ITFPS-2 Age 9 Extension SSA Rev 1.4.2022
ITFPS-2 Age 9 Extension SSA Rev 1.4.2022.docx
WIC Infant and Toddler Feeding Practices Study-2 (WIC ITFPS-2)Year 9 Extension
OMB: 0584-0580
SUPPORTING STATEMENT PART A FOR
Revision to OMB Number 0584-0580
WIC Infant and Toddler Feeding Practices Study-2 (WIC ITFPS-2):
Year 9 Extension
Amanda Reat
Office of Policy Support
Food and Nutrition Service
US Department of Agriculture
1320 Braddock Place
Alexandria, VA 22314
Phone: 703-305-2539
E-mail: [email protected]
10-27-2021
Table of Contents
A Justification 1
A.1 Circumstances making the collection of information necessary 1
A.2 Purpose and Use of the Information 3
A.3 Use of Information Technology and Burden Reduction 9
A.4 Efforts to Identify Duplication and Use of Similar Information 10
A.5 Impacts Small Business or other Small Entities 10
A.6 Consequences of Collecting the Information Less Frequently 11
A.7 Special Circumstances relating to the Guidelines of 5 CFR 1320.5 12
A.8 Responses to the Federal Register Notice and Efforts to Contact Outside Agencies 13
A.9 Explanation of Any Payment or Gift to Respondents 14
A.10 Assurance of Confidentiality Provided to Respondents 18
A.11 Justification for Sensitive Questions 19
A.12 Estimates of Respondent Burden Including Annualized Hourly Cost 20
A.13 Estimates of Other Total Annualized Cost Burden 24
A.14 Annualized Cost to the Federal Government 24
A.15 Explanation for Program Changes or Adjustments 25
A.16 Plans for Tabulation and Publication and Project Time Schedule 26
A.17 Reason Display of OMB Expiration Date is Inappropriate 28
A.18 Exceptions to Certification for Paperwork Reduction Act Submissions 28
Appendices
A1 Year 9 Study Consent Form – English
A2 Year 9 Study Consent Form – Spanish
B1 Study extension letter – English
B2 Study extension letter – Spanish
Contents (continued)
Appendices (continued)
C1. Contact Information Form – English
C2. Contact Information Form – Spanish
D1. Contact Information Form Reminder – English
D2. Contact Information Form Reminder - Spanish
E1 Year 9 Interview advance letter – English
E2 Year 9 Interview advance letter – Spanish
F1 Year 9 interview telephone survey - English
F2 Year 9 interview telephone survey - Spanish
F3 Year 9 replicate dietary intake interview - English
F4 Year 9 replicate dietary intake interview - Spanish
G1a Reminders for Year 9 interview from Study Liaison - English
G1b Reminders for Year 9 interview from Study Liaison - Spanish
G2a Reminders for Year 9 non-locatable active interview – English
G2b Reminders for Year 9 non-locatable active interview – Spanish
G3a Reminders for Year 9 study participation refusal conversion – English
G3b Reminders for Year 9 study participation refusal conversion – Spanish
G4a Reminders for Year 9 study participant not answering calls – English
G4b Reminders for Year 9 study participant not answering calls – Spanish
G5a Reminders for Year 9 Interview Telephone Research Center voicemail, first message – English
G5b Reminders for Year 9 Interview Telephone Research Center voicemail, first message – Spanish
G6a Reminders for Year 9 Telephone Research Center voicemail, expiring interviews – English
Contents (continued)
Appendices (continued)
G6b Reminders for Year 9 Telephone Research Center voicemail, expiring interviews – Spanish
H1 Year 9 H/W measurement card – English
H2 Year 9 H/W measurement card – Spanish
I1 Height and weight reminders – English
I2 Height and weight reminders – Spanish
J1 Year 9 text or email provider measures – English
J2 Year 9 text or email provider measures – Spanish
K1 Year 9 thank you – English
K2 Year 9 thank you – Spanish
L1 Birthday card respondent year 9 – English
L2 Birthday card respondent year 9 – Spanish
M1 Birthday card child age 9 – English
M2 Birthday card child age 9 – Spanish
N1 Announcement of study extension to WIC State agencies
N2 Announcement of study extension to WIC sites
O Study extension webinar
