2288ss04
2288ss04.docx
Pesticides Data Call In Program (Renewal)
OMB: 2070-0174
November 17, 2021
Supporting Statement for an Information Collection Request (ICR)
Under the Paperwork Reduction Act (PRA)
EXECUTIVE SUMMARY
Identification of the Information Collection – Title and Numbers
Title: Pesticide Data Call-Ins (DCIs)
ICR Numbers: EPA No. 2288.04; OMB Control No. 2070-0174
Docket ID No.: EPA-HQ-OPP-2020-0693
Abstract:
This ICR covers the information collection activities associated with the issuance of data-call-ins (DCIs) under section 3(c)(2)(B) of the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA).1
EPA regulates the use of pesticides under the authority of two federal statutes: FIFRA and the Federal Food, Drug and Cosmetic Act (FFDCA)2, both as amended by the Food Quality Protection Act (FQPA) of 1996. In general, before manufacturers can sell pesticides in the United States, EPA must evaluate the pesticides thoroughly to ensure that they meet federal safety standards to protect human health and the environment. EPA grants a "registration" or license that permits a pesticide's distribution, sale, and use only after the company meets the scientific and regulatory requirements.
In evaluating a pesticide registration application, EPA assesses a wide variety of potential human health and environmental effects associated with the use of the product. Applicants, or potential registrants, must generate or provide the scientific data necessary to address concerns pertaining to the identity, composition, potential adverse effects, and environmental fate of each pesticide. The data allow EPA to evaluate whether a pesticide has the potential to cause harmful effects on certain non-target organisms and endangered species (including humans, wildlife, and plants) and on surface water or ground water.
Through a rigorous scientific and public process, EPA specifies the kinds of data and information necessary to make regulatory judgments about the risks and benefits of pesticide products under FIFRA §§ 3, 4 and 5, as well as the data and information needed to determine the safety of pesticide chemical residues under FFDCA §408. The regulations in 40 CFR part 158 describe the minimum data and information EPA typically requires to support an application for pesticide registration or amendment; support the reregistration of a pesticide product; support the maintenance of a pesticide registration by means of the data call-in process (e.g., as used in the registration review program); or establish or maintain a tolerance or exemption from the requirements of a tolerance for a pesticide chemical residue.
As described in 40 CFR 158.30, however, FIFRA provides EPA with flexibility to require, or not require, data and information for the purposes of making regulatory judgments for individual pesticide products, thereby allowing for the data required to be modified on an individual basis to fully characterize the use and properties, characteristics, or effects of specific pesticide products under review. The Agency encourages each applicant to consult with EPA to discuss the data requirements particular to its product prior to and during the registration process. In addition, the Agency cautions applicants that the data routinely required by the regulations may not be sufficient to permit EPA to evaluate the potential of the product to cause unreasonable adverse effects on man or the environment. EPA may, therefore, require the submission of additional data or information beyond that specified in the regulations if such data or information are needed to evaluate a pesticide product as required by FIFRA and FFDCA.
EPA uses the DCIs issued under this ICR to acquire the data that has been deemed necessary for the Agency’s statutorily mandated review of a pesticide’s registration, which require it to assess whether the continued registration of an existing pesticide causes an unreasonable adverse effect on human health or the environment and whether the Agency will pursue appropriate regulatory measures. The key program areas are described in more detail in this ICR, along with the Agency’s estimates of the information collection burden and costs associated with issuing DCIs under those key program areas.
SUPPORTING STATEMENT
1. Explain the circumstances that make the collection of information necessary.
All the programs and DCI activities represented in this ICR share a common statutory authority, FIFRA §3(c)(2)(B), which authorizes EPA to require pesticide registrants to generate and submit data to the Agency, when such data are needed to maintain an existing registration of a pesticide.
A pesticide product may be registered or remain registered only if it meets the statutory standard for registration given in FIFRA §3(c)(5), which is as follows: the composition is such as to warrant the proposed claims for it; its labeling other material required to be submitted comply with the requirements of this Act; it will perform its intended function without unreasonable adverse effects on the environment and when used in accordance with widespread and commonly recognized practice it will not generally cause unreasonable adverse effects on the environment.
FIFRA §2(bb) defines “unreasonable adverse effects on the environment'' as (1) any unreasonable risk to man or the environment, taking into account the economic, social, and environmental costs and benefits of the use of any pesticide, or (2) a human dietary risk from residues that result from a use of a pesticide in or on any food inconsistent with the standard under section 408 of the Federal Food, Drug and Cosmetic Act (FFDCA).
FIFRA §3(c)(2) directs EPA to publish guidelines specifying the kinds of data that applicants and registrants must submit to support the EPA regulatory determinations established under FIFRA. EPA identifies the majority of the data requirements in 40 CFR part 158, and in the context of individual actions as allowed by FIFRA §3(c)(2).
EPA regulations at 40 CFR part 152, subpart E, describe a variety of means by which an applicant may satisfy EPA’s data requirements and requests for data. Persons submitting data must request inclusion on an Agency-maintained Data Submitters list as the means for asserting their rights to offers of compensation from applicants who cite their data. Procedures also allow an applicant to cite data previously submitted by another person that are relevant to that applicant’s product. When the latter option is selected, an applicant may be required to either obtain permission or offer compensation to cite the data, depending upon whether the data at issue are entitled to the exclusive use or data compensation provisions of FIFRA §3(c)(1)(F).
In addition, 40 CFR part 152, subpart E spells out the circumstances under which certain applicants are exempt from data submission or citation obligations (i.e., the formulators’ exemption provided by FIFRA §3(c)(2)(D)).
All the programs and DCI activities represented in this ICR share a common statutory authority, FIFRA §3(c)(2)(B), which authorizes EPA to require pesticide registrants to generate and submit data to the Agency, when such data are needed to maintain an existing registration of a pesticide.
Before the Agency determines that specific data are needed under this ICR, the Agency will first search for available information (e.g., EPA databases for information that may have been submitted to EPA under another ICR, submitted voluntarily, or submitted by another respondent; information that has otherwise published in the literature; or information that is otherwise publicly available).
EPA has also established a transparent and participatory process that allows for public dialogue on EPA’s risk characterizations under these pesticide registration review programs, including the consideration related to the need for other data or information in order to make the required statutory determinations for that pesticide. Only if the needed data are not found to be otherwise available will EPA require the submission or generation of the specific data needed in a particular case. Such data, which are described in more detail later in this document, may include toxicology studies, fish and wildlife studies, environmental fate studies, chemistry studies, endocrine disruptor screening data and/or other data needed to analyze the potential risks and benefits associated with pesticide chemicals.
2. Indicate how, by whom, and for what purpose the information is to be used. Except for a new collection, indicate the actual use the Agency has made of the information received from the current collection.
EPA uses the information collected under this ICR to carry out its statutory responsibilities under FIFRA §§ 4, 3(g), 6(b), and FFDCA §408. The data collected allows EPA to consider the data or information in making a registration decision and assess whether the continued registration of an existing pesticide causes an unreasonable adverse effect on human health or the environment. The data and information collected under this ICR are used by Agency scientists to assess and characterize pesticide risks, and to determine whether the pesticide continues to meet the standards established by federal law.
Through a rigorous scientific and public process, EPA specifies the kinds of data and information necessary to make the regulatory judgments required under FIFRA and FFDCA.
Some of these judgments include, but are not limited to:
Determine if a pesticide can be registered or remain registered because it does not cause an unreasonable adverse effect on human health or the environment.
Determine if a pesticide has the potential to interact with the endocrine system and meeting the safety standard of FFDCA §408.
Determine if a pesticide might harm a listed species, under the Endangered Species Act, or its designated critical habitat.
Determine if pesticide residues in food or feed will result in a reasonable certainty of no harm to human health from aggregate exposure through dietary, non-occupational, and drinking water routes of exposure.
