XXXX NAHMS AMR SS Part B (20210630)
XXXX NAHMS AMR SS Part B (20210630).docx
On Farm Monitoring of Antimicrobial Use and Resistance in U.S. Broiler Production
OMB: 0579-0481
B. Collections of Information Employing Statistical Methods
1. Describe (including a numerical estimate) the potential respondent universe and any sampling or other respondent selection method to be used. Data on the number of entities (e.g., establishments, state and local government units, households, or persons) in the universe covered by the collection and in the corresponding sample are to be provided in tabular form for the universe as a whole and for each of the strata in the proposed sample. Indicate expected response rates for the collection as a whole. If the collection had been conducted previously, include the actual response rate achieved during the last collection.
The structure of the poultry industry is hierarchical and the sample selection reflects this reality. Companies have one or more processing plants. A complex consists of the processing plants and farms that feed it. Therefore, complexes are embedded within company.
Farms raise broilers and deliver them to specific processing plants. A farm is composed of one or more houses. The house is the structure where the actual broilers are raised. Typically, all birds on a farm (in all houses) are placed and then slaughtered at the same time, representing an all-in all-out strategy.
Each group of birds raised in the house is known as a flock. Therefore, a single house will have multiple flocks during the year (typically 5-6). See the diagram below for a visualization of this hierarchy.
This study consists of repeated sampling performed at the complex level. University of Minnesota will collect antimicrobial use (AMU) information for each farm selected within complex. Additionally, producers will collect litter samples at a specific house on each participating farm and ship these samples to the University of Minnesota.
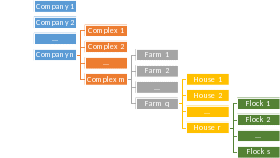
Figure 1: General outline of the hierarchy of the high-volume broiler industry.
The target population is the top 30 broiler producer companies in the United States and the broilers they raise, as published by WATT Global Media1. These companies account for greater than 95% total live weight slaughtered broilers (57,170,360,000 lb. reported from the WATT list out of 58,286,944,000 lb. reported from NASS). They also account for greater than 95% of young chickens slaughtered in 20192 (9,050,080,000 head reported from the WATT list out of 9,224,243,000 head reported from NASS). Because these companies represent the majority of commercial broiler production in the U.S., APHIS is comfortable describing the target population as the principal commercial broiler producers in the U.S.
APHIS will not perform statistical survey analysis techniques to extrapolate results from this study to the U.S. broiler industry. Results from this study cannot be considered representative of all broiler producers in the U.S. because companies not on the WATT list have no ability to participate.
Even though the sample is not a probability sample below the level of company, this study is the most comprehensive study investigating AMU and AMR in U.S. broiler production. We target the 30-top broiler-producing companies, which produce the majority (approximately 95%) of live weight slaughtered and young chickens slaughtered in the U.S.
APHIS will perform post-stratification estimates as appropriate. We will use population-level information such as slaughter estimates and antimicrobial use policies in U.S. broiler production for these estimates.
The primary objective of this study is to measure antimicrobial use patterns and their relationship to antimicrobial resistance of select agents over time. As a longitudinal study (at the complex level), our emphasis is not primarily to select statistically representative samples using random selection. Instead, our emphasis is to select a sample of companies and complexes who can complete all the study activities and provide the complete data we need to make comparisons across time while controlling for variability at the various levels of the broiler industry.
The University of Minnesota will attempt to make contact with all 30 companies from the list to solicit participation in the study and will enroll as many as are willing to participate. We expect that approximately 10-15 (estimated mid-point of about 12 reflected on the APHIS 71 and 79 forms) companies will enroll. Collectively, this comprises at least 50% of the total production on the WATT list. We can enroll up to 36 complexes.
The University of Minnesota will select the number of complexes proportional to the size of the company. They will enroll a maximum of 5 complexes for the larger companies. From each enrolled complex, they will select approximately 4 farms (ranging between 4 and 8, depending on complex enrollment) and sample each quarter.
Only one house on each selected farm will contribute litter samples, as inter-farm variability is much higher than intra-farm variability. No farms will be sampled more than once during a calendar year. In total, we expect to sample approximately 144 farms per quarter, with a total of approximately 576 farms during the calendar year.
For each selected farm, the data collector (an employee of the participating company) will complete a short questionnaire. This questionnaire asks questions regarding antibiotic use in broilers between 21 days of age and slaughter age, with preference being communicated to the data collectors that sampling closer to slaughter age is preferred to represent the bacteria that are most likely to enter the slaughter plant. In addition, the data collector will collect two litter samples from one house on the farm, one from each side of the house.
The design we are using for this study is an evolution of a design that the University of Minnesota has used since 2016. We have incorporated the feedback from the data providers, industry experts, and researchers to adjust the design to its current state.