P Agenda for conference call on extension with State Agency and sites
Q1 Study extension summary and agreement – Correspondence to State Agency with copy to site POC
Q2 Addendum A to study extension summary
Q3 Addendum B to study extension summary
R Participant contact information request
Contents (continued)
Appendices (continued)
S Administrative data on Lost to Followup Participants
T Crosswalk of previously approved and revised materials
U1a Federal Register Notice comment #1
U1b Federal Register Notice comment #2
U1c Federal Register Notice comment #3
U2a Federal Register Notice agency response to comment #1
U2b Federal Register Notice agency response to comment #2
U2c Federal Register Notice agency response to comment #3
V1 NASS review
V2 NASS review response
W Confidentiality and nondisclosure agreement
X Westat IRB approval letter
Y Burden table
Z Base study sampling methods
AA Details of imputation, calculation of the survey weights, and nonresponse bias analysis
Tables
A2.1 Overview of Data Collection Activities 4
A8.1 Consultants from Outside the Agency 14
A9.1 Participant Incentives by Event 17
A9.2 Grant Amounts for WIC Sites 18
A16.1 Data Collection and Reporting Schedule 23
A16.2 Research Questions and Principal Data Sources for Year 9 Report 24
Part A
Justification
A.1 Circumstances making the collection of information necessary
Explain the circumstances that make the collection of information necessary. Identify any legal or administrative requirements that necessitate the collection. Reference the appropriate section of each statute and regulation mandating or authorizing the collection of information.
This is a revision of a currently approved study, the Supplemental Nutrition Program for Women, Infants, and Children (WIC) Infant and Toddler Feeding Practices Study-2 (WIC ITFPS-2), ICR Reference Nos. 201208-0584-002, 201306-0584-008, 201404-0584-005, 201408-0584-007, 201601-0584-008, and 201810-0584-001; expiration date: 03/31/2022. The revision is informally named the “Year 9 Extension” and referred to herein as the Y9 Extension. This revision seeks to encompass two (2) activities related to the on-going WIC ITFPS-2. The first activity, the Year 9 Follow-up Study or Y9FU Study, will extend the data collection timeframe of WIC ITFPS-2 up to 9 years of age for study children. The second activity, the Lost to Follow-up Study or L2FU Study, involves examining WIC administrative data to gather programmatic administrative data on former WIC ITFPS-2 participants.
The Base Study, Age 3 Extension, Age 5 Extension, Age 6 Extension and, now, the Y9 Extension of the WIC ITFPS-2 (OMB No. 0584-0580, expiration date: 03/31/2022) affirm the U.S. Department of Agriculture (USDA) Food and Nutrition Service’s (FNS’) mission to increase food security and reduce hunger by providing children and low-income people access to food, a healthful diet, and nutrition education in a way that supports American agriculture and inspires public confidence.1 The Healthy, Hunger-Free Kids Act of 2010 (Public Law 111-296, Sec. 305) mandates programs under its authorization, including WIC, to cooperate with USDA program research and evaluation activities. The Y9FU Study will follow up with children four years after the end of their WIC age eligibility, providing data to answer research questions relevant to WIC program and policy as well as the nutrition and wellbeing of children up to the month of their ninth birthdays.
The L2FU Study will allow FNS to explore if participants who left WIC ITFPS-2 in the early years of the study were systematically different than that those who continued WIC ITFPS-2, and it will allow FNS to determine if these study participants left WIC at the same rate as their counterparts who remained in WIC ITFPS-2. This is an important question because WIC retention rates have fallen over the last few rates, and FNS needs to understand characteristics of those who leave WIC even though they may still be eligible for services.
