020-0124.
(ii) Respondent PRA activities.
The following table describes the expected paperwork related activities that the submitter might engage
Respondent Paperwork Activity |
Description |
1. Read instructions |
Read germane statutory provisions and implementing regulations in 40 CFR parts 158.
|
2. Plan activities |
Decide whether the product qualifies is subject to the proposed data requirements; |
3. Gather information |
Canvass/contact other companies holding EPA registrations or marketing similar products, if any, to determine whether it would be appropriate to share or rely on testing data already submitted by another company; |
4. Create information
|
If submitting study data, arrange for testing of product performance and efficacy data required by germane regulations to support registration;
|
5. Compile and review |
Assemble data, evaluate for accuracy, appropriateness, and completeness;
|
6. Complete paperwork |
Complete all appropriate application documents;
|
7. Submit notification to EPA |
Using preferred option for submission, submit required information to the EPA. |
8. Store/maintain data |
File and maintain copies of all registration data submitted to the Agency. |
5. THE INFORMATION COLLECTED – AGENCY ACTIVITIES, COLLECTION METHODOLOGY, AND INFORMATION MANAGEMENT.
5(a) Agency Activities
The proposed rule would not change the Agency’s current registration practice. In general, the degree and level of Agency activities in the review of data submissions will depend on the complexity of the product being registered, and whether it is identical or substantially similar to other products already registered. Products containing active ingredients present in currently registered products and proposed for uses currently registered (“me-too” registrations) may require only a minimal review for completeness of the application, the adequacy of the labeling, and the satisfaction of data compensation requirements.
New Registrations
A product containing a new active ingredient will require multiple data reviews related to physical chemistry, toxicology, environmental fate, ecological effects, worker exposure, residue chemistry, environmental chemistry, and product performance prior to approval. Therefore many divisions may be actively involved in the data analysis and agency determination of OPP registration actions. For conventional pesticides, the application is reviewed by ITRMD, the Registration Division (RD), the Health Effects Division (HED), and the Environmental Fate and Effects Division (EFED), and Biological and Economic Analysis Division (BEAD). For biological/biopesticide pesticides, the application is reviewed by ITRMD, and the Biopesticides and Pollution Prevention Division (BPPD). Applications for antimicrobial pesticide products are reviewed by ITRMD and the Antimicrobials Division (AD). The Agency notifies an applicant when an application is incomplete or is found to be deficient. The applicant is permitted to correct the deficiencies and submit the corrections.
Amendments
Once issued, a registration also may be amended in various ways, such as adding or deleting uses and pests, modifying the labeling, or altering the product composition in minor ways. To request these changes, the registrant is required to submit an application for amended registration on EPA Form 8570-1, along with all appropriate additional forms, labeling and supporting data.
Label Approvals
Label reviews are most often accomplished by a Product Manager, or Team Leader, in one of the three regulatory divisions within EPA’s Office of Pesticide Programs (OPP) responsible for pesticide registration: the Registration Division, the Antimicrobials Division, and the Biopesticides and Pollution Prevention Division. These divisions ensure that revisions comply with the applicable labeling requirement or guidance.
A general category of OPP’s activities related to the information collection described in this ICR is summarized below:
Receive Application
The pesticide registration application package, complete with the required forms, necessary data, and proposed labels, is received by the Front-End Processing Unit in the Information Technology and Resources Management Division (ITRMD). After screening the application for administrative completeness, ITRMD refers the complete application and any accompanying data to the appropriate regulatory division. ITRMD is responsible for entering the registration action into OPP’s central tracking database system, called OPPIN. If the application form is accompanied by data to support the registration application (e.g., new active ingredients and new uses), ITRMD will forward the registration data package to a contractor for inputting into the tracking database. After this is completed, the data package is routed to the appropriate regulatory division for processing.
Review Application
The regulatory divisions, based on the registration action, assign the packages for appropriate evaluation. Each scientific discipline reviews the data and may develop a Data Evaluation Report (DER) and appropriate risk assessments that summarize the data review.
If the registration application is clearly for a “me-too” pesticide product or use, then the product may be registered on an expedited basis by the reviewer. If its similarity to a pesticide currently registered by the EPA is questionable, it may be sent for a short interdisciplinary review. The Program Manager or Team Leader ensures that the database is updated by identifying where it is sent for review. If the registration action is clearly not for a “me-too” pesticide product or use, then action is taken to correct the assignment of the registration action and to route the data to the appropriate scientific evaluation group for full data reviews.
Make Registration Decision
The Program Manager or Team Leader examines all of the scientific reviews and proposed labeling and determines whether the product may be registered. If the product contains an active ingredient not currently registered by EPA, the review summary is included as part of a decision package and referred to the Director of OPP for a final decision on whether or not to register a pesticide. When a new food use is sought, a tolerance or exemption is established for an already registered active ingredient (e.g., new use), the final decision is made by the Division Director of the registering division.
