Ethylene Oxide Commercial Sterilization Facilities
Ethylene Oxide Commercial Sterilization Facilities National Emission Standards for Hazardous Air Pollutants (NESHAP) Technology Review (New)
EtO_114ICR_Instructions
Ethylene Oxide Commercial Sterilization Facilities
OMB: 2060-0733
OMB Control Number: 2060-NEW
Expiration: MM/DD/YYYY
Ethylene Oxide Commercial Sterilization
Section 114 Information Collection Request (ICR)
Instructions Document
Paperwork Reduction Act Burden Statement
This collection of information is approved by OMB under the Paperwork Reduction Act, 44 U.S.C. 3501 et seq. (OMB Control No. 2060-NEW). Responses to this collection of information mandatory under section 114(a) of the Clean Air Act. An agency may not conduct or sponsor, and a person is not required to respond to, a collection of information unless it displays a currently valid OMB control number. The average public reporting and recordkeeping burden for this collection of information is estimated to be approximately 108 hours per response. Send comments on the Agency’s need for this information, the accuracy of the provided burden estimates and any suggested methods for minimizing respondent burden to the Regulatory Support Division Director, U.S. Environmental Protection Agency (2821T), 1200 Pennsylvania Ave., NW, Washington, D.C. 20460. Include the OMB control number in any correspondence. Do not send the completed form to this address. |
Introduction
Under the authority of Section 114 of the Clean Air Act, this Section 114 ICR is to be completed for operations at all facilities wholly owned by your company that are subject to the requirements of 40 CFR part 63, subpart O. The operations for sterilization may include sterilizer chamber vents, chamber exhaust vents, aeration room vents, and fugitive emissions.
This Section 114 ICR consists of a main questionnaire and three (3) supplements, in the form of Microsoft Excel workbooks, to allow for submission of information requested for operations in the ethylene oxide (EtO) commercial sterilization source category. The supplements only need to be used if additional space is needed. This Instructions Document includes instructions for providing and submitting data and documents requested in this Section 114 ICR.
All recipients must complete and return the questionnaire, along with any of the supplements that were used, by the date specified in the Section 114 transmittal letter. If you have questions regarding this request, please contact Mr. Matthew Witosky, Office of Air and Radiation, at (919) 541-2865 or [email protected].
About the Questionnaire and Supplements
Once downloaded from the U.S. EPA website, you must create and complete a main questionnaire for your EtO commercial sterilization facility. The file name must be changed to reflect the actual company, city and state (see Section V below).
In addition, there are 3 supplements to the main questionnaire should you need more space than what is available in the original tables (Section B, Table 3; Section B, Table 4; and Section I, Table 1) to provide the data requested. If you prefer to fill out any supplement in lieu of the original table, please leave the original table blank in the main questionnaire. Be sure to select “Yes” in the designated cell above each original table where a supplement will be used, and the data fields will be automatically shaded in gray. The file name(s) of your supplement(s) must also be changed to reflect the actual company, city and state (see Section V below).
Once you open the questionnaire and supplements, you must scroll down and across each worksheet and complete all sections, except Section M (which may be used should you need space to provide any additional information). You may use the checklist at the end of this Instructions Document to confirm that you have filled out all sections in the main questionnaire. More details may be found on the “Introduction” worksheet of the main questionnaire.
Please note, in the main questionnaire and supplements, that data fields for “annual” costs do not mean “annualized” cost. Annual costs represent expenses incurred annually to perform routine activities. You must specify the dollar year for each cost-related data field in the designated column. Please use a consistent dollar year throughout the survey, if possible.
About the Additional Documents Requested
In addition to completing the main questionnaire and any supplements, we are requiring the submission of addition documents and information to complement the information requested. The “Documents” worksheet of the main questionnaire contains a full list of additional documents that are requested. Specifically, these documents include:
Facility diagram(s) that shows all EtO commercial sterilization operations at the facility up to and including the shipment of sterilized and fumigated products away from your facility;
Process flow diagram(s) for all EtO commercial sterilization operations at your facility;
The most recent air permit(s) for your facility;
Permit application documents associated with the initial air permit and any subsequent permit application documents submitted for the purpose of revisions to the air permit, if applicable, up to and including any permit applications for the most recent air permit(s) for your facility;
A copy of your facility’s Startup, Shutdown, and Malfunction (SSM) plan, or set of plans if more appropriate, for all EtO commercial sterilization operations;
Documentation for the calculations and supporting information for all emissions factors and approaches used to determine the annual EtO emissions at your facility;
All performance test(s) conducted over the last 5 years for each air pollution control device (APCD);
All engineering test(s) conducted over the last 5 years for each APCD;
Records for parametric monitoring conducted on each APCD for the last calendar year (CY2019);
Action levels and standard operating procedures (SOP) for room area monitoring;
Results and records of any other EtO monitoring efforts conducted at your facility, such as near-source, ambient air, fenceline monitoring, or dispersion modeling;
Documentation of studies done on quantifying EtO residuals in your products; and
Any process and instrumentation diagrams (P&IDs) that are not included in other documents requested.