WIC serves a highly-vulnerable population: low-income pregnant and post-partum women, infants, and children through their fifth birthday who are at nutritional risk. The program provides supplemental food packages, health referrals, and nutrition education for participants. The goal of WIC ITFPS-2, which includes the Base Study, the Age 3 Extension, the Age 5 Extension and the Age 6 Extension (ICR Reference No. 201208-0584-002 , 201306-0584-008, 201408-0584-007; 201601-0584-008; and 201810-0584-001; expiration date: 03/31/2022) is to examine feeding practices and associated nutrition outcomes of study children from birth to 6 years of age. The Y9 Extension extends the goal until study children are 9 years of age.
A.2 Purpose and Use of the Information
Indicate how, by whom, how frequently, and for what purpose the information is to be used. Except for a new collection, indicate the actual use the agency has made of the information received from the current collection.
Purpose of Information Collection. Information collected by WIC ITFPS-2 through study child age 5 will provide USDA FNS with data on the factors that influence feeding practices and the nutrition and health outcomes of children in the first five years of their lives, during the time that the study child is age-eligible for WIC. The Age 6 Extension study expands the data collection to their sixth year of life, the first year in which study children can no longer receive WIC services. The Y9FU Study of the Y9 Extension will involve: (1) conducting one additional follow-up telephone interview with the mother/caregiver around the time the child is 9 years old; (2) conducting a second, replicate dietary intake interview with a 10 percent subsample of caregivers who complete the first interview, and (3) obtaining height and weight (H/W) measurements around age 9 years on each child from caregiver provision to the study of measurements taken by health-care professionals or from direct measurements taken at WIC sites. The L2FU Study will involve obtaining WIC administrative data on the WIC participation patterns of participants who left WIC ITFPS-2 in the first five years of the study during which the study child would have been age-eligible for WIC.
The Y9FU Study telephone interview will include questions to address four research domains: the dietary behaviors and health outcomes of study children around age 9, four years after they age out of WIC; the food security status of the children’s households around age 9 years, considering the role of other food assistance programs from which they benefit; the feeding practices of caregivers for their children during the child’s ninth year of life; and, the food and health-related environmental characteristics for children who formerly participated in WIC.
From whom the information will be collected. This collection is completely voluntary for caregivers of former WIC participants who enrolled in WIC ITFPS-2, and State agencies and local agencies administering the WIC program. Although the Healthy, Hunger-Free Kids Act of 2010 requires cooperation by State and local WIC agencies in USDA studies, we have previously and will continue to allow States and sites to choose to reaffirm their cooperation.
Table A2.1. Overview of Data Collection Activities
Instrument |
Mode |
Purpose |
Number of Respondents |
Frequency |
Caregivers of former WIC Children |
||||
Contact Information Form (Appendix C1/C2) |
Hardcopy/electronic |
Obtain current contact information of study participants |
1,067 |
Up to 4 timesa |
Year 9 Interview Telephone Survey (Appendix F1/F2) |
Telephone |
Collect data on feeding practices, family circumstances, and nutrition and health outcomes |
1,068 |
Once |
Year 9 (Y9) Replicate Dietary Intake Interview (Appendix F3/F4) |
Telephone |
Collect replicate data on dietary intake |
107 |
Once |
Y9 Height/Weight (H/W) Measurements at WIC Site or Provider’s Office (Appendix H1/H2) |
In Person |
Collect data on child height/weight |
748 |
Once |
WIC Site Staff |
||||
Y9 H/W Measurements at WIC Site (Appendix H1/H2) |
In Person |
Collect data on child height/weight |
80 |
4 times |
Health care professional |
||||
Y9 H/W Measurements at Healthcare professional’s workplace (Appendix H1/H2) |
In Person |
Collect data on child height/weight |
428 |
Once |
WIC Agencies |
||||
Administrative Data on Lost to Follow-up (L2FU) Study Participants |
Electronic |
Collect administrative data |
27 |
Once |
a Respondents are asked to complete Contact Information Forms up to the time of the 9 Year Interview. As the study children’s birthdays span 15 months, the number of times a respondent is asked to complete a Contact Information Form varies.
How the information will be collected. The current cohort, recruited for the Base Study, was a national probability sample of WIC participants (see Appendix Z for more details). The target study participant is the child, but all materials are sent to the participating caregiver. We notify the caregivers in the existing cohort of the extension of the study to child age 9, and will collect data on the participants around the study child’s ninth birthday using two methods, telephone interview and direct measurement.