In previous iterations, approximately 10 companies out of 15-20 companies that the University of Minnesota contacted enrolled in the study. Once a company enrolled, the researchers encouraged them to choose complexes for participation that were geographically representative of all of their complexes. Also, while not explicitly required, researchers encouraged the companies to select complexes which were proportional to antimicrobial use policy practiced (compared to estimates of the distribution of antimicrobial use policies in the population). Given that a complex was enrolled to participate, nearly all farms selected to participate contributed usable information. Attrition occurred at most 5% of the time due primarily to logistical issues involving the inability of a data collector to visit the farm in time to collect the survey and biological sample information.
2. Describe the procedures for the collection of information including:
Companies will enroll in the study. We will provide all 30 of the companies from the WATT list the option to participate. The companies that agree to participate will select complexes to enroll. The number of complexes enrolled will be proportional to the size of the company. We will not enroll more than 5 complexes per company and expect to enroll a total number of 36 complexes. We will select approximately 4 (between 4 and 8, depending on complex enrollment) farms per quarter, with no farms selected more than once during a calendar year. Producers will collect litter samples from only one house per farm.
APHIS and the University of Minnesota will post-stratify the estimates based on sample data using known slaughter population totals, antimicrobial use policy categories, and other information (if enough data are available after collection). We will generate descriptive summary estimates, in the form of counts, proportions, and means, for antimicrobial use – such as antimicrobials used, duration and dosage, and indications for use –for the sample using the information collected on the NAHMS 471 form.
The University of Minnesota will produce descriptive summary estimates using the biological testing results by culturing Salmonella, Campylobacter, Enterococcus, and E. coli from the litter samples. This will include estimates of Salmonella and Campylobacter prevalence, along with the Salmonella genotypes and Campylobacter and Enterococcus speciation information for the isolates. They will also summarize resistance profiles by isolate and by antimicrobial. Additionally, the University of Minnesota will construct multivariable and multivariate models using resistance metrics as dependent and use metrics as independent variables while accounting for correlation and variability at the various levels of hierarchy in the population.
The University of Minnesota will analyze data from the qPCR analysis using a variety of multivariate statistical analysis techniques, including:
graphics for displaying distributions of gene frequencies across farms within complex and across complexes and over time;
network analyses to investigate relationships among gene quantities;
ordination techniques, such as principal components and principal components regression analysis regressing principal components describing variability in resistance on use variables.
APHIS and the University of Minnesota will perform data management and analysis using Microsoft Access, Excel, Stata, and R.
Participating companies from the WATT Global Media list of top U.S. broiler producers account for at least 50 percent of live weight slaughtered in the U.S.
APHIS estimated sample sizes based on the number of anticipated samples that would yield positive isolates for Salmonella and Campylobacter. Based on work performed in previous iterations of the study, we anticipate house-level prevalence values for Salmonella and Campylobacter to be approximately 40% and 50% for the organisms respectively. Assuming these prevalence values, and assuming that measurements of flocks within house are independent, we estimate standard errors of farm-level prevalence estimates to be approximately 0.022 in any given year (a partial dependence model predicted a higher, but comparable estimated standard error).
Unusual problems requiring specialized sampling procedures and any use of periodic (less frequent than annual) data collection cycles to reduce burden
This is a longitudinal study based on observing and comparing trends in antimicrobial use in broilers and its relationship to antimicrobial resistance metrics in the same populations. Because antimicrobial use can vary by season, as diseases often have a variable incidence throughout the year, it is important to ensure that data collection occurs throughout the year. Further, Salmonella and Campylobacter exhibit a seasonality in poultry, again
supporting the need for sampling throughout the year. For these reasons, companies will sample approximately 4 (between 4 to 8 depending on complex enrollment) farms within each complex during each quarter. This stratification of the calendar year will ensure representative sampling and data collection are conducted throughout the year and capture seasonal variation.
3. Describe methods to maximize response rates and to deal with issues of non-response:
Questionnaire design and training
The questionnaire is designed to minimize the amount of data collected to that which is absolutely necessary to meet the stated objectives.
We developed questionnaires in partnership with industry and trade associations experts, governmental researchers, and academic researchers.
We used industry input to develop a questionnaire that was consistent with how large broiler companies record data during ordinary operations.
To minimize collection burden, the questionnaire design captures information that the data providers have readily available as part of normal commercial operation.
Contacting respondents
Many respondents have taken part in a previous iteration of this study through the University of Minnesota. The relationship they have with the researchers at the University of Minnesota is valuable as it has helped both the researchers and the companies involved to understand the burden required to be a part of the study and the benefits to the company and the industry as a result of the study.
The National Chicken Council and the U.S. Poultry and Egg Association support this study. We expect this support to continue to help marketing the study to broiler companies and their dependents in the target population.