Please refer to the “Documents” worksheet of the main questionnaire for more details.
Handling of Confidential Business Information (CBI)
In order to ensure safe and appropriate handling of any CBI that facilities may provide, each “green” worksheet (where facility information is to be entered) in the main questionnaire and supplements contains a question in Cell N2 asking whether any CBI is entered in this specific worksheet. This question must always be completed.
Specifically, if any CBI data and documents are included in your main questionnaire and in any supplements if used:
Be sure to select “Yes” from the dropdown menu in Cell N2 on each “green” worksheet, and shade in red each cell that contains CBI. Do not shade the cells that do not contain CBI, or the entire table in which only certain cells contain CBI;
Complete the main questionnaire and any supplements in their entirety. Be sure to clearly mark up all cells that contain CBI, across all worksheets, based on the relevant instructions;
In the “Certification” worksheet, check the applicable boxes to acknowledge appropriate handling of CBI in your questionnaire and any supplements;
Save this Excel workbook (main questionnaire or supplement) as the CBI version following the naming convention explained in Section V below;
Create a non-CBI version of this Excel workbook (main questionnaire or supplement). Select “Save As” to save another copy of this Excel workbook using the naming convention for non-CBI versions explained in Section V below;


 In
the non-CBI version of your Excel workbook (main questionnaire or
supplement), select and copy the Sample CBI Cell (Cell O2 on each
“green” worksheet except “Documents” –
see the example below), and paste directly into each cell that
contains CBI. Make sure that all "CBI" cells are shaded in
red. In the “Documents” worksheet, make sure that any
embedded CBI document is deleted.
In
the non-CBI version of your Excel workbook (main questionnaire or
supplement), select and copy the Sample CBI Cell (Cell O2 on each
“green” worksheet except “Documents” –
see the example below), and paste directly into each cell that
contains CBI. Make sure that all "CBI" cells are shaded in
red. In the “Documents” worksheet, make sure that any
embedded CBI document is deleted.
When you save the non-CBI version of the Excel workbook, all data fields that contained CBI before should look like the example shown below in your completed non-CBI workbook;
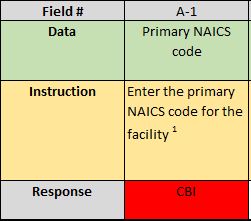
Before you submit, be sure to go through the non-CBI file to confirm that all CBI data and documents have been removed as instructed. Repeat Step (3) with the non-CBI version of the Excel workbook.
Once completed, the CBI version of your Excel workbook (main questionnaire or supplement) must contain the full data and documents that you wish to submit, while the non-CBI version must only contain the non-CBI portion of the data and documents. Please submit both the CBI and non-CBI versions of your questionnaire and supplements (if used) to U.S. EPA following the submittal procedures specified in Section VI below.
Naming Conventions for Your Questionnaire, Supplements, and Documents
Before submitting your questionnaire and supplements (if used) to U.S. EPA, please ensure that you adhere to the following naming conventions:
Non-CBI version of Excel workbooks with responses
[Company]_[CityState]_EtO_114ICR_Main_NonCBI
[Company]_[CityState]_EtO_114ICR_Sup1_NonCBI
[Company]_[CityState]_EtO_114ICR_Sup2_NonCBI
[Company]_[CityState]_EtO_114ICR_Sup3_NonCBI
CBI version of Excel workbooks with responses
[Company]_[CityState]_EtO_114ICR_Main_CBI
[Company]_[CityState]_EtO_114ICR_Sup1_CBI
[Company]_[CityState]_EtO_114ICR_Sup2_CBI
[Company]_[CityState]_EtO_114ICR_Sup3_CBI
Documents
[Company]_[CityState]_[Field # in the main questionnaire (dash) numbering, if multiple documents are provided]
Example filename: Acme_JonestownMN_A-21-2.xlsx
Instructions for Submitting Your Responses
For the non-CBI version of your completed Excel workbooks (main questionnaire and supplements if used), you may submit using one of the following methods. If you choose to submit your documents as standalone PDF files, please only send the non-CBI documents along with your main questionnaire and any supplements.