Notification to Participating Cohort of Study Extension: The study team will mail the study extension consent (Appendix A1/A2), and the study extension letter (Appendix B1/B2), within two weeks after OMB approval. All participants will be asked to sign and return one copy of the consent. Any caregivers who do not return the hard copy consent form will have another opportunity to consent verbally at the time of the Y9 telephone interview. In total, an estimated 3,020 caregivers from the original cohort of live births will receive a study extension letter and study extension consent.
Contact Information Form: The respondents will be asked to complete Contact Information Form (CIF) (Appendix C1/C2) about once every four months up to the Y9 telephone interview. They will receive periodic CIF reminders as well (Appendix D1/D2). The study team estimates 2,668 respondents will be asked to complete a CIF up to four times and 1,067 will respond.
Interviews: As mentioned, study participants will be asked to complete a telephone interview when their child is 9 years old. Interview windows extend from two weeks prior to the child’s ninth birthday to four weeks after. One to two weeks before the interview window opens, participants will receive an advance letter notifying them of the upcoming interview (Appendix E1/E2). The study team expects 2,668 participants from the existing cohort will receive the advance letter. The Y9 interview (Appendix F1/F2) will be administered by telephone, conducted using a Computer-Assisted Telephone Interview (CATI). The study team will attempt to reach participants through outgoing calls, and participants will also have the option of calling in at their own convenience to complete the interview. Participants whose interviews are not completed within 14 days of the opening of the window will receive reminders while the interview window is open and the interview is not complete (Appendices G1a/G1b, G2a/G2b, G3a/G3b, G4a/G4b, G5a/G5b, G6a/G6b). The study team expects that 1,068 study participants will complete the Y9 telephone interview.
A subsample (160 participants) of participants eligible for the Y9 telephone interview will be randomly selected to complete the Y9 Replicate Dietary Intake Interview (Appendix F3/F4) within 2 to 10 days of completing the Y9 telephone interview. Participants will be notified of their selection for the replicate interview at the end of the Y9 telephone interview, and the interviewer will schedule a time to call the participant for the replicate interview, also administered using CATI. The study team expects 10 percent (n=107) of participants completing the Y9 telephone interview will complete a replicate dietary intake interview.
Height and Weight (H/W) Measurements: The study will collect data on children’s H/W measurements around 9 years of age to calculate body mass index (BMI) in order to assess the impact of feeding practices on weight status. The study team will send the study participants information on the measurements in the Y9 advance letter (Appendix E1/E2) along with a self-addressed postage-paid measurement card (Appendix H1/H2) and reminders to complete the measurement cards (Appendix I1/I2). The letter will ask caregivers to take the card to a WIC site and have the WIC site measure the study child, record the measurements, and send the sealed card back to the study team. For those caregivers who are unable to visit a WIC clinic, we will ask the study caregivers to go to the child’s health-care professional to be measured. For study participants who do not wish to visit either WIC or their child’s health-care professional (or who went to their professional’s office but neglected to bring their measurement card) but who have a printout of a recent measurement by the health-care professional, the study will accept a copy of that printout in lieu of the measurement card (Appendix J1/J2). Based on experience with the mode of return of the 72-month measurement cards and acknowledging that respondents may be less likely to return to WIC as their WIC experience becomes more distant, the study team expects a maximum of 320 (less than 50% of those who are expected to return the measurements) study participants to return measurement cards completed by WIC, and 428 study participants to return a measurement card completed at a health-care professional’s office or send a printout of measurements taken recently at the professional’s office.
WIC Administrative Data: Following OMB approval, the study will inform the 27 participating WIC State agencies of the study extension and request their continued participation. We will hold a Webinar (Appendix O) with the state agencies and sites to orient them to the Y9 Extension. We will schedule individual calls with the WIC agencies to discuss their participation in the Y9FU and L2FU Studies. We will provide the WIC agencies with a summary of what study participation entails (Appendix Q1, Q2, and Q3). For the L2FU Study, the study team will securely transmit a list with identifying information (e.g., name, and date of birth) of the participants who left the study in the first five years to the WIC agencies, and request that they return the list with the study participants’ WIC participation status up to the child’s fifth birthday (Appendix S).
