SSB STEADI eval primary care _10_9_19
SSB STEADI eval primary care _10_9_19.docx
Evaluation of CDC’s STEADI Older Adult Fall Prevention Initiative in a Primary Care Setting
OMB: 0920-1281
SUPPORTING STATEMENT: PART B
OMB #
Evaluation of CDC’s STEADI Older Adult Fall Prevention in a Primary Care Setting
September 19, 2019
Point of Contact:
Gwen Bergen
Behavioral Scientist
Contact Information:
Centers for Disease Control and Prevention
National Center for Injury Prevention and Control
4770 Buford Highway NE MS F-64
Atlanta, GA 30341-3724
phone: 770.488.1394
fax: 770.488.1317
email: [email protected]
Contents
B. Collections of Information Employing Statistical Methods 3
B.1 Respondent Universe and Sampling Methods 3
B.2. Procedures for the Collection of Information 11
B.3 Methods to Maximize Response Rates and Deal with Nonresponse 12
B.4 Test of Procedures or Methods to be Undertaken 13
B.5 Individuals Consulted on Statistical Aspects and Individuals Collecting and Analyzing Data 13
B. Collections of Information Employing Statistical Methods
B.1. Respondent Universe and Sampling Methods
The respondent universe for this data collection will consist of two groups: (1) Patient Surveys - Patients 65 years old and over who attend one of the selected primary care health clinics and are randomized to one of four (three intervention and one control) groups, and (2) Process evaluation interviews - Clinical staff (physicians, nurses, and operations managers) who interact with the STEADI process at one of the selected primary care health clinics.
Patient Surveys
Information will be collected from older adults attending the selected clinics using a fall risk screener (Attachment D), baseline survey (Attachment B1), three follow-up patient surveys (Attachment B2). Older adults will also be asked to complete a falls tracking log (12 months total) to aid recall of falls for older adults to use while completing the surveys (Attachment B3). Older adults (≥ 65 years) who are at risk for a fall (defined as those that screen at high risk based on the STEADI Stay Independent Fall Risk Screener (Attachment D), are English-speaking, community dwelling, and ambulatory will be eligible for the study. Exclusion criteria include known diagnosis of dementia, complaining of fever or acute illness, or are with limited life expectancy receiving hospice care. All patients identified at high risk for a fall who agree to be contacted for the study will be given the consent form (Attachment C), a baseline survey (Attachment B1), three follow-up surveys (Attachment B2), and monthly falls tracking log (12 total) over one year (Attachments B3).
Two study clinics (A, B) within the same health system were chosen based on willingness to participate, a large proportion of older adult patients, and a stable practice environment over the study period. There will be three intervention arms in the study and one control group (Table B.1.1). Implementation of each of the three interventions arms will be rotated weekly between the two clinics. The medical assistant or office coordinator will screen older adults for their fall risk during both intervention and control weeks. The research nurse will conduct the appropriate fall risk assessments for the intervention arm. Depending on risk factors identified by the assessments, the research nurse will make recommendations to the physician for further assessment or intervention (Figures B.1.2-B.1.5) The physician will review the recommendations while examining the patient and will prescribe the appropriate intervention(s) for the patient.
The control group will consist of older adults from the two participating clinics who screened as high risk for a fall but who will receive the clinic’s standard of care for fall prevention instead of receiving one of the STEADI components.
The sampling method for the patient surveys is best understood in context of the timing of the patient’s first visit to a study clinic after the study starts. Randomization is based on the clinic visited and the timing of the visit (e.g. week visited of a six week cycle). The study uses a block-randomized design in which the clinical research nurse rotates through two clinic locations, switching clinics weekly. In this manner, each arm of the intervention will be implemented in each clinic, and each clinic will provide members of the control group. Randomization is achieved based on the week of the patient’s primary care appointment under the assumption that over the data collection period, week of appointment is not systematically related to falls risk.