EPA must also consider the cumulative effects of pesticides that share a “common mechanism of toxicity,” and consider the special sensitivities of infants and children
EPA evaluates the data submitted by registrants to ensure that residues in or on food are not above the residue levels relied on for establishing the tolerance. If the submitted residue data demonstrates that the residue levels are above the levels relied on for establishing the tolerance, EPA will take appropriate action to modify or revoke the tolerance.
Inherent in EPA’s review of most of the programs is an evaluation of the risks posed by the pesticide, which may also result in risk mitigation considerations.
The availability of data and information about the pesticide is essential to perform quality and accurate risk assessment that impact Agency decisions, which may result in a more or fewer restriction on pesticide use. The lack of data will mean that there would be a higher degree of uncertainty and the potential for effects or exposures cannot be accurately characterized, often requiring the use of conservative assumptions in lieu of the data. Use of conservative assumptions may result in overestimates of potential effects or exposures or limit the flexibility the registrants and Agency have when complying with other mandates, e.g., ESA. Limited flexibility can result in use restrictions that could have otherwise been avoided. If new data or information show that the risk is increased, then additional mitigation may be needed to address potential risks of concern. However, if the new data or information show that the risk is less than previously assumed, then the registrants may be able to expand uses, or the Agency may be able to reduce restrictions previously imposed.
The issuance of a DCI may also help registrants assert their rights to the exclusive use or data compensation provisions under FIFRA §3(c)(1)(F); as well as facilitate collaborative efforts to generate data when such opportunities are available.
In general, the practical utility of the data that might be collected through a DCI has been determined to be necessary in order to answer specific questions about the safety of the pesticide product before the Agency can register it. Although the issuance of a DCI is based on the circumstances presented by the individual pesticide chemical, EPA has established data requirements based on the pesticide type and intended use patterns, while maintaining flexibility to address individual circumstances when appropriate.
Under any of the programs covered by this ICR, a request for data may serve a specific purpose, either in terms of its function or use in the assessment that supports the Agency’s regulatory judgments about the risks and benefits of the pesticide under FIFRA §§ 3, 4 and 5, and to determine the safety of pesticide chemical residues under FFDCA §408.
3. Describe whether, and to what extent, the collection of information involves the use of automated, electronic, mechanical, or other technological collection techniques or other forms of information technology, e.g., permitting electronic submission of responses.
After initiating a statutorily mandated pesticide review whether a Special Review, closeout of a Reregistration Review, a Registration Review, or an Anticipated Residue (AR) or Percent Crop Treated (PCT) Review, and determining that additional data are needed, the Agency will issue a DCI when the need for additional data has been identified.
The Pesticide Registration Information System (PRISM) software application developed within OPP integrates the functionality necessary to support the Registration Review and EDSP (Endocrine Disrupter Screening Program)3 programs. PRISM supports many of the Registration Review and EDSP processes associated with tracking, including DCIs, 408(p) orders s, and data submissions. PRISM serves as a replacement for the equivalent functionality provided by the Office of Pesticide Programs Information Network (OPPIN) application. PRISM was enhanced to accept electronic registration (e-Registration) documents. The e-Submission module of PRISM supports the processing of specific application documents (e.g. FIFRA §3 new applications, §3 amendments, experimental use permits, petitions for tolerances, and applications for supplemental distributor products) required for pesticide applications. OPP continues to track Reregistration program information, including DCIs, registrant responses, and reregistration data submissions through OPPIN. Currently, OPPIN also lists the bibliography of data submitters for all the DCIs. All correspondence associated with the issuance and response to the DCI is filed in the master registration file or ‘registration jacket’ of affected products. Failures to comply with DCI requirements are referred to EPA's Office of Enforcement and Compliance Assurance for appropriate follow-up actions.
Although the Agency does not publish the submitted information, public access to the OPPIN bibliography is made through the National Pesticides Information Retrieval System (NPIRS). NPIRS supports searches of the OPPIN database by chemical, subject, submission date, laboratory, guideline number, and document type. The public may request copies of non-confidential studies through the Freedom of Information Act (FOIA).
4. Describe efforts to identify duplication
This information collection is specific to the needs of the federal pesticide laws negating the need for similar data by other federal agencies or any other office within EPA. Prior to requesting any information, the Agency must review existing records for the availability of the information that it is considering requesting. The Agency maintains files on all pesticide chemicals, which include all correspondence and information/data submitted. Before any DCI is issued, these files are referenced to determine whether the necessary data are already on hand, thereby eliminating duplicative data requests. For example, most of the percent-crop-treated information can be obtained internally, and DCIs will only be issued when more data is necessary. The data for anticipated residues, on the other hand, are unique to the requirements of FIFRA, and must be submitted to the Agency.
In addition, EPA facilitates a variety of public comment periods for all the review programs covered by this ICR, the result of which may modify the data that is included in a specific DCI if warranted by information provided by registrants or the public.
OPP also encourages cost-sharing agreements among manufacturers of specific pesticide chemicals to minimize the potential duplication of laboratory tests, minimize animal testing, and reduce the costs of developing the data. All DCI notices explain the statutory provisions for cost-sharing agreements under FIFRA, as well as the various response opportunities, which include the citation to or submission of other scientifically relevant data that they believe satisfies the DCI.
5. If the collection of information impacts small businesses or other small entities, describe the methods used to minimize burden.
Currently, pesticide registrants may be divided into two groups. Approximately 10 percent of registrants manufacture or import chemical active ingredients intended for use as pesticides, sell these active ingredients to other firms for formulation into pesticide products, and/or make the end products themselves. The second and much larger group consists of registrants that purchase the active ingredients in their pesticide products from members of the first group and combine them with pesticide inert ingredients, or sometimes simply repackage them to make their end‑use products.
This second group is primarily composed of small businesses. When small businesses use a registered source of the active ingredient to formulate their products, they are generally exempt from generating health and safety data for pesticide active ingredients ("generic data"). Consequently, they usually only need to respond to a DCI for active ingredient data by claiming the "generic data exemption" and do not incur any other information burden associated with the data call‑in.
6. Describe the consequence to Federal program or policy activities if the collection is not conducted or is conducted less frequently, as well as any technical or legal obstacles to reducing burden.
The frequency of collection is on occasion in response to the receipt of a DCI requesting specific information. DCIs are issued on occasion based on the Agency’s determination of an identified need for the data for a particular pesticide. As such, a specific DCI is typically issued once per respondent (i.e., pesticide, data, and the respondent combination is unique). Given the “on-occasion” and one-time frequency of this collection, there is not an opportunity to consider a less frequent collection.
AR or PCT information is collected one time within the five years preceding the reliance on such data. The AR or PCT information collection is required by FFDCA §§ 408(b)(2)(E)(I) and 408(b)(2)(F) and cannot be collected less frequently.
For each DCI issued, the respondent provides an initial response, and, as determined by the nature of that initial response, may also provide a study status report for multi-year studies, and then submit the final data.
7. Explain any special circumstances that require the collection to be conducted in a manner inconsistent with OMB guidelines.
The only PRA guideline that may be exceeded in this collection is the time period for retaining records related to the studies conducted to generate the data that is submitted to EPA. Pursuant to FIFRA §8, EPA recordkeeping requirements in 40 CFR 169.2(k) state that records containing research data relating to registered pesticides be retained as long as the registration is valid, and the producer remains in business. Registrations are valid until they are either voluntarily canceled or withdrawn by the registrant or until EPA has cause to suspend or cancel the registration. Since the average period of marketability of a pesticide ranges from 15 to 30 years, the PRA guidelines specifying that data other than health, medical or tax records not be required to be retained for more than three years will be exceeded in this collection activity.