In addition to the data collection activities, the study team will communicate with study participants to express appreciation for their engagement with the study. Study participants will receive a thank you note after the completion of the Y9 telephone interview (Appendix K1/K2). Continuing a practice already in place on the study, the caregivers will receive birthday cards from the study on their own birthdays (Appendix L1/L2), and the children will receive birthday cards from the study on their ninth birthdays (Appendix M1/M2).
The study team will send an announcement of the Y9 Extension to the 27 WIC State Agencies and 80 WIC site points-of-contact who have been collaborating on the study to-date (Appendix N1 and Appendix N2). The study team will present a webinar for the points-of-contact (Appendix O), and follow up with conference calls to answer any questions (Appendix P). Following the webinar and conference calls, the study team will send a study extension summary and agreement and addenda (Appendices Q1,Q2 and Q3) describing study activities and roles and responsibilities requested from WIC, including providing updated contact information for participants with children still enrolled in WIC (Appendix R).
A crosswalk indicating which of the materials for the Y9 Extension are revisions of previously approved materials is presented in Appendix T.
Purpose of the Information: The information through age 9 will be a valuable asset to policymakers, WIC Program Staff, health professionals, and the research community. The Y9FU Study will complement other large nutrition-related studies and will provide additional insights given the study’s longitudinal design, including detailed infant and young child feeding information, and participant sample. The study continues the FNS follow-up for the first time of a sample of WIC recipients followed longitudinally at four years beyond the period of WIC age eligibility, including those who leave WIC prior to becoming age-ineligible. Policymakers and WIC Program Staff will use the findings to design and shape the program to ensure participants’ long-term health and nutrition needs are being met. Health professionals will be able to use the information to shape their interactions with this highly-vulnerable population and to better understand their needs in the years following the cessation of services. Researchers will be able to further analyze the study data and further contribute to the knowledge base regarding this high-risk, vulnerable population. Reports on Base Study results were released to the public by FNS: prenatal findings (2015), infant year findings (2016) and second year findings (2018), third year findings (2019) and fourth year findings (2020) . The results have been presented at conferences to scientific audiences and stakeholder organizations interested in WIC, breastfeeding, nutrition, and maternal and child health, and have formed the basis for journal articles submitted to peer reviewed journals. The findings are available to the public on the FNS website both as full reports and 2-page summaries.
The L2FU Study will help FNS understand if those who left the longitudinal study are fundamentally different from those who remain in the study and the extent of this new data’s impact on the study cohort’s WIC participation rates.
Information shared with any other organizations inside or outside USDA or the government. Results will be presented in aggregated form in the study reports, which will not seek to make generalizations beyond the study sample. We will prepare de-identified public use quantitative data files that are associated with the final report. FNS will publicly share the resulting reports and data files on its website: http://www.fns.usda.gov/ops/research-and-analysis.
A.3 Use of Information Technology and Burden Reduction
Describe whether, and to what extent, the collection of information involves the use of automated, electronic, mechanical, or other technological collection techniques or other forms of information technology, e.g., permitting electronic submission of responses, and the basis for the decision for adopting this means of collection. Also, describe any consideration of using information technology to reduce burden.
FNS is committed to complying with the E-Government Act of 2002 to promote the use of technology. The WIC participant interviews are designed to reduce participant burden through the use of Information Technology. Specifically, for the telephone interviews, WIC participants will speak with an interviewer on the phone and will not have to write down or enter any information other than notes to help with recalling information. We anticipate approximately 1,068 respondents across the Y9 telephone interview, and the replicate dietary interview with a subset of participants, will submit responses that are directly entered into an electronic database by the telephone interviewers through the Y9 instrument. Thus considering respondents and non-respondents, 37 percent of the interview responses will be collected electronically. The participants also have the option to submit the CIF electronically through the study website and based on the previous trends, FNS estimates that about 75 percent of the CIF responses (3,201 over four rounds) will be submitted electronically. With regards to the data collected for the L2FU Study, the 27 State agencies will also submit their responses to the administrative data request electronically. All other responses will be non-electronic. Measurements taken at WIC or health-care professionals’ offices will be returned through sealable self-addressed postage-paid cards. These approaches are consistent with the data transfer protocols used in the Age 6 Extension study for study participants no longer receiving WIC services. Out of a total of 35,293 responses for this study, FNS estimates that 4,941 responses (13%) will be submitted electronically.