Table B.1.1 Experimental Block-Randomization Design |
Clinic |
|
Time period |
Clinic A |
Clinic B |
Week 1 |
Research nurse in clinic conducting FULL assessments and clinical staff conducting intervention. (Intervention week) |
Control week |
Week 2 |
Control week |
Research nurse in clinic conducting PARTIAL (Medication Management) assessments and clinical staff conducting intervention. (Intervention week) |
Week 3 |
Research nurse in clinic conducting PARTIAL (Physical Therapy) assessments and clinical staff conducting intervention. (Intervention week) |
Control week |
Week 4 |
Control week |
Research nurse in clinic conducting FULL assessments and clinical staff conducting intervention. (Intervention week) |
Week 5 |
Research nurse in clinic conducting PARTIAL (Medication Management) assessments and clinical staff conducting intervention. (Intervention week) |
Control week |
Week 6 |
Control week |
Research nurse in clinic conducting PARTIAL (Physical Therapy) assessments and clinical staff conducting intervention. (Intervention week) |
Repeat 6 week cycle until recruitment objectives for all arms are met |
||
Figure B.1.2 Full STEADI Intervention

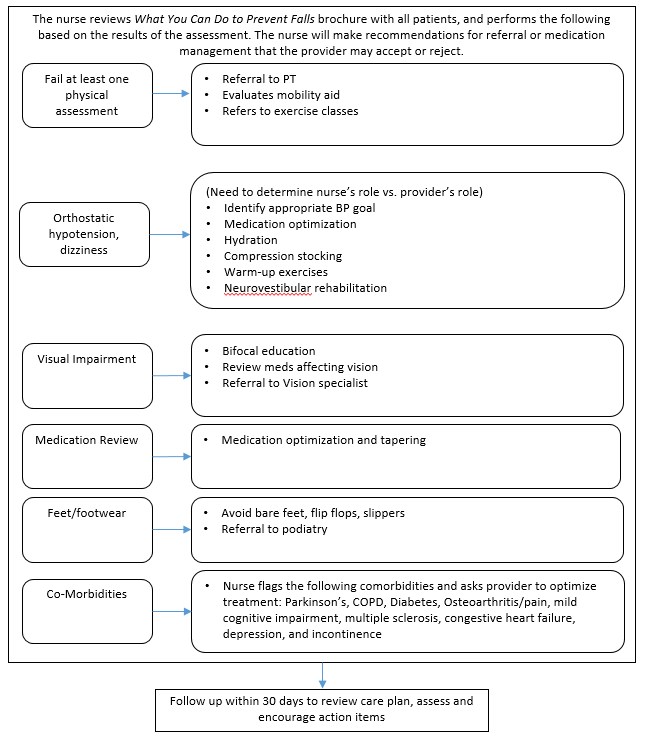
Figure B.1.3 Medication Management Component of STEADI
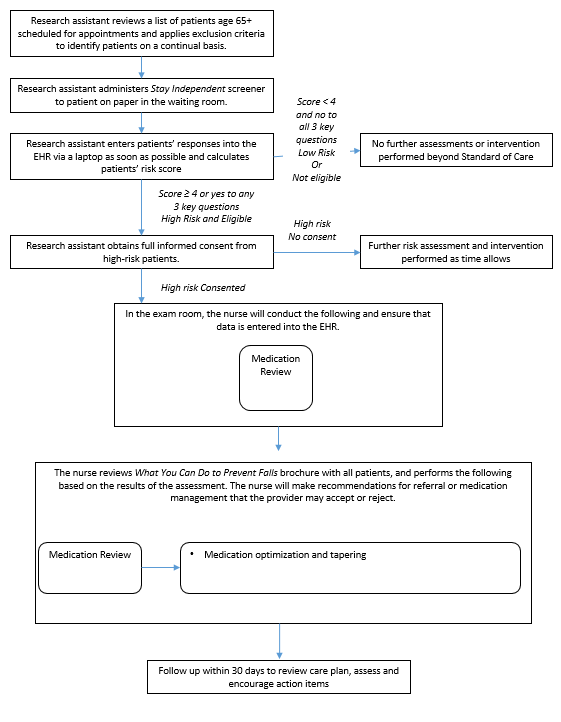
Figure B.1.4 Physical Therapy Component of STEADI
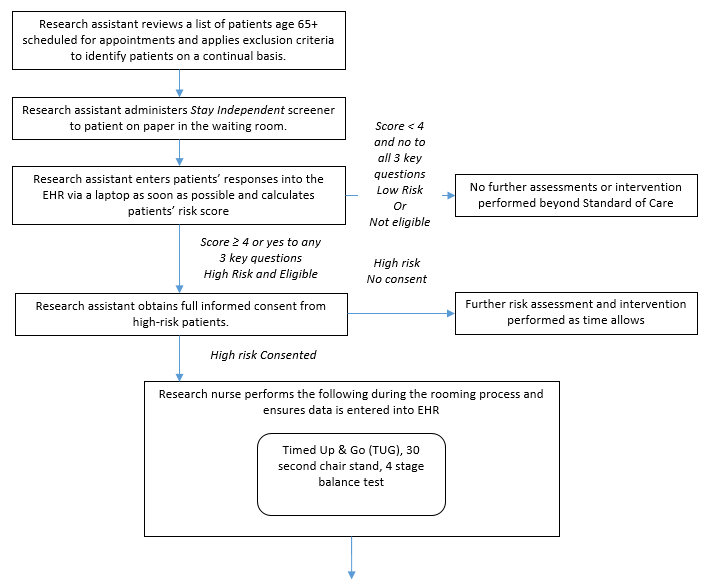

Figure B.1.5 Control Groups
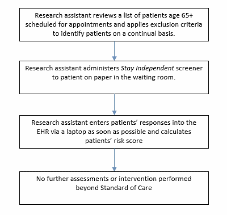
Patients who attend the clinic during the same week the nurse is present will be given the assigned clinic intervention and serve as part of the intervention group. Patients who attend the clinic the week, when the nurse is at the other clinic will be assigned to the control group and be recruited for the patient survey.
At the initial screening, patients will give verbal consent to be contacted for the study. If they agree to be contacted for the study and screen as high risk for a fall, as per the Stay Independent Fall Risk Screener (Attachment D), patients will be included in the study and assigned to either the intervention or control group of that clinic, depending on the week as described above. Participants will complete be asked to read and complete the study consent form (Attachment C) and the baseline survey shortly after their appointment (Attachment B.1) and will be asked to complete three follow-up surveys at 4, 8, and 12 months after the baseline survey (Attachment B2). They will also be asked to keep track of their falls during the 12-month period in the falls tracking log (Attachment B3).