If the registration action is for revised labeling in response to a Pesticide Registration Notice, the revised labeling submitted along with appropriate EPA forms will be reviewed by a Program Manager or Team Leader for compliance with the applicable Pesticide Registration Notice and, following the registration decision, entered into the tracking database.
Notify Applicant
OPP sends a Notice of Registration to the applicant informing the applicant that the product has been registered and specifying any conditions of registration. For labeling amendments, a letter is sent to the applicant stating approval/disapproval. If the label amendment is approved, a stamped master label is sent to the registrant.
Store and Maintain Data
OPP stores, files, and maintains copies of any registration notices and labeling information.
5(b) Collection Methodology and Management
All registration and regulatory determination actions are entered into OPP’s central database system, called OPPIN, to track progress toward preapplication determination or registration. All registration actions are entered into OPP’s central database system, called OPPIN, to track progress toward registration. Registration actions accompanied by data (e.g., products containing new active ingredients or new uses) are also entered into the database to track progress toward registration. Once a product has been registered, pertinent status information regarding the product is revised in the tracking database.
5(c) Small Entity Flexibility
EPA offers direct assistance to small entities, facilitating their compliance with the requirements for obtaining an exemption.
5(d) Collection Schedule
Not applicable. This information is only conducted as a registration application is received for consideration. There is no set schedule for submission of this information to EPA.
6. ESTIMATING BURDEN AND COST OF THE COLLECTION
The paperwork burden associated with the proposed rule is based on the information and activities identified in the existing ICR and is considered to off-set or serve as an alternative burden for that estimated in the existing ICR. The methodology and approach presented here is consistent with that used for the existing ICR.
6(a) Respondent Burden Hours and Cost from Submission Process
As defined in the Application for New and Amended Pesticide Registration ICR, there are two main categories of applicants for registration: those requiring submission of a full complement of supporting data (e.g., new active ingredients); and those requiring submission of less data (e.g., amendments, for currently registered chemicals). These have been described as Types A and B, respectively. EPA considers requests for label claims against pests of significant public health importance, wood-destroying, and invasive species pests to be Type B actions. The following table presents the estimated burden and cost estimate per submission for these actions.
Table 1: Estimated Average Burden and Cost per Type B Submission
Collection
Activities, |
Burden Hours |
Total |
|||
Mgmt. |
Technical |
Clerical |
Hours |
Costs |
|
$121.72/hr |
$72.01/hr |
$40.93/hr |
|||
Read Instructions |
7 |
0 |
0 |
7 |
$852 |
Plan activities |
0.5 |
0 |
0 |
0.5 |
$61 |
Gather/create information |
0 |
1.5 |
0 |
1.5 |
$108 |
Compile and review |
0.5 |
0.5 |
0 |
1 |
$97 |
Complete paperwork |
0 |
0 |
3 |
3 |
$123 |
Submit information |
|
|
|
|
|
Store/maintain data |
0 |
0 |
1 |
1 |
$41 |
Third party disclosure |
|
|
|
|
|
TOTAL |
8 |
2 |
4 |
14 |
$1,282 |
*Numbers may not sum due to rounding.
EPA does not expect the passage of the rule as proposed to significantly change the estimates of the paperwork burden from the submission process. According to the Application for New and Amended Pesticide Registration ICR, the Agency reviews 7,273 Type B actions annually. Product efficacy reviews account for about 60 reviews, on average, every year, or less than one percent of the total; thus, the burden of these particular actions has little measurable effect on the overall average. EPA has not estimated the burden associated with applications for efficacy reviews specifically, but it is probably greater than average. In particular, the burden associated with the first two steps, Reading Instructions and Planning Activities, may be high because requirements have not been codified and it may be difficult for applicants to ascertain what information should be submitted. Codifying the requirements would make it easier for applicants to obtain the information they need, reducing the burden of seeking that information and identifying the appropriate studies.
EPA estimates that annually 12 data packages are submitted to the EPA that lack the appropriate efficacy data to support the requested label claims. EPA expects the proposed rule to eliminate this category of waste because applicants would know what data are required. A reduction of 12 applications per year with an estimated paperwork burden of $1,282 implies a cost savings of approximately $15,0001 annually resulting from the rule as proposed. Each action is estimated to take 14 hours – a reduction of 12 applications per year implies a reduction in burden hours of 1682 annually. If the rule were to be finalized, EPA would expect the number of Type B actions to decline from 7,273 to 7,261 per year over the next three years.