University of Minnesota will recruit companies, complexes, and farms via phone or email communications. They will send a letter to interested or potential respondents to notify them of the purpose, burden, and benefits of the study. Because many of the companies in the target population have participated in a previous iteration of this study, they are likely already familiar with the data collection mode, process, and schedule.
In the event of a lapse in survey completion, University of Minnesota will make phone calls and emails with enrolled company contacts.
Non-response adjustment
We do not have significant concern of non-response of the farms because farms are selected by the companies and are only sampled once per year.
Participation is voluntary. Companies may enter and leave throughout the study period. Further, if circumstances require a change (e.g., disease issues within a complex that prevent farm access by company personnel), companies are free to do so. We will use models to account for the unbalanced data in our data analysis, in addition to fixed and random effects in the statistical models can be used to help account for the time periods when specific companies and complexes were sampled.
If enough data are available after data collection, we will post-stratify estimates using known slaughter population totals and antimicrobial use policies as appropriate.
Sampling and design strategies
Data collectors will collect samples between 21 days-of-age and slaughter. This gives the company flexibility to sample all houses in the complex on the same day. Companies are encouraged to collect the samples as close to slaughter age as possible.
We designed collection time points to align with broiler production. This increases the potential of successfully completing the questionnaire and biological sampling as well as reducing attrition.
Biological sampling depends on composite samples (litter sample collections) to reduce collection burden on participants.
University of Minnesota will deliver questionnaires in the sample kit as farm-level surveys. If needed, they will contact companies to finalize the farm record after the flock has been shipped to slaughter.
At most, we will sample a specific farm once per year. This reduces burden on that farm operator and is congruent with the feedback proposed under previous iterations of this study.
4. Describe any tests of procedures or methods to be undertaken.
This is a new iteration of a study that began in 2016. The participant recruitment methods and the biological sampling processes have all been used since 2016 and have been refined based on participant and researcher feedback. APHIS and the University of Minnesota consulted with broiler experts in industry and academia to revise the study design and the data collection form to better meet the needs of the participants and to reduce burden on the data collectors while maintaining data quality. This is important, especially in a market environment during the coronavirus disease 2019 (COVID-19) pandemic.
5. Provide the name and telephone number of individuals consulted on statistical aspects of the design and the name of the agency unit, contractor(s), grantee(s), or other person(s) who will actually collect and /or analyze the information for the agency.
The statistical aspects of the design were coordinated by:
Matthew Branan (970-494-7349), Mathematical Statistician, National Animal Health Monitoring System, USDA, APHIS, VS, S&P, CEAH, Fort Collins, CO.
Data collection, entry, and management will be conducted by University of Minnesota personnel. Contact persons for data collection are:
Dr. Randall Singer (612-625-6271), Professor of Epidemiology, University of Minnesota, Department of Veterinary and Biomedical Sciences, St. Paul, MN, 55108.
Analysis of the data will be accomplished by University of Minnesota and APHIS-NAHMS veterinarians, epidemiologists, and statisticians under the direction of:
Dr. Amy Delgado (970-494-7256), Associate Director, Center for Epidemiology and Animal Health, USDA, APHIS, VS, S&P, Fort Collins, CO.
Dr. Randall Singer (612-625-6271), Professor of Epidemiology, University of Minnesota, Department of Veterinary and Biomedical Sciences, St. Paul, MN, 55108.
NASS review of the OMB package submission will be coordinated with:
David Hancock (202-690-2388), OMB Clearance Officer, Methodology Division Standards and Survey Development Methodology Branch, USDA, NASS, Washington, DC.
Appendix – Brief overview of laboratory sample analysis
The litter samples will be cultured for Salmonella, E. coli, Campylobacter and Enterococcus using standard methods. All bacterial isolates will be evaluated for their antibiotic susceptibility using a broth microdilution assay (Sensititre). Salmonella isolates will be confirmed by PCR and then serotyped using an ISR sequencing approach. E. coli isolates will be confirmed with biochemical tests. Campylobacter and Enterococcus isolates will be confirmed and speciated with a multiplex PCR.
DNA will be extracted from one litter sample per house using standard protocols. Quantitative PCR will then be performed using a microfluidic qPCR assay targeting different antibiotic resistance genes, genes associated with horizontal gene transfer, and the 16S rRNA gene. A customized database will be used to process the sample results, develop standard curves for each assay, and then normalize the sample results to the standard curve.
1 List of top 30 U.S. broiler producer companies available here: https://www.wattglobalproducts.com/products/top-us-broiler-producers-of-2019.
2 NASS estimates of total live weight slaughtered from the NASS Poultry Slaughter Report dated April 24, 2019: https://downloads.usda.library.cornell.edu/usda-esmis/files/3197xm04j/n009w967m/2v23w3090/psla0419.pdf.
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | swarnken |
| File Modified | 0000-00-00 |
| File Created | 2021-07-15 |
© 2026 OMB.report | Privacy Policy