This sampling approach results in block randomization in a way that should not bias survey results because patients will have no knowledge of the study when they are selecting the week they will schedule an appointment. An intervention capacity of 500 patients per arm (1,500 patients total) and 1,500 controls was selected as the amount of falls risk assessments the research nurse could conduct assuming on average, 4.8 assessments could be conducted per day. In order to reach 3,000 total fall risk assessments, 12,106 patients will need to complete the STEADI Falls Risk Screener (Figure B.1.6). From a previous study conducted in a similar population of older adults, an estimated 36% of the study population will screen as high risk for a fall (Eckstrom et al., 2017). The number of eligible participants will likely exceed the number of fall risk assessments the research nurse can conduct which will provide an adequate pool of eligible patients for recruitment. We will screen and recruit at each site until our sample requirements for all arms are met.
Figure B.1.6 Sample Flow Chart
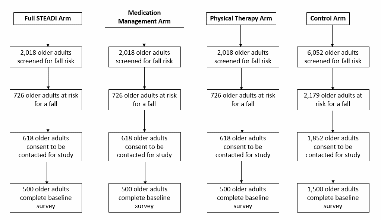
Process Evaluation Interviews
The study is also designed to assess feasibility and fidelity of the STEADI implementation. Information will be collected from STEADI implementation staff through in person or telephone interviews (Attachments E1and E2). Provider interviews will be conducted to determine the providers’ opinions about the feasibility of implementing STEADI into their clinical practice (Attachments E1 and E2). Providers will include physicians and physician assistants/nurse practitioners who interacted with STEADI and helped to implement STEADI in their clinics and clinic operations managers of the clinics. The information collected in these interviews will offer insight into the process of implementing STEADI into a large health system, the barriers and facilitators of implementing STEADI in the selected clinics, the perceived efficacy and patient engagement, and lessons learned that could provide useful information to other clinics implementing STEADI into their practice should it demonstrate a reduction in falls. Table B.1.5 summarizes the potential respondent universe, the targeted respondents, and the methods for selecting respondents for both patients and implementation staff.