In the long run, EPA expects the rule as proposed would lead to increased clarity among registrants about EPA data requirements, and the reduction in uncertainty resulting from the rule may lead registrants to submit more data packages. If registrants increased the number of data packages submitted to the Agency by more than 12 annually, this would result in an increased total submissions, and associated total burden, but with fewer incomplete data packages. The Agency does not have information on whether the clarity resulting from the rule as proposed will result in an increase or decrease in the number of total submissions to the Agency relative to prior to the publication of the rule.
6(b) Respondent Paperwork Burden Hours and Cost from Data Generation
Because this proposed rule would largely codify current agency practices (i.e., the same data was being collected via a different regulatory mechanism [see 40 CFR 158.30 and 158.75]), the agency generally expects respondents to provide product performance data that they would have provided to the Agency respective of the existence of this rule. However, the current lack of clarify regarding the requirements for efficacy data may lead applicants to generate and submit more data than the agency needs; codification would increase clarity and reduce the submission of unnecessary data. Data generation test costs range from $18,400 for a premise treatment product claiming efficacy against cockroaches to $197,700 for an on-animal treatment product claiming efficacy against flies. EPA estimates that the proposed rule would result in a $900,000 annual reduction in data generation costs. In the existing Application for New and Amended Pesticide Registration ICR, paperwork costs are assumed to be 35% of data generation costs, and so EPA calculates a $315,000 annual reduction in data generation paperwork as a result of the proposed rule. Figure 1 illustrates the method for calculating the paperwork burden of data generation.
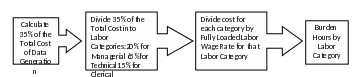
Figure 1: Method for Calculating Paperwork Burden from Test Costs
EPA estimates in the Application for New and Amended Pesticide Registration ICR that 20% of paperwork burden cost is managerial, 65% is technical, and 15% is clerical. Using fully loaded labor wage rates3, EPA estimates that a reduction of $315,000 annually will translate to a reduction in burden hours of 4,515 annually – 518 managerial hours, 2,843 technical hours, and 1,154 clerical hours.
Because of increased clarity resulting from the publication of the rule as proposed, registrants may choose to submit more data packages and the Agency could see an increase in the number of actions. The overall direction of total per-registrant burden is thus uncertain – lower burden per action, but potentially a higher number of actions. In the next three years covered by the ICR, EPA does not expect a measurable increase in number of actions from the rule due to the fact that bringing products to market is time consuming, and the full impact of the rule may not show up in actions for some time.
EPA estimates that the total average annual cost of paperwork burden from data generation for new and amended products is $33.7 million. This rule will save $315,000 annually, or 1% of the total paperwork burden from data generation. EPA estimates that the total average annual burden hours from data generation for new and amended products is 483,000 hours. The rule will reduce the burden by 4,515 hours, or 1% of the total hourly burden from data generation.
6(c) Estimating Agency Burden and Cost
The incremental burden for the agency associated with codifying the product performance data requirements is uncertain. The proposed rule would result in clarity for registrants which may lead to registrants submitting fewer incomplete packages, thus reducing the burden for the agency. However, given the small number of product performance data submissions relative to the total number of submissions reviewed by the agency, the burden will not change measurably. Moreover, the increase in clarity may encourage registrants to submit more packages and this may translate to an increased agency burden. Therefore, the direction and extent of Agency burden in the long term is uncertain but likely to be very small.
The underlying Agency burden for product registration is discussed in more detail in the existing ICR and briefly summarized below:
The estimated number of Agency FTEs dedicated to Section 3 registration and registration support activities is approximately 24 managerial FTEs, 146 technical FTEs, and 7 clerical FTEs. The aggregated Agency estimated FTE dedicated to Section 3 activities is 177 and the burden hours are 369,1274. The fully loaded hourly mean wage rate estimate is $132.14 for managerial occupations, $87.24 for technical occupations, and $48.83 for clerical occupations. To calculate the Agency’s estimated annual cost of Section 3 activities, the number of FTE’s allocated to registration activities is multiplied by these fully loaded labor rates and by 2,080 hours per FTE, which is estimated to be about $6.68 million for management occupations; $26.56 million for technical occupations; and $689 thousand for clerical occupations. The total estimated Agency cost is $33.93 million.
6(d) Bottom Line Burden Hours and Cost
EPA estimates that the publication of the rule as proposed will result in an average of 12 fewer submissions of incomplete data packages that do not support desired label claims, saving registrants $15,000 in paperwork costs related to application submission. Further, the Agency estimates that the publication of the rule as proposed will reduce unnecessary data generation, leading to a $315,000 reduction in paperwork costs related to data generation. This is a total annual reduction in paperwork burden of $330,000 on average, equivalent to an annual reduction in burden hours of 4,683.