In addition, pursuant to 5 CFR 1320.5(a)(1)(iii)(C), EPA previously sought, and OMB provided approval for the elimination of expiration dates on the forms that may be used as part of the DCI activities covered by this ICR. The justification for doing so included the statutory basis for the collections; the stability of the information collected; the general use of the forms for multiple purposes; and the use of pre-populated or numbered forms. Although these forms are not established under this ICR, EPA notes that it will continue to omit the expiration dates on these forms.
8. If applicable, provide a copy and identify the date and page number of publication in the Federal Register of the agency’s notice, required by 5 CFR 1320.8(d), soliciting comments on the information collection prior to submission to OMB. Summarize public comments received in response to that notice and describe actions taken in response to the comments. Specifically address comments received on cost and hour burden. Describe efforts to consult with persons outside EPA to obtain their views on the availability of data, frequency of collection, the clarity of instructions and recordkeeping, disclosure, or reporting format (if any), and on the data elements to be recorded, disclosed, or report.
Pursuant to 5 CFR 1320.8(d), EPA published a notice in the Federal Register on March 31, 2021 (86 FR 16718; FRL-10021-09), announcing the planned renewal of this information collection activity, soliciting public comment on specific aspects of the ICR and providing a 60-day public comment period.
The EPA also consulted 9 stakeholders, specifically seeking their assessment of the regulatory burden estimates expressed by the Agency in this ICR. EPA consulted with the following entities and received 1 comment:
Pyxis Regulatory Consulting, Inc.
Lewis and Harrison
Household and Consumer Products Association
ACC Center for Biocide Chemistries
Steptoe & Johnson
TSG Consulting
Certis USA, LLC (Responded)
Valent Biosciences LLC
Bayer Environmental Science
The stakeholder commented that while they had not vetted the entire ICR, they agreed that the overall burden estimate provided by the Agency is reasonable. The burden calculated and estimated by the Agency has remained unchanged. A full summary of the consultation is in Attachment E.
9. Explain any decision to provide any payment or gift to respondents, other than remuneration of contractors or grantees.
This question is not applicable to this ICR
10. Describe any assurance of confidentiality provided to respondents and the basis for the assurance in statute, regulation, or agency policy.
Except as provided in FIFRA §10(d)(1)(A), (B) or (C), health and safety data submitted by registrants under FIFRA must be made available by the Agency upon request from anyone not affiliated with a multinational pesticide firm. These exceptions, however, specifically prohibit disclosure of the inert ingredients in a pesticide or of its manufacturing, quality control processes, sales and production data, or trade secrets.
Registrants may claim at the time of submission that specific data are subject to treatment as confidential for reasons other than falling within the exclusions for mandatory release. All data subject to such claims, or falling within FIFRA §10(d)(1)(A), (B), or (C) are handled strictly in accordance with the provisions of the FIFRA Confidential Business Information Security Manual. The manual requires that all CBI must be marked or flagged as such, all CBI must be kept in secure (double‑locked) areas, and all CBI intended to be destroyed must be cleared by a Document Control Officer and shredded.
11. Provide additional justification for any questions of a sensitive nature, such as sexual behavior and attitudes, religious beliefs, and other matters that are commonly considered private.
No information of a sensitive or private nature is requested in conjunction with these information collection activities, and these information collection activities comply with the provisions of the Privacy Act of 1974 and OMB Circular A-108.
12. Provide estimates of the hour burden of the collection of information.
Several programs involve the issuance of DCIs and share both a common statutory authority, FIFRA § 3(c)(2)(B), and the same basic information collection activities. As such, this section of the ICR will present the basic information collection activities and estimates common to all the DCIs that are addressed in this ICR, followed by a presentation of the program specific activities and estimates.
The IC activities and procedures associated with the issuance of a DCI are a subset of the overall activities related to DCIs generally. The following is a brief list of the overall activities common to DCIs and the basic IC activities covered by this ICR:
EPA identifies the chemical as part of the related program.
EPA identifies the potential need for data.
Registrant & public involvement/comment as part of the related program.
EPA completes final data needs determination.
EPA issues the DCI to the chemical’s registrants of the chemical.
Registrant submits an initial response to EPA, indicating how they plan to comply with the DCI.
As appropriate, the registrant may consult with EPA on their plans, e.g., data requested, protocols for studies, timeframes for submissions, etc.
If multi-year studies are involved, the registrant may be asked to submit an annual status report to EPA, reporting on progress towards compliance with DCI due date(s).
Registrant submits the data/final study reports identified in the DCI to EPA.
EPA reviews submission to determine if it satisfies the DCI.
EPA processes the data for consideration and uses in applicable assessments and decision-making.
The collection methodology for these IC activities, including the initial response options, is diagramed in Attachment B.
The data requirements are organized in 40 CFR part 158 in a series of subparts that address individual scientific disciplines or data types and describe general policies and procedures associated with the submission of data in support of a pesticide regulatory action. These general provisions include definitions, applicability, flexibility, confidential data (e.g. confidential business information, or CBI), how to submit data, use of other data, format of data submissions, flagging of studies for potential adverse effects, waivers, and minor use data policies, among other provisions. By applying the data requirements based both on the pesticide type and identified use patterns, the data collected can be tailored to ensure that the relevant data is available to support the regulatory decisions for that registration.
In establishing the data requirements in 1984, EPA adopted a stepwise approach to assist the applicant in determining the data needed to support the registration of a product. This approach, which is described in 40 CFR part 158, subpart B, involves the use of “data tables” to facilitate the identification of the applicability of the data requirements. The data requirements illustrate the questions the registrant will need to answer regarding the safety of the pesticide product before the Agency can register it. Because of the variety of chemicals and use patterns, and because EPA must retain the flexibility to tailor data requirements as appropriate, only qualitative descriptors are in the tables. Test notes provide more specific information on the applicability of specific data requirements.
The table descriptors NR (not required), R (required), and CR (conditionally required) should be viewed as a general presentation, indicating the likelihood that the data requirement applies.
The use of R does not necessarily indicate that a study is always required, but that it is more likely to be required than not. For example, if the applicant wanted to apply his pesticide to apples, then crop field trials would almost always be required on apples. However, if the physical/chemical properties of the chemical did not lend themselves to the test, such as performing an inhalation test with a chemical that is a solid and has an extremely low vapor pressure, then a waiver might be granted. Generally, test notes for R studies discuss any particular circumstances when the testing might not be required.
The use of CR means a study is less likely to be required. Triggers in the test notes indicate the circumstances under which the Agency has learned through experience that the information is needed. Although only an approximation, if percentages were to be assigned to indicate the probability that a particular study is needed, R could be viewed as representing that the submission of a study is required approximately 50% to 100% of the time and CR would indicate that a study is required 50% of the time or less.
Thus, NR, R, and CR are used for convenience to make the table format feasible but serve only as a general indication of the applicability of a data requirement. In all cases, the test notes referred to in the table must be consulted to determine the actual need for the data.
The table format includes a column heading entitled “Guideline,'' which refers to the OCSPP Harmonized Test Guidelines4. Guideline numbers are provided as information/guidance to applicants. These Guidelines set forth recommended instructions and test methods for performing a study to generate the required data. Since these are guidance documents, the applicant is not required to use these Guidelines, but may instead seek to fulfill the data requirement by other appropriate means, such as alternative test methods, submission of an article from open literature, or use of modeling. The applicant may submit a protocol of their own devising for the Agency to review. However, the OCSPP Harmonized Guidelines have been developed through a rigorous scientific process, including extensive peer review by the FIFRA Scientific Advisory Panel. Additionally, many of the Guidelines have been harmonized internationally. As such, they represent the recommended approach to developing high-quality data that should satisfy EPA's data needs for risk assessment.