Email
For files that are less than 10 MB, email your non-CBI responses to [email protected] with a subject line of “EtO Section 114 ICR Response for [Company] [CityState]”
Mail
You may save your files on a media such as thumb drive, CD or DVD, and mail to
Ethylene Oxide Commercial Sterilization Section 114 ICR Response
U.S. EPA Office of Air Quality Planning and Standards
Sector Policies and Programs Division, Fuels and Incineration Group
Mail Code E143-05
109 T.W. Alexander Drive
Research Triangle Park, NC 27711
For the CBI version (full version) of your completed Excel workbooks (main questionnaire and supplements if used), you must save your files on a separate media such as thumb drive, CD, or DVD. Please clearly mark the media with “Confidential Business Information”, and mail to:
U.S. EPA Office of Air Quality Planning and Standards
U.S. EPA Mailroom (C404-02)
Attn: Ms. Tiffany Purifoy, Document Control Officer (ESD #322)
109 T.W. Alexander Drive
Research Triangle Park, NC 27711
If you choose to submit your documents as standalone PDF files, all the CBI and non-CBI documents must be saved on the media along with your main questionnaire and any supplements.
DO NOT ELECTRONICALLY TRANSMIT CBI (e.g., via email, fax or ftp) TO U.S. EPA.
If you have questions regarding this request, please contact Mr. Matthew Witosky, U.S. EPA, Office of Air and Radiation, at (919) 541-2865 or [email protected].
Checklist of Tables in the Main Questionnaire of the Section 114 ICR
|
A. Facility Details |
☐ |
Table 1. Facility Information |
☐ |
Table 2. Parent Company Information |
☐ |
Table 3. Facility Documents |
☐ |
Table 4. Facility Buildings |
☐ |
Table 5. Facility-level Data |
☐ |
Table 6. Materials Sterilized with EtO |
☐ |
Table 7. Materials Sterilized with Non-EtO Techniques and Approaches |
|
B. Individual Room Area (All Areas where EtO is Used or Emitted) |
☐ |
Table 1. Characteristics of Room Areas |
☐ |
Table 2. Natural Draft Openings (NDO) |
☐ |
Table 3. Leak Checks of Components in EtO Service (Optional Supplement 1) |
☐ |
Table 4. Room Area Controls (Optional Supplement 2) |
☐ |
C. EtO Drum and Container Storage |
☐ |
D. Ethylene Glycol (EG) Tanks |
|
E. Sterilization Chambers |
☐ |
Table 1. Summary for Sterilizer Chambers |
☐ |
Table 2. Sterilizer Chamber Operation and Monitoring Characteristics |
☐ |
Table 3. Control Characteristics for Sterilizer Chambers |
☐ |
Table 4. Control Characteristics for Sterilizer Chambers (continued) |
☐ |
Table 5. Vacuum Pumps |
|
F. Aeration |
☐ |
Table 1. Aeration that Occurs in Separate Unit (Aeration Room & Aeration Cell/Chamber) |
☐ |
Table 2. Aeration that Occurs within Sterilizer Chamber |
☐ |
Table 3. Movement of Sterilized Products through the Facility |
|
G. Summary of Air Pollution Control Devices |
☐ |
Table 1. APCD Characteristics |
☐ |
Table 2. Emissions and CEMS |
|
H. Details of Air Pollution Control Devices |
☐ |
Table 1. Wet Scrubber and Glygen Absorber Unit |
☐ |
Table 2. Dry-bed Scrubber |
☐ |
Table 3. Catalytic Oxidizer & Combination Water Balancer/Catalytic Oxidizer |
☐ |
Table 4. Thermal Oxidizer |
☐ |
Table 5. Other APCDs |
|
I. EtO Monitoring |
☐ |
Table 1. Personal Monitoring (Badges) for EtO (Optional Supplement 3) |
☐ |
Table 2. Room Area Monitoring for EtO |
☐ |
Table 3. Other Monitoring for EtO |
☐ |
J. Wastewater |
☐ |
K. Unique Cycles and EtO Reduction |
☐ |
L. Other Questions regarding EtO Commercial Sterilization |
☐ |
Table 1. EtO and Facility Operation |
☐ |
Table 2. Standalone Non-Colocated Warehouse, Distribution Center, or Enclosed Building for Sterilized Products |
☐ |
Table 3. Alternative Sterilization |
☐ |
M. Additional Information |
☐ |
N. Documents |
|
Certification |
☐ |
Acknowledgment of CBI Handling |
☐ |
Certification by Reporter |
☐ |
Certification by Facility Personnel |
☐ |
Certification by Professional Engineer |
☐ |
Certification by Certified Industrial Hygienist |
EtO Commercial Sterilization Section 114 ICR - Instructions Document
Page
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| File Title | Instructions |
| Author | Eastern Research Group |
| File Modified | 0000-00-00 |
| File Created | 2021-05-11 |
© 2026 OMB.report | Privacy Policy