The rule as proposed will reduce the cost of registrant paperwork burden from the submission process by 0.15%. The rule as proposed will reduce the average cost of paperwork burden from data generation for new and amended products by 1%. Due to the relatively small impact of the rule, the Agency concludes that the proposed rule would not substantively change the burden estimates calculated in the Application for New and Amended Pesticide Registration ICR.
The Agency does not expect an immediate substantial increase in the number of registrations due to the codification of the rule as there is a significant effort of resources and time involved in developing new products. For this reason, EPA expects that the savings presented above will remain accurate for the period covered by this ICR. However, in the long run, the clarity resulting from the rule as proposed may encourage registrants to submit more data packages to the Agency. The Agency does not possess information to indicate whether registrants would submit more packages after the publication of the rule as proposed, nor does the Agency possess information to indicate how many more packages registrants would submit.
6(e) Reasons for Change in Burden
This ICR presents the estimated annual respondent paperwork burden and costs for the proposed data requirements. This paperwork burden and cost will serve as an off-set of existing paperwork burden and costs associated with the existing requirements. Taken as a whole, EPA estimates a total burden reduction of $330,000 annually. At the final rule stage, the Agency will determine the extent to which the existing approved burden hours and costs in OMB’s inventory may be reduced. That change will be classified as a program change.
6(f) Burden Statement
The total respondent burden calculated in the Application for New and Amended Pesticide Registration ICR is $108.7 million annually. If the proposed rule is finalized, that burden will be lowered to $108.4 million annually. This is primarily existing burden not attributable to this proposed rule and covered under EPA ICR No. 0277 and approved under OMB Control No.: 2070-0060. Burden is defined in 5 CFR 1320.3(b). An agency may not conduct or sponsor, and a person is not required to respond to a collection of information unless it displays a current and valid OMB control number. The OMB control numbers for EPA’s regulations in title 40 of the CFR, after appearing in the Federal Register, are listed in 40 CFR part 9 and included on the related collection instrument or form, if applicable.
6(g) Docket Information
Since this ICR is accompanying a proposed rule, this supporting statement and related materials are available in the rulemaking docket established under number EPA-HQ-OPP-2020-0124, which is available for online viewing at http://www.regulations.gov or in person viewing at the EPA Docket Center Public Reading Room, EPA West Building, Room 3334, 1301 Constitution Avenue, NW, Washington, DC 20004. The EPA/DC is open from 8:30 a.m. to 4 p.m., Monday through Friday, excluding federal holidays. The docket telephone number is (202) 566-1744.
You may submit comments regarding the Agency's need for this information, the accuracy of the provided burden estimates and any suggested methods for minimizing respondent burden, including the use of automated collection techniques. Submit your comments to both EPA and OMB, referencing the Docket ID No. identified above and RIN 2070-AJ49, as follows:
To EPA online using http://www.regulations.gov (our preferred method) or by mail to: EPA Docket Center, Environmental Protection Agency, Mail Code 28221T, 1200 Pennsylvania Ave., NW, Washington, DC 20460, and
To OMB via email to [email protected]. Address comments to OMB Desk Officer for EPA.
7. ATTACHMENTS TO THE SUPPORTING STATEMENT
Attachments to the supporting statement are available in the docket identified in Section 6(g) of the Supporting Statement. These attachments are available for online viewing at http://www.regulations.gov or otherwise accessed directly as identified in the following index.
Attachment A: |
7 U.S.C. 136a – Section 3 of FIFRA. EPA provides a summary of this law, along with a link to the U.S. Code, on our website: https://www.epa.gov/laws-regulations/summary-federal-insecticide-fungicide-and-rodenticide-act |
Attachment B: |
40 CFR part 158 – Current Regulations. An electronic version of part 158, entitled “Data Requirements for Pesticides,” is maintained by the Government Printing Office here: https://www.ecfr.gov/ |
Attachment C: |
Proposed Rule – The proposed rule is in the docket. |
Attachment D: |
Currently Approved ICR |
Attachment E: |
PRIA 4 |
1 12 data package submissions x $1,282/submission
2 12 data package submissions x 14 hours/submission
3 Fully loaded hourly labor wage rates are $121.72 for managerial labor, $72.01 for technical labor, and $40.93 for clerical labor. See Attachment J of the New and Amended Pesticide Registration ICR for information on how fully loaded labor wage rates are calculated.
4 2,080 hours/FTE x 177 FTEs
Page
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| File Title | Clean ICR 04_08_2020_BEAD |
| Author | newuser |
| File Modified | 0000-00-00 |
| File Created | 2021-03-26 |
© 2026 OMB.report | Privacy Policy