Table B.1.5 Summary of STEADI data collection activities
Data Collection Method |
Respondent Universe |
Targeted Respondents |
Methods for Selection |
Stay Independent Fall Risk Screener (Attachment D) |
Older adults 65 and older who attend one of the selected clinics |
Older adults 65 and older who attend one of the selected clinics |
Patient over 65 who meet the inclusion criteria |
Patient Surveys (Baseline and Follow-up) Falls Tracking Log (Attachments B1-B3) |
Older adults 65 and older who attend one of the selected clinics |
All patients who screened at high risk for a fall and meet the inclusion criteria from EHR records: Based on the EHR, those diagnosed with dementia, non-ambulatory, are not complaining of fever or acute illness, and are not receiving hospice care are excluded |
Patients over 65 who screen at risk will be asked to complete the surveys. We estimate 89.6% of those recruited will answer at least 2 of the 3 follow-up surveys, We expect 3,000 individuals to screen at risk for a fall, agree to participate in the study, and meet the inclusion criteria, for an estimated total of 2,688 patients with enough data to consider complete. |
Provider Interview (Attachments E1-E2) |
All medical staff that interact with the STEADI process at one of the selected clinics |
Providers, clinic operations managers, (total of 16 staff) |
Convenience sample. The selected clinics will identify and recruit participants. |
B.2. Procedures for the Collection of Information
Patient Surveys. Eligible patients who meet the inclusion criteria (65 or older, community dwelling, excluding those with known diagnosis of dementia, on hospice care, and are being seen for an acute illness) will be identified using the clinics’ schedule and EHR records. Potential participants will be given the Stay Independent Fall Risk Screener at their scheduled appointment (Attachment D). Those who meet the criteria for fall risk and agree to be contacted for the study will be given a consent form (Attachment C) and the baseline survey shortly after their visit (Attachment B1). Follow-up surveys will be distributed by the patient’s choice of web, mail, or telephone calls (Attachment B2). Non-responders will be contacted by mail and telephone to ensure maximum response rate. The baseline and follow-up surveys will take an estimated 10-15 minutes to complete. An estimated 3,000 older adults will be asked to complete the baseline and three follow-up surveys. In addition to the patient surveys, all study participants will be asked to keep a falls tracking log (Attachment B3) they can reference while filling out the follow-up surveys. The web surveys and follow-up mail surveys will be self-administered. For those participants who do not complete the web or mail surveys, telephone surveys will be administered by the contractor. These surveys will be administered at 4, 8, and 12 months after the baseline survey.
Unique identifiers will be assigned to each case in the data files as data are collected and participants removed from contact lists when their interview participation is complete. Survey data will be stored by the contractor in secure servers. All respondents will be told during the consent process that the data they provide will be treated in a secure manner to the extent allowed by law. They also will be informed that participation is voluntary, that they may refuse to answer any question, and can stop at any time without risk. In addition, names of participants in any component of the study will not be provided to the federal government. Instead, a unique ID will be assigned to each participant.
Participants who complete at least two out of the three follow-up surveys will be included in the analysis. Assuming a response rate of 80% for each follow-up survey, 89.6% of participants will complete at least two of the three surveys (an estimated 51.2% will complete all three surveys and an additional 38.4% will complete any two of the three surveys). We will have adequate power (80%) to detect a minimum of an 8.7% difference in falls between each intervention arm and control group for the patient surveys assuming a sample of 500 intervention patients and 1,500 control patients (3,000 patients total) , a response rate of 89.6% (2,688 patients ) and a design effect of 1.1 (Table B.2.1). Based on these calculations, we conclude that the study is sufficiently powered to detect meaningful differences in the annual rate of falls observed in the intervention group as compared to the control group. As this is the most important outcome measured by the patient survey, we conclude that the study is sufficiently powered to meet its scientific objectives.
Table B.2.1. Estimated Minimum Detectable Difference (MDD), Risk Ratio (RR), and Odds Ratio (OR) for Each Arm of STEADI with 80% Power Given a Response Rate of 89.6%
Study Recruitment |
Study Completion |
Design Effect |
Effective Sample Size |
MDD* |
RR |
OR |
1,000 |
896 |
1.0 |
896 |
0.083 |
0.82 |
0.71 |
1,000 |
896 |
1.1 |
814 |
0.087 |
0.81 |
0.70 |
1,000 |
896 |
1.2 |
746 |
0.091 |
0.80 |
0.69 |
*Assuming a one-tailed test, an alpha of 0.05, a desired power of 80%, and a sample split evenly between interventions and controls. A one-tailed test is appropriate, as the intervention is only desirable if it results in a lower rate of falls.