Since it is not possible to sufficiently delineate all circumstances in test notes, consultation with EPA is encouraged. Applicants are encouraged to visit the Agency's website at http://www.epa.gov/pesticide-registration/data-requirements.
The Agency may also require the submission of studies to generate data that are not codified in 40 CFR part 158 (i.e. non-guideline studies) where critical information is needed about the risks and benefits of the pesticide in order to support its registration. Agency requests for these studies are based on the particular characteristics of the chemical and the Agency’s need for the information to make the required statutory finding. In cases where the Agency has determined that there is a need for specific data not yet codified in 40 CFR part 158, EPA may already requestor accept voluntary submission of the data in order to facilitate making sound regulatory decisions, while minimizing the burden and costs associated with a delayed or conditioned decision. Attachment C provides a listing of the non-codified studies (i.e. non-guideline studies) that EPA has recently or is currently requesting for certain data call-ins (DCIs) already issued, or expected to be issued by EPA, along with a rationale for requiring the data, and an explanation of the practical utility of the data . The studies are grouped by scientific discipline.
The data that EPA may collect and review under this ICR will likely vary for each DCI because the DCI is tailored to address the specific needs of the individual chemical or active ingredient under review. However, the data request will be primarily based on the data requirements that are found in 40 CFR part 158, which includes a provision that allows the Agency to seek additional non-codified data that is determined to be necessary to make the risk-based decisions mandated by federal law. In codifying the requirements in 40 CFR part 158, EPA provided substantiation and support to demonstrate the need and practical utility for the data in terms of its use to assess the risks for particular chemicals based on the different use patterns and pesticides, and in order to make the required registration decisions.
The Agency uses two basic approaches to calculating the potential burden and costs for this ICR:
1) For the data generation activities, EPA calculated the paperwork burden as a percentage of the testing costs; and
2) For the rest of the paperwork activities EPA estimated the average amount of time required to complete the specific activity, considering estimates provided in other approved ICRs involving the same activity, feedback from stakeholders, and EPA's overall experience with such activities.
Method Used to Calculate the Burden and Costs for Data Generation. EPA calculates the paperwork burden and costs for the data generation activities as a percentage of the testing costs. This percent-based estimate of paperwork associated with conducting a test was initially established in consultation with OMB in the 1980's in an effort to provide a reasonable estimate of the burden associated with the paperwork component of data generation, which may vary based on the complexity of the test performed.
This methodology as described in detail in the 2007 document entitled “General Methodology Used to Estimate Paperwork Burden Hours and Costs by the Office of Pesticide Programs for Submission of Required Data/Information for Responding to a Data Call-In Notice.”5 Based on feedback received at the time, EPA concluded the methodology was a reasonable and fair alternative to simply setting a single estimate for data generation burden or perhaps using some set criteria like a high, medium or low burden, neither of which may fairly reflect potential differences in burden.
In December 2013, the Agency held a DCI Response Burden Assessment Workshop with industry stakeholders. As part of the reassessment, EPA consulted with industry about the Agency burden assumptions, the methodology used to estimate the burden, the time estimates for conducting PRA activities, and the accuracy of and appropriate distribution of the labor rates. 6 As a result, the Agency redefined some of the 2007 methodologies by revising the number of DCI recipient groups and calculations for those groups to reassess the PRA burden. For more detail on the revised burden methodology, see Attachment B.
In summary, to calculate the burden and costs associated with the paperwork activities involved in conducting the tests, the Agency starts with the cost of the test, typically the market price for the test as identified by laboratories that offer testing services. For this ICR, the Agency maintains an archive of the basic FIFRA study cost estimates that were developed through surveys of independent testing laboratories, Agency economic analyses, and registrant comments during ICR renewal periods. To the extent possible, EPA uses multiple sources to provide test cost estimates, which are updated as needed.
Based on the existing methodologies, EPA used 35% of the estimated total test cost to calculate the total potential cost of the paperwork activities related to data generation. The 35% of test cost is disaggregated by labor category, and then burden hours are extrapolated by using the loaded labor rates. To disaggregate by labor category, the Agency considered the estimated distribution of paperwork activity across the labor category represented and the existing methodology assumption that paperwork activities for data generation mostly involve the technical staff to perform the tests, with a few activities related to management and clerical. See Figure 1 for an illustrated outline of the Agency burden calculation process for data generation.
Figure 1 – Method for Calculating Paperwork Burden from Test Costs
This
approach assumes and incorporates the following core considerations:
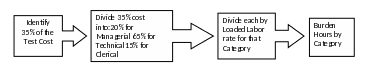
(1) Recipients generate all of the data as specified in the DCI without any changes.
(2) All data generation is performed by an independent laboratory.
(3) Paperwork burden is disaggregated by labor category as follows:
a. Managerial (20%)
b. Technical (65%)
c. Clerical (15%)
(4) Labor rates are fully loaded, meaning that they include the estimated costs of wages, overhead, and benefits paid to an employee.
See Attachment B, Section II, B. Methodology for Calculating Labor Wage Rates, for more details about this method.
Method Used to Calculate the Burden and Costs for Other Activities. For the other activities, EPA estimated the burden hours by considering the activities themselves and the average expected amount of time that the activity is anticipated to involve. These estimates consider the Agency's experience with similar data collection activities and direct experience in conducting the tests in EPA Laboratories. The methodology used to calculate cost are identified in Appendix D, section VI, Is the burden for those not generating data covered?
For each program DCI (e.g. reregistration and registration review, special review, etc.), each DCI will involve the same essential document preparation and submission activities, which are grouped into the following three information collection categories for purposes of presenting the burden and costs in this ICR:
DCI Recipients
Data Generators
Consortium Participants
DCI Recipients - After receiving a DCI, each recipient has 90 days to provide an initial response that indicates how the recipient plans to comply with the DCI. A registrant may avoid generating the data if they qualify for a generic data exemption, for example if they use a registered pesticide as the source of the active ingredient in their own product, cancel the product’s registration, submit or cite existing data, or are granted a waiver by EPA in response to their request. These initial response options are generally available under the pesticide program, and the activities, along with the paperwork burden and costs associated with those activities, are already addressed under other ICRs. Not all DCI recipients will generate data in response to a DCI. The DCI recipient is assumed to be involved in the four burden activities listed in Table 1. The burden for DCI recipients only will be lower than the burden estimates for the DCI recipients who are also part of the data generation group.
Data Generators - Regardless of the response option that the DCI recipient might select, the Agency has assumed that some data will be generated for each chemical. The data generator is assumed to be involved in the nine burden activities listed in Table 1. While Agency records indicate that not all the studies requested in a DCI are, in fact, generated (data generators can request waivers, submit or cite existing data like the DCI recipients), for the most part, the data generator group will assume the highest DCI response burden among the three respondent groups.
Consortium Participants - The Agency assumes that whenever more than one company receives identical DCIs for the same chemical, companies will work together to generate one set of data by participating in a consortium or task force. Generally, the Agency calculations for these paperwork burdens are accounted for as part of the 35% of the cost of generating studies.7 However, in addition to the cost of data generation, consortium participants are subject to costs associated with operating a consortium or task force (e.g., communication, attending meetings, etc.). The seven additional consortium burden activities and operating costs are accounted for in Table 1.
Table 1 identifies the paperwork activities that would typically be performed by a DCI recipient. Each recipient group is expressed by the activities of the corresponding categories.