A.4 Efforts to Identify Duplication and Use of Similar Information
Describe efforts to identify duplication. Show specifically why any similar information already available cannot be used or modified for use for the purpose described in item 2 above.
Every effort has been made to avoid duplication. Through careful review of the data requirements, we have determined that no current data are similar to that proposed for collection in this study. Although we are asking the same or similar questions to those posed in the base study and/or the extensions through age 6, the aim is to obtain current data to continue to track issues in children’s nutrition, growth, and environments as they unfold over the years after WIC age-eligibility ends, and to examine new issues that may arise during the ninth year.
A.5 Impacts Small Business or other Small Entities
If the collection of information impacts small businesses or other small entities (Item 5 of OMB Form 83-I), describe any methods used to minimize burden.
Information being requested or required has been held to the minimum required for the intended use. The data collection plan has no impact on small businesses or other small entities. Out of the total 3,203 respondents for this collection, FNS estimates that approximately 40 percent of the 428 health-care professionals conducting measurements are small entities.
A.6 Consequences of Collecting the Information Less Frequently
Describe the consequence to Federal program or policy activities if the collection is not conducted or is conducted less frequently, as well as any technical or legal obstacles to reducing burden.
If the study is not conducted at this time, FNS will not have information on the enduring effects of prior WIC participation four years after participants’ are age-eligible for WIC. The early years of WIC ITFPS-2 provide recent data on dietary intake and feeding practices and how WIC services influence these, but there is no known longitudinal data facilitating the examination of the influence of prior WIC participation on children’s dietary intakes and nutritional outcomes years after their WIC eligibility ends. These data are vital to information for WIC policy, services, and nutrition education.
With over 45 percent of the nation’s infants enrolled in WIC and increasing rates of obesity in young children, it is critical to understand the enduring effects of WIC services and nutrition education on nutritional intakes and feeding patterns of former WIC participants. This extension provides continuation of the opportunity for FNS to understand nutrition and feeding patterns of former WIC children during the years when those children are no longer eligible for WIC services – national data on this population has never before been available through age 9. We are proposing collecting data at a the same frequency (once during the extension year) as compared to that approved for the Age 6 Extension. We anticipate important changes as the children’s diets, health, and environments diversify, and a one-year follow-up of dietary intake, household circumstances, health, and feeding behavior should be sufficient for longitudinal follow-up. Anything less than annual data may miss important turning points as the children transition fully into the elementary school environment. The information is essential for policymakers and WIC program staff making decisions about program operations. They will use the information to develop appropriate and effective prevention strategies aimed at improving the health of young children.
A.7 Special Circumstances relating to the Guidelines of 5 CFR 1320.5
Explain any special circumstances that would cause an information collection to be conducted in a manner:
Requiring respondents to report information to the agency more often than quarterly;
Requiring respondents to prepare a written response to a collection of information in fewer than 30 days after receipt of it;
Requiring respondents to submit more than an original and two copies of any document;
Requiring respondents to retain records, other than health, medical, government contract, grant-in-aid, or tax records for more than three years;
In connection with a statistical surveys, that is not designed to produce valid and reliable results that can be generalized to the universe of study;
Requiring the use of a statistical data classification that has not been reviewed and approved by OMB;
That includes a pledge of confidentiality that is not supported by authority established in statute or regulation, that is not supported by disclosure and data security policies that are consistent with the pledge, or which unnecessarily impedes sharing of data with other agencies for compatible confidential use; or
Requiring respondents to submit proprietary trade secret, or other confidential information unless the agency can demonstrate that it has instituted procedures to protect the information’s confidentiality to the extent permitted by law.