No statistical methods will be used to draw a sample for the provider interviews. The selected clinics will identify the providers and clinic operations managers who are eligible to complete the interviews. We will conduct interviews with up to 16 total staff (10 providers and 6 clinic operations managers). Statistical sampling methods are not necessary, as the interviews will collect only qualitative information.
B.3 Methods to Maximize Response Rates and Deal with Nonresponse
To maximize response rates for the patient surveys, patients will be offered multiple options for completing the baseline survey and follow-up surveys. They will first be offered a self-administered web survey option. If they do not complete the web survey within two weeks, they will receive a mailed version of the patient survey they can complete and return to the contactor in a prepaid envelope. Patients who do not complete either the web survey or the mail survey within six weeks will receive a telephone call from a trained telephone interviewer to prompt them to complete the survey or to complete the survey over the telephone at that time. Patients’ preferences for completing the follow-up surveys will be recorded and used for additional follow-up surveys.
In addition, patients will receive an incentive with a value of $3 per completed survey, in the form of postage stamps. As there are four patient surveys, each patient will receive up to $12 in postage stamps for their participation in the study. The stamps offer a small token of appreciation for patients to complete the baseline survey, and each subsequent follow-up survey. Attrition from the baseline through the three follow-up surveys would be detrimental to the results of this study. Older adults who are offered incentives had a 22.5% increase in their response rate (McGonagle et al., 2017).
If the cumulative response rate for the baseline and three follow-up patient surveys is found to be less than 80%, the contractor will calculate and report unit and item non-response rates and carry out a non-response bias analysis following the guidelines in Standard 3.2 of the OMB Standards and Guidelines for Statistical Surveys (OMB 2006). Using electronic health record data the contractor will assess and measure non-response bias by evaluating the demographic characteristics of the baseline and final survey participants compared to those who screened at risk for falls, but did not complete the surveys. The sample composition of the baseline and follow-up survey participants will also be compared to evaluate whether any within-study attrition is contributing to non-response bias.
For the provider interviews, the selected clinics will identify and recruit the providers and operations manager staff to complete the process evaluation interviews. To account for any potential nonresponse, replacement staff will be recruited to complete the interviews.
B.4 Test of Procedures or Methods to be Undertaken
Approximately 7 to 9 participants aged 65 and older will be recruited from the Chicago area to pretest the patient surveys. This testing will help determine the length of time participants will spend answering the questions as well as clarity of questions. Based on the pretesting performance, questionnaires will be modified to reduce respondent burden and enhance usability. The data collection system will also be tested prior to the study to ensure correct skip patterns and data storage.
B.5 Individuals Consulted on Statistical Aspects and Individuals Collecting and Analyzing Data
CDC staff were consulted about subject matter and statistical methods for collecting and analyzing data. Gwen Bergen PhD, MPH, MS, Senior Behavioral Scientist, and Terri Head, Project Officer, National Center for Injury Prevention and Control (NCIPC), Division of Unintentional Injury Prevention (DUIP) are the technical contacts for this project in addition to Cora Peterson, PhD, NCIPC, Health Economist, Division of Analysis, Research and Practice Integration. Staff members from NORC were consulted about statistical methods and for implementation of STEADI. David Rein PhD, MPA, Laurie Imhof MPP, and Michelle Dougherty, MPH from NORC at the University of Chicago developed the implementation plan, evaluation plan, and questionnaires.
REFERENCES
Eckstrom E, Parker EM, Lambert GH, Winkler G, Dowler D, Casey CM. Implementing STEADI in Academic Primary Care to Address Older Adult Fall Risk. Innovation in aging. 2017 Sep;1(2):igx028.
McGonagle KA, Freedman VA. The Effects of a Delayed Incentive on response rates, Response Mode, Data Quality, and Sample Bias in a Nationally Representative Mixed Mode Study. Field Methods. 2017;29(3):221-237.
Office of Management and Budget. (2006). Office of Management and Budget Standards and Guidelines for Statistical Surveys. Available at: https://georgewbushwhitehouse.archives.gov/omb/inforeg/statpolicy/standards_stat_surveys.pdf
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | Moreland, Briana (CDC/ONDIEH/NCIPC) |
| File Modified | 0000-00-00 |
| File Created | 2021-08-17 |
© 2026 OMB.report | Privacy Policy