Form FDA 3926 Change Request
0814_Change Request Form FDA 3926_JUN 2021.docx
Individual Patient Expanded Access Applications
Form FDA 3926 Change Request
OMB: 0910-0814
UNITED STATES FOOD & DRUG ADMINISTRATION
Individual Patient Expanded Access Applications
OMB Control No. 0910-0814
Non-substantive Change Request to an existing information collection: Form FDA 3926, “Individual Patient Expanded Access Investigational New Drug Application.”
I. Background:
This information collection supports FDA regulations as well as Agency forms and associated guidance. Provisions in section 561 of the Federal Food, Drug, and Cosmetic Act (as codified in 21 U.S.C. 360bbb) set forth general requirements relating to expanded access to unapproved therapies and diagnostics. Sometimes called “compassionate use,” expanded access is a potential pathway for a patient with a serious or immediately life-threatening disease or condition to gain access to an investigational medical product (drug, biologic, or medical device) for treatment outside of clinical trials when no comparable or satisfactory alternative therapy options are available. To facilitate expanded access to investigational drugs by patients, regulations in 21 CFR part 312; subpart I (Expanded Access to Investigational Drugs for Treatment Use) establish submission requirements that include demonstrating certain criteria have been met, and that content and format requirements have been satisfied. Because of FDA’s long history of facilitating expanded access to investigational drugs for treatment use for patients with serious or immediately life-threatening diseases or conditions, our efforts in this regard are ongoing. Form FDA 3926, entitled, “Individual Patient Expanded Access – Investigational New Drug Application (IND)” was developed to assist respondents to the information collection. Form FDA 3926 requires the completion of data fields that enable us to uniformly collect the minimum information necessary from licensed physicians who want to request expanded access as prescribed in the applicable regulations. To supplement the form instructions, we issued guidance, most recently updated in October of 2017, entitled, “Individual Patient Expanded Access Applications: Form FDA 3926,” available at – https://www.fda.gov/regulatory-information/search-fda-guidance-documents/individual-patient-expanded-access-applications-form-fda-3926. As discussed in the guidance, 21 CFR 312.310(b) contains additional submission requirements for individual patient expanded access requests.
Form FDA 3926 is available as a fillable PDF document on FDA’s website. Form FDA 3926 and accompanying instructions may be found at FDA’s website at https://www.fda.gov/about-fda/reports-manuals-forms/forms. The current coding of the form includes a feature that makes certain fields grayed out and unfillable as a pdf, if they are not required for the type of submission selected. This feature was intended as a time-saving measure for physicians, so they would not have to fill in unnecessary fields on the form.
II. Proposed change:
FDA proposes to revise the form to clarify what fields should be filled out, depending on the type of submission (either initial or follow-up) and does not intend to change any of the information collected under the already approved ICR. Specifically, FDA proposes to include a statement that selecting the submission type in field 3 (initial or follow-up) will turn on only the fields that should be filled out on the form. This feature was intended as a time-saving measure for physicians, so they would not have to fill in unnecessary fields on the form. However, some users were unclear about Field 3 and either left it unchecked or checked one of the boxes and still tried to fill in the other fields on the form. This is exacerbated by the increase in expanded access requests due to the COVID-19 pandemic. This clarifying statement about the functionality will help physicians fill out the form correctly, and also eliminate the need to fill in unnecessary fields on the form FDA.
III. Screen shots:
We have included a screenshot below of the proposed new language (the only proposed change to Form FDA 3926) shown in highlight on Image 1 below:
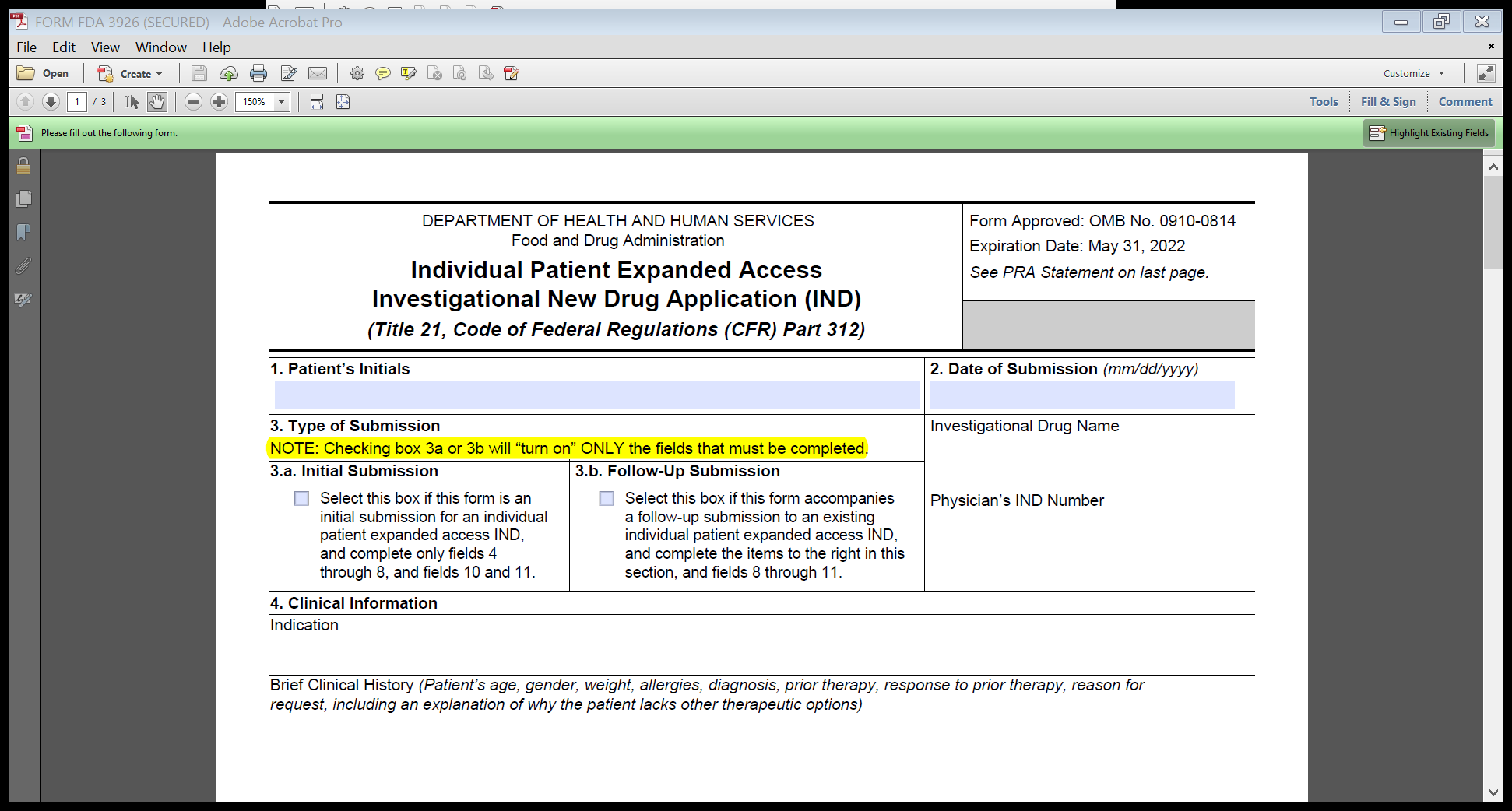
Image 1: Proposed edit to Form FDA 3926
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | Almeida, Cecilia |
| File Modified | 0000-00-00 |
| File Created | 2021-06-23 |
© 2026 OMB.report | Privacy Policy