Table 1: Expected DCI Recipient Activities and Categories
DCI Recipient |
|
Collection Activity |
Collection Category |
1) Read Instructions |
Reporting |
2) Plan Activities |
|
3) Complete Paperwork |
|
4) Store/maintain Data |
Recordkeeping |
Data Generator |
|
Collection Activity |
Collection Category |
1) Read and discuss test requirements |
Reporting |
2) Discuss test and protocol with Agency |
|
3) Plan activities |
|
4) Create information |
|
5) Gather information |
|
6) Process, compile, review information for accuracy |
|
7) Complete written forms |
|
8) Record, disclose, display information |
Recordkeeping |
9) Store, file, or maintain information |
|
Consortium Activities |
|
1) Negotiate/establish consortium/task force agreements/select administrator |
Paperwork burden associated with operating a consortium |
2) Establish/conduct appropriate technical working groups |
|
3) Participate in consortium discussions |
|
4) Plan logistics for calls or meetings |
|
5) Schedule and participate in discussions with Agency |
|
6) Review Agency assessments, participate in public comment activities |
|
7) Store, file, or maintain consortium information |
|
The estimated paperwork burden and costs for DCI recipients vary from DCI to DCI because of the variations in the individual studies that are part of the DCI program group (e.g., reregistration registration review, special review, etc.) and the combination of activities (waivers, exemptions, consortium participation, data generation etc.) each DCI manifests. As discussed, there are multiple ways of responding to a DCI, and not all DCI recipients will generate and submit data as part of the DCI response. Until the Agency receives the 90-day response letters to the DCI notice from the registrants indicating what studies, if any, they will conduct, it is not possible to accurately estimate the burden and costs of developing the data. Nor can the Agency accurately predict the number of DCI recipients who will generate data or the amount of data that might be submitted to EPA. The Agency’s burden estimates are based on past patterns of DCI response activities.
DCI Recipients - DCI recipients are subject to burden from having to provide an initial response to the EPA for a DCI regardless of whether or not they generate data. The methodology EPA used for calculating the burden for this group is derived from the 2007 Methodology,8 Phase 1 requirements outlined in Case Study #1, Attachment A of that document reflects the activities that all DCI recipients would have to conduct regardless of whether or not they generate data.
Given that a single DCI can be sent to several companies, DCI recipient burden is calculated at the company level—not at the DCI level. To estimate the number of companies that are DCI recipients, EPA conducted a search of companies that received a DCI request in its Pesticide Registration Information System (PRISM) to determine the average annual number of impacted entities.
Data Generators - The paperwork burden and costs for data generators are based in part on the average cost of paying a laboratory to conduct the test(s) necessary to generate the data requested in the DCI. To estimate paperwork activities for each type of labor category (managerial, technical, and clerical), the disaggregated paperwork burden costs are multiplied by their corresponding labor wage rates ($/hr). As previously mentioned, some DCIs do not follow the Agency’s methodology of paperwork being 20%-65%-15% Managerial-Technical-Clerical as certain IC Groups have paperwork burden that falls disproportionately on different labor categories. For details regarding the methodology used for calculating data generation paperwork burden for each of the IC Groups, refer to Attachment B, Appendix B.
EPA has also assumed that for each DCI, companies are combining resources when responding to a DCI and data generation is necessary—thus, it is expected that only one set of data is being submitted to the EPA in response to each DCI request. EPA understands that this assumption may not be accurate and solicits industry input to clarify this assumption.
Consortium Participants - Although consortium members encumber burden from consortium activities, the cost savings from avoiding study generation are expected to far exceed the burden of such activities. Furthermore, EPA assumes that no business would opt to join a consortium if the cost of consortium activities would result in a higher cost per DCI. Thus, for each consortium member, the upper bound (i.e., maximum) total cost per DCI submitted by a consortium is expected to be less than or equal to the per DCI burden incurred by a recipient who chooses to submit their DCI data independently. In this case, the burden per consortium member would be equal to that in Table 3 for Data Generators. Unlike typical data generators, however, consortiums face additional paperwork burden activities, such as meetings and correspondence to coordinate consortium activities. Industry provided EPA with information to support that approximately 21 consortiums exist and typical consortium activities that result in paperwork burden. Details on consortium activities and the methodology used for calculating total consortium paperwork burden are located in Attachment B, Appendix C.
The Agency estimates that 122 companies will receive a DCI request annually. For more information on methodologies used in estimating the total number of DCI recipients and burdens to DCI recipients, see Attachment B, Appendix A.
EPA expects to issue approximately 268 DCIs annually over the next three years that will require data generation. This estimate for data generators does not include voluntarily submitted data as they are not DCIs.
The breakdown of the regulatory decisions for DCIs that EPA expects to make over the next three years (2021-2024) is as follows:
Table 2: Estimated Number of Annual DCIs by IC Group (FY 2021 – 2024)
IC Number |
IC Group |
Total DCIs 1-Year Period* |
Total DCIs 3-Year Period* |
1 |
Reregistration DCIs: Confirmatory Data |
0.3 |
1 |
2 |
Reregistration: Voluntarily Submitted Data |
0.3 |
1 |
(Low Burden Studies) |
|||
3 |
Reregistration: Voluntarily Submitted Data |
||
(High Burden Studies) |
|||
4 |
Reregistration DCIs: Product Specific Data |
0.3 |
1 |
5 |
Maintenance DCI1 |
75 – NTIP |
225 – NTIP |
1 - Efficacy |
3 - Efficacy |
||
6 |
Registration Review DCIs |
110.7 |
332 |
7 |
Registration Review Resistance Management Plans |
79 |
237 |
8 |
Registration Review: Voluntarily Submitted Data (Low Burden Studies) |
5 |
15 |
9 |
Registration Review: Voluntarily Submitted Data (High Burden Studies) |
||
10 |
Anticipated Residue DCIs: Base Set of Data |
0.3 |
1 |
11 |
Anticipated Residue DCIs: Verification of Use Data |
0.3 |
1 |
12 |
Anticipated Residue DCIs: Updated Public Source Monitoring Data |
0.3 |
1 |
13 |
DCIs for Percent Crop Treated Estimates |
0.3 |
1 |
Total DCIs* |
267.5 |
803 |
|
Total Voluntarily Submitted Data |
5.3 |
16 |
|
1Includes Non-Target Insect Pollinator (NTIP) and Efficacy Studies
* Counts for IC Groups 2, 3, 8, and 9 are for voluntarily submitted data—i.e., they are not DCIs. Therefore, the total DCI count does not include these estimates. Numbers may not add due to rounding.
The following programs involve reviews of existing registrations that could result in a determination that additional data are necessary for a decision, and which would be sought through the issuance of a DCI under FIFRA §3(c)(2)(B). 9
Reregistration Program
FIFRA §410 requires EPA to re-assess the health and safety data for all pesticide active ingredients registered before November 1, 1984, to determine whether these “older” pesticides meet the criteria for registration that would be expected of a pesticide being registered today for the first time. FIFRA §4 directs EPA to use FIFRA §3(c)(2)(B) authority to obtain the required data. While Reregistration Eligibility Decisions (REDs) were completed by 2006 for food-use pesticide ingredients and 2008 for non-food use pesticide ingredients, the Agency still has one DCI to issue after FY 2021 to close out the program (e.g., to obtain product specific chemistry, acute toxicity, and efficacy data).
Reregistration Review Program
FIFRA, as amended by the Food Quality Protection Act (FQPA) of 1996, mandates the continuous review of existing pesticides (FIFRA §3(g)11). All pesticides distributed or sold in the United States must be registered by EPA based on scientific data showing that they will not cause unreasonable risks to human health or to the environment when used as directed on product labeling. The registration review program is intended to make sure that, as the ability to assess and reduce risk evolves and as policies and practices change, all registered pesticides continue to meet the statutory standard of no unreasonable adverse effects. Changes in science, public policy, and pesticide use practices will occur over time. Through the registration review program, the Agency periodically re-evaluates pesticides to make sure that as these changes occur, products in the marketplace can continue to be used safely. Information on this program is provided at http://www.epa.gov/pesticide-reevaluation. In 2006, the Agency implemented the registration review program pursuant to FIFRA § 3(g) and will review each registered pesticide every 15 years to determine whether it continues to meet the FIFRA standard for registration (40 CFR Part 155, subpart C12). The Pesticide Registration Improvement Act (PRIA)13 requires EPA to complete registration review decisions by October 1, 2022 for all pesticides registered as of October 1, 2007. Each chemical will need to repeat registration review no later than 15 years following the date of completion of the initial registration review or following the date the chemical was first registered (for chemicals registered after October 1, 2007). FIFRA §3(g) instructs EPA to use the FIFRA §3(c)(2)(B) authority to obtain data determined to be necessary to complete the assessments, reviews, and decisions called for under FIFRA §3(g).
In addition, EPA intends for these reviews to also involve the review of data related to endangered species and endocrine effects:
Endangered Species Protection Program (ESPP): EPA regards the ESPP, which concerns endangered species assessments (effects determinations) required under the Endangered Species Act (ESA)14, as part of the risk characterization of the pesticide under Registration Review. FIFRA §3(g) instructs EPA to use the FIFRA §3(c)(2)(B) authority to obtain the required data.
Endocrine Disruptor Screening Program (EDSP): EPA considers endocrine effects pursuant to FFDCA §408(p)15 as part of the risk characterization of the pesticide under Registration Review.16 FFDCA §408(p) mandates the issuance of Orders requiring screening of substances for their potential endocrine disruptor effects. FIFRA §3(c)(2)(B) of FIFRA also provides a means of obtaining needed data for pesticides. Thus two types of data collection authorities allow the Agency to address endocrine disruptor screening and testing data needs: DCIs and 408(p) orders. Currently, EPA is using DCIs to obtain the endocrine disruptor screening and testing data on as a needed basis for pesticide chemicals.
The Agency has updated the estimated wages, benefits and overhead for all labor categories for affected industries, state government, and EPA employees based on publicly available data from the US Bureau of Labor Statistics. The formulas used to estimate the labor rates and formulas used to derive the fully loaded rates and overhead costs for this ICR renewal are listed in Attachment D. Tables 3 and 4 provide information on the burden and costs faced by DCI recipients, data generators, and consortium participants. Respondent costs are based on managerial, technical, and clerical wage rates estimated at $145.34, $76.35, and $49.61 per hour, respectively. These wage rates are based on 2015 wage rates estimated by the Bureau of Labor Statistics (BLS) for the North American Industry Classification System (NAICS) for pesticide registrants (NAICS code 325300).
Table 3 outlines burden and cost to these three groups per company or DCI. For DCI recipients, burden is estimated by company since companies are responsible for responding to the 90-day notice. For data generators, the burden assumes that only one data package is being submitted by one or more companies for each DCI. Methods used for calculating the cost and burden for cases under each IC Group vary. For a review of methods used in these calculations, refer to Attachment B, Appendix A, B, and C.
Table 3: Estimated DCI-Related Annual Respondent Burden and Costs per Company/DCI*
Activity Category |
Clerical |
Technical |
Manager |
Burden Totals |
||||
Hrs. |
$49.61/hr |
Hrs. |
$76.35/hr |
Hrs. |
$145.34/hr |
(hrs) |
Costs ($) |
|
IC Category – DCI Recipients1 |
||||||||
Reporting |
0 |
$0 |
7 |
$534 |
12 |
$1,744 |
19 |
$2,278 |
Recordkeeping |
1 |
$50 |
0 |
$0 |
0 |
$0 |
1 |
$50 |
IC Category – Data Generators1 |
||||||||
Reregistration Program DCIs |
||||||||
1) Confirmatory DCIs |
||||||||
Reporting |
724 |
$35,897 |
3,821 |
$291,760 |
501 |
$72,829 |
5,046 |
$400,486 |
Recordkeeping |
634 |
$31,432 |
0 |
$0 |
117 |
$16,943 |
750 |
$48,375 |
2) Product Specific DCIs |
||||||||
Reporting |
110 |
$5,461 |
581 |
$44,348 |
75 |
$10,866 |
766 |
$60,675 |
Recordkeeping |
96 |
$4,773 |
0 |
$0 |
19 |
$2,780 |
115 |
$7,553 |
3) Reregistration: Voluntarily Submitted Low Burden Studies |
||||||||
Reporting |
76 |
$3,749 |
396 |
$30,252 |
57 |
$8,274 |
529 |
$42,275 |
Recordkeeping |
65 |
$3,232 |
0 |
$0 |
7 |
$1,034 |
72 |
$4,266 |
4) Reregistration: Voluntarily Submitted High Burden Studies |
||||||||
Reporting |
414 |
$20,549 |
2,191 |
$167,287 |
284 |
$41,331 |
2,890 |
$229,167 |
Recordkeeping |
364 |
$18,056 |
0 |
$0 |
70 |
$10,142 |
434 |
$28,198 |
Maintenance and Registration Review DCIs |
||||||||
5) Maintenance DCIs |
||||||||
Reporting |
390 |
$19,322 |
2,055 |
$156,889 |
279 |
$40,536 |
2,723 |
$216,747 |
Recordkeeping |
340 |
$16,883 |
0 |
$0 |
53 |
$7,737 |
394 |
$24,621 |
6) Registration Review DCIs |
||||||||
Reporting |
3,355 |
$166,410 |
17,812 |
$1,359,881 |
2,386 |
$346,734 |
23,552 |
$1,873,026 |
Recordkeeping |
2,972 |
$147,408 |
0 |
$0 |
493 |
$71,691 |
3,465 |
$219,099 |
7) Registration Review Resistance Management Plans |
||||||||
Reporting |
0 |
$0 |
32 |
$2,407 |
3 |
$481 |
35 |
$2,888 |
Recordkeeping |
0 |
$0 |
4 |
$321 |
0 |
$0 |
4 |
$321 |
8) Registration Review: Voluntarily Submitted Low Burden Studies |
||||||||
Reporting |
76 |
$3,749 |
396 |
$30,252 |
57 |
$8,274 |
529 |
$42,275 |
Recordkeeping |
65 |
$3,232 |
0 |
$0 |
7 |
$1,034 |
72 |
$4,266 |
9) Registration Review: Voluntarily Submitted High Burden Studies |
||||||||
Reporting |
414 |
$20,549 |
2,191 |
$167,287 |
284 |
$41,331 |
2,890 |
$229,167 |
Recordkeeping |
364 |
$18,056 |
0 |
$0 |
70 |
$10,142 |
434 |
$28,198 |
Anticipated Residue/Percent Crop Treated DCIs |
||||||||
10) AR DCIs: Base Set of Data |
||||||||
Reporting |
3 |
$128 |
11,173 |
$852,992 |
4 |
$598 |
11,179 |
$853,717 |
Recordkeeping |
1 |
$43 |
0 |
$0 |
0 |
$0 |
1 |
$43 |
11) AR DCIs: Verification-of-use Data |
||||||||
Reporting |
13 |
$650 |
34 |
$2,590 |
16 |
$2,308 |
63 |
$5,548 |
Recordkeeping |
2 |
$82 |
0 |
$0 |
0 |
$0 |
2 |
$82 |
12) AR DCIs: Updated Public Source Monitoring Data |
||||||||
Reporting |
12 |
$615 |
95 |
$7,216 |
14 |
$1,977 |
121 |
$9,807 |
Recordkeeping |
2 |
$77 |
0 |
$0 |
0 |
$0 |
2 |
$77 |
13) DCIs for Percent Crop Treated Estimates |
||||||||
Reporting |
3 |
$125 |
45 |
$3,403 |
1 |
$189 |
48 |
$3,716 |
Recordkeeping |
1 |
$64 |
0 |
$0 |
0 |
$0 |
1 |
$64 |
Consortiums |
||||||||
Paperwork burden associated with operating a consortium |
510 |
$25,299 |
800 |
$61,078 |
810 |
$117,722 |
2,120 |
$204,098 |
* Numbers may not add due to rounding. Please refer to text for information on calculations presented in this table.
1 Note that these estimates reflect burden and costs per company when referring to DCI recipients (122) and per DCI (268) when referring to data generators. Methods used for calculating the cost and burden for cases under each IC Group vary. For a review of methods used in these calculations, refer to Appendix A, B, and C.
Table 4 presents the total respondent burden hours for DCI recipients, data generators, and consortium participants (excluding voluntary data submissions). These calculations reflect recordkeeping, reporting, and total burden numbers for each IC group universe. The per DCI/company respondent burden and costs in Table 3 are scaled by the 1-year expected number of DCIs per IC Group in Table 2 to calculate the respondent bottom-line annual costs by IC Group in Table 4. Refer to Appendices A, B, and C for methodologies and formulas demonstrating how these estimates were calculated.
Table 4: Respondent Bottom line: Costs (Annual Totals)
|
Burden Hours |
Costs |
|||||
Reporting |
Recordkeeping |
Total |
Reporting |
Recordkeeping |
Total |
||
Data Recipients |
2,318 |
122 |
2,440 |
$277,971 |
$6,052 |
$284,023 |
|
Data Generators |
|||||||
Reregistration Program DCIs |
|||||||
|
Confirmatory DCIs |
1,682 |
250 |
1,932 |
$133,495 |
$16,125 |
$149,620 |
Product Specific DCIs |
255 |
38 |
294 |
$20,225 |
$2,518 |
$22,743 |
|
Voluntarily Submitted Low Burden Studies |
176 |
24 |
200 |
$14,092 |
$1,422 |
$15,514 |
|
Voluntarily Submitted High Burden Studies |
963 |
145 |
1,108 |
$76,389 |
$9,399 |
$85,788 |
|
Maintenance and Registration Review DCIs |
|||||||
|
Maintenance DCIs |
199,995 |
28,887 |
228,881 |
$15,918,069 |
$1,805,799 |
$17,723,868 |
Registration Review DCIs |
2,606,450 |
383,446 |
2,989,896 |
$207,281,499 |
$24,247,001 |
$231,528,500 |
|
Registration Review Resistance Management Plans |
2,752 |
332 |
3,084 |
$228,160 |
$25,351 |
$253,511 |
|
Registration Review: Voluntarily Submitted Low Burden Studies |
2,644 |
361 |
3,005 |
$211,374 |
$21,331 |
$232,705 |
|
Registration Review: Voluntarily Submitted High Burden Studies |
14,449 |
2,169 |
16,618 |
$1,145,834 |
$140,991 |
$1,286,825 |
|
Anticipated Residue/Percent Crop Treated DCIs22 |
|||||||
|
AR DCIs: Base Set of Data |
3,726 |
0.3 |
3,727 |
$284,572 |
$14 |
$284,587 |
AR DCIs: Verification-of-use Data |
21 |
0.5 |
22 |
$1,849 |
$27 |
$1,877 |
|
AR DCIs: Updated Public Source Monitoring Data |
40 |
0.5 |
41 |
$3,269 |
$26 |
$3,295 |
|
DCIs for Percent Crop Treated Estimates |
16 |
0.4 |
17 |
$1,239 |
$21 |
$1,260 |
|
DCI Data Generator Total |
2,814,937 |
412,955 |
3,227,892 |
$223,872,378 |
$26,096,882 |
$249,969,260 |
|
|
Operating Activities Burden Hours |
Operating Activities Cost |
|||||
Consortium Members |
- |
- |
44,520 |
- |
- |
$4,286,061 |
|
Total Burden |
2,817,255 |
413,077 |
3,274,852 |
$224,150,350 |
$26,102,934 |
$254,539,344 |
|
Numbers may not add due to rounding. Voluntary submissions are not included in the Data Generator Total. Please refer to text for information on calculations presented in this table. Methods used for calculating the cost and burden for cases under each IC Group vary. For a review of methods used in these calculations, refer to Appendices A, B, and C.
13. Provide an estimate for the total annual cost burden to respondents or recordkeepers resulting from the collection of information.
There are no operational and/or maintenance costs.
14. Provide estimates of annualized cost to the Federal government.
While Agency burden activities for processing all DCIs is substantially similar, the Agency burden and cost are commensurate with the amount of data to be analyzed and the specific DCI. A detailed breakout of the Agency burden and cost for the different types of DCIs for the Reregistration, Registration Review, Special Review, AR and PCT programs are in Attachment B, Appendices A, B and C. The Agency labor, and wage rate calculations are in Attachment D.
Table 5 provides a summary of the annual total estimated Agency burden and cost for all DCI programs. Annual Agency burden hours for DCI activities are estimated at 7,179 hours at a cost of $550,781.
Table 5. Summary of Agency DCI Paperwork Burden and Cost (annual totals)
|
Burden Hours |
Costs |
||||
Reporting |
Recordkeeping |
Total |
Reporting |
Recordkeeping |
Total |
|
Reregistration Program DCIs |
326 |
5 |
330 |
$25,956 |
$207 |
$26,164 |
Maintenance DCI |
12,384 |
171 |
12,555 |
$986,347 |
$7,883 |
$994,230 |
Registration Review DCIs |
7,587 |
1,005 |
8,592 |
$581,194 |
$46,343 |
$627,537 |
Anticipated Residue/Percent Crop Treated DCIs |
53 |
7 |
60 |
$4,086 |
$326 |
$4,412 |
Total Annual Agency Burden |
20,350 |
1,188 |
21,538 |
$1,597,583 |
$54,760 |
$1,652,342 |
Numbers may not add due to rounding. Please refer to text for information on calculations presented in this table. Methods used for calculating the cost and burden vary for each type of DCI. For a review of methods used in these calculations, refer to Appendices B.
15. Explain the reasons for any program changes or adjustments reported in Items 13 (or 14) of OMB Form 83-I.
Estimates of burden hours and costs are substantially larger than in the most recent ICR. This is primarily due to an error in past ICRs where annual totals were misreported as 3-year totals underestimating the total approved burden hours and costs by a factor of 3. The Agency has corrected this error and is now reporting annual total burden hours and costs throughout this ICR. Additionally, due to a clerical error, a burden of 58,206 hours was approved rather than the submitted 625,669 burden hours from the currently approved ICR by OMB. The Agency has corrected these errors and this ICR represents an increase of 2,649,183 hours (3,274,852 – 625,669) in the total estimated annualized burden compared with what is currently approved by OMB. The burden increase is a result of several factors, including an increase in DCIs issued annually (Previous to Current Numbers), the addition of high-test costs for certain DCIs, and an increase in non-government wage rates. All these activities have contributed to the significant increase in burden. Thus, this change represents a program adjustment and correction as show in Table 6.
Table 6. Summary of Adjustments
|
Total Annual Burden Hours |
Labor Wage Rates (Year) |
Number of DCIs Issued |
Total Annual Test Costs |
Current 2021 DCI Renewal ICR |
3,274,852 |
2019 |
268 |
$254,539,344 |
Current approved DCI 2018 ICR |
625,669 |
2015 |
221 |
$44,890,390 |
Difference |
2,649,183 |
|
47 |
$209,648,954 |
16. For collections whose results will be published, outline the plans for tabulation and publication.
There is not a collection schedule per se. DCIs are issued when the need is identified. The time frame in which the respondents must then submit the requested material is established for each DCI based on the individual circumstances surrounding the DCI and applicable review. However, as discussed in Section 3(b) Programs Involving DCIs, a variety of FIFRA programs require EPA to conduct periodic reviews to ensure the pesticide continues to pose no risk of unreasonable adverse effects on human health or the environment. These review processes generate the bulk of the DCI determinations. For a variety of reasons, most manufacturers wait to generate new data and/or submit new/existing data until EPA issues the DCI. One of the most important reasons for this is that EPA’s issuance of a DCI is a public statement that the data are needed, and will be relied on, thus “triggering” the data compensation provisions of FIFRA §3(g)(1)(B).
As part of the consultation and public participation process, EPA generally works with respondents to ensure that sufficient time is built into the individual DCIs to allow for respondents to gather and submit the requested information. However, the timing of AR/PCT-related DCIs and respondent data submissions is somewhat different.
AR DCIs will generally be issued whenever ARs data is relied upon, either to establish new tolerances or to reassess existing tolerances. Registrants have five years before data must generally be submitted in support of the ARs used. Data must also be periodically reviewed when PCT estimates are relied upon, but in most cases the Agency will be able to collect internally or generate this data. EPA will issue PCT DCIs in cases where the Agency is unable to obtain the information on its own. In these cases, the registrant must submit data within five years of the use of PCT estimates. Additional time is provided for the development of new studies appropriate to the nature of the studies required.
17. If seeking approval to not display the expiration date for OMB approval of the information collection, explain the reasons why display would be inappropriate.
This question not applicable to this ICR
18. Explain each exception to the certification statement identified in Item 19 of OMB Form 83-I.
EPA does not request an exception to the certification of this information collection.
SUPPLEMENTAL INFORMATION
The annual public burden for this collection of information is estimated to range between 20 and 8182 hours per response over the three-year period. According to the Paperwork Reduction Act, “burden” means the total time, effort, or financial resources expended by persons to generate, maintain, retain, or disclose or provide information to or for a Federal agency. For this collection it includes the time needed to review and understand instructions; prepare and submit reports (including searching data sources); complete and review the collection of information; transmit the information; and keep records.
To comment on the Agency's need for this information, the accuracy of the provided burden estimates, and any suggested methods for minimizing respondent burden, including the use of automated collection techniques, EPA has established a public docket for this ICR under Docket ID Number EPA-HQ-OPP-2020-0693, which is available at http://www.regulations.gov. This site can be used to submit or view public comments, access the index listing of the contents of the public docket, and to access those documents in the public docket that are available electronically. When in the system, select “search,” then key in the Docket ID Number identified above.
You can also provide comments to the Office of Information and Regulatory Affairs, Office of Management and Budget via http://www.reginfo.gov/public/do/PRAMain. Find this particular information collection by selecting ‘‘Currently under 30-day Review—Open for Public Comments’’ or by using the search function.
All comments received by EPA will be included in the docket without change, including any personal information provided, unless the comment includes profanity, threats, information claimed to be Confidential Business Information (CBI), or other information whose disclosure is restricted by statute. Do not submit electronically any information you consider to be CBI or other information whose disclosure is restricted by statute.
Please note that due to the public health concerns related to COVID-19, the EPA Docket Center (EPA/DC) and Reading Room is closed to visitors with limited exceptions. The staff continues to provide remote customer service via email, phone, and webform. For the latest status information on EPA/DC services and docket access, visit https://www.epa.gov/dockets.
LIST OF ATTACHMENTS
The attachments listed below can be found in the docket for this ICR or by using the hyperlink that is provided in the list below. The docket for this ICR is accessible electronically through http://www.regulations.gov using Docket ID Number: EPA-HQ-OPPT-2020-0693.
Attachment |
Description |
|
A |
Forms that are commonly associated with Data Call-ins are available electronically at: https://www.epa.gov/pesticide-registration/label-review-manual in the forms section. |
|
|
|
EPA Form No. 8570-4 - Confidential Statement of Formula |
|
|
EPA Form No. 8570-27 - Formulator's Exemption Statement |
|
|
EPA Form No. 8570-28 - Certification of Compliance with Data Gap Procedures |
|
|
EPA Form No. 8570-32 - Certification of Attempt to Enter into an Agreement with Registrants for Development of Data Form |
|
|
EPA Form No. 8570-34 - Certification with Respect to Citation of Data Form |
|
|
EPA Form No. 8570-35 - Data Matrix Form |
|
|
EPA Form No. 8570-36 - Summary of the Physical/Chemical Properties Form |
|
|
EPA Form No. 8570-37 - Self-Certification Statement for the Physical/Chemical Properties |
|
|
The remaining forms are computer generated and uniquely pre-populated and sent directly to individual registrants. The forms below are part of the multipage DCI notice which contain samples of the forms and instructions below. This information has been provided to OMB directly. EPA Form No. 6300-3 - Requirements Status and Registrant’s Response. EPA Form No. 6300-4 - Data Call-In Response Form. |
B |
Office of Pesticide Programs 2015 Revised General Methodology and assumptions Used to Estimate Paperwork Response Burden for Pesticide Data Call-In Recipients, November 2015. This methodology includes the calculations for paperwork burden and costs of data generation activities. |
|
|
|
Appendix – A Estimated Burden Hours and Costs for DCI Recipients |
|
|
Appendix – B Estimated Burden Hours and Costs for DCI Collection Activities for Data Generators, by IC Group |
|
|
Appendix – C Estimated Burden Hours and Costs for Consortium Activities |
|
|
Appendix – D General Methodology used to Estimate Paperwork Burden Hours and Costs by the Office of Pesticide Programs for Submission of Required Data/Information for Responding to a Data Call-in Notice, October 2007. |
C |
Non-Codified Study Justifications, February 10, 2021 |
|
D |
Work sheets to Calculate Industry and EPA Labor Costs (2019) |
|
E |
Consultation Summary |
|
F |
Generic ICR Template for future Supporting Statements for a Generic Information Collection Request (ICR) under the Paperwork Reduction Act (PRA) |
|
3 As noted earlier in section 3(b)(ii), the information collection activities associated with EDSP are already covered by another ICR.
4 The OCSPP Harmonized Test Guidelines are available at http://www.epa.gov/test-guidelines-pesticides-and-toxic-substances/master-list-test-guidelines-pesticides-and-toxic
5 See Appendix D.
6 On December 12, 2013, The Office Pesticide Programs sponsored a DCI Response Burden Assessment Workshop. Industry participants included, but were not limited to: representatives from BASF, the DOW Chemical Company, the American Chemistry Council Biocides Panel, Steptoe and Johnson, LLP, Technology Sciences Group Inc., Monsanto, and SC Johnson. Meeting materials and Industry comments are part of the docket for the ICR renewal at: EPA-HQ-OPP- 2016-0109.
7 As part of the 2007 methodology, the Agency identified three response phases: Phase 1: the initial response; Phase 2: data generation and Phase 3: data submission to EPA. The Phase 1, Phase 2 and Phase 3 response activity burden hours and costs are accounted for as subsets of the paperwork burden estimates for information collection activities that are related to generating data to respond to a DCI. These burdens are accounted for as part of the 35% of the test burden and cost.
8 See Appendix D.
9 Though rarely used, EPA may conduct a Special Review (40 CFR 154.7) if EPA believes that a pesticide poses risks of unreasonable adverse effects on human health or the environment. Section 3(c) (2) (B) of FIFRA provides a means of obtaining any needed data. However, for this ICR renewal no burden is calculated for this program since the EPA has not conducted a special review for over a decade.
13 7 USC 136w-8. For more information about PRIA, go to Pesticide Registration Improvement Extension Act of 2018 (PRIA 4) | US EPA
1416 USC 1531 et seq. For information about the ESPP, go to https://www.epa.gov/endangered-species.
16 For information about the EDSP, go to https://www.epa.gov/endocrine-disruption
Page
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| File Title | July 8, 2021 |
| Author | Katherine Sleasman |
| File Modified | 0000-00-00 |
| File Created | 2021-12-03 |
© 2026 OMB.report | Privacy Policy