There are no special circumstances relating to the Guidelines of 5 CFR 1320.5. The collection of information is conducted in a manner consistent with the guidelines in 5 CFR 1320.5.
A.8 Responses to the Federal Register Notice and Efforts to Contact Outside Agencies
If applicable, identify the date and page number of publication in the Federal Register of the agency’s notice, soliciting comments on the information collection prior to submission to OMB. Summarize public comments received in response to that notice and describe actions taken by the agency in response to these comments.
Describe efforts to consult with persons outside the agency to obtain their views on the availability of data, frequency of collection, the clarity of instructions and recordkeeping, disclosure, or reporting form, and on the data elements to be recorded, disclosed, or reported.
In accordance with 5 CFR 1320.8(d), FNS published a notice on May 5, 2021 in the Federal Register Volume 86, Number 85, Pages 23914-23916. The public comment period ended on July 6, 2021. Three comments were received in response to the notice. Public comments in response to the 60-day Federal Register Notice (FRN) (Appendices U1a, U1b, and U1c), and the FNS response to the comments (Appendices U2a, U2b, and U2c).
In general, the three commenters showed support for the Y9FU and emphasized the need for longitudinal data among the WIC population to ensure programmatic efficacy. One commenter highlighted the need to address internal and external validity given that three years will have passed since the last follow up (at year 6) with the study cohort. Responses from FNS shared the steps we have taken in the design phase of the study and the steps we will take in conducting analysis to ensure validity.
The information collection request has been reviewed by David Hancock and a team of staff at the Sampling and Frame Development Section at the National Agricultural Statistics Service (NASS) of USDA with special reference to clarifying the sampling strata design and the recruitment window for the study as well as asking for further descriptions about the study timeline and data collection components (Appendix V1). The response from FNS clarified the sampling and recruitment questions and provided further details for the study timeline and data collection; FNS also further explained sample size calculations (Appendix V2). Expert guidance was sought from researchers with expertise in child nutrition, development, and school performance for design and research questions intended to maximize the successful conduct of the Y9FU Study. The external researchers supported the design and scope of the study and provided suggestions for survey questions regarding food security, child physical activity, and child development. Their suggestions were incorporated into the final participant survey for this study.
Table A8.1. Consultants from outside the agency
Name |
Affiliation |
Area of Expertise |
Deanna M. Hoelscher, PhD, RDN, LD, CNS, FISBNPA |
Regional Dean, UTHealth School of Public Health at Austin 512.391.2510 |
Child nutrition, child development, WIC |
Angela Odoms-Young, PhD |
Associate Professor, University of Illinois at Chicago 312-413-0797 |
Child nutrition, child development, WIC |
Jeanne Brooks-Gunn, PhD |
Co-Director, National Center for Children and Families, Teachers College, Columbia University |
Child development |
Peter Quan Alison Black Beth Schlein Linette Lanclos Bayazid Sarkar Duan Franklin |
National Agricultural Statistics Service |
Statistical procedures |
A.9 Explanation of Any Payment or Gift to Respondents
Explain any decision to provide any payment or gift to respondents, other than remuneration of contractors or grantees.
This study’s complex longitudinal design requires participation of the same individuals over time to produce high quality estimates of longitudinal patterns of behavior. Respondents will be asked to engage in multiple data collection events during the year, during specified windows of time, and during a period in their lives when they face competing demands from work and as parents to young children. These respondents are exerting unusual effort, and therefore, the potential for response bias among subsets of participants must be avoided proactively to ensure high quality data. Preventing response bias in a highly mobile sample of low-income caregivers of young children can be particularly challenging, and incentives ensure that participants feel their burden is recognized, acknowledged, and appreciated. As approved under the previous OMB package, in the Base Study, WIC participant respondents were provided with incentives of $20 on their prepaid gift card for each interview from the prenatal through the 24-month interview, and an additional $10 when using their own cell phones to offset the cost of use of personal cell phone minutes. During the 30-36 month period, respondents received an incrementally increasing incentive of $30 on their prepaid gift card for the 30-month interview and $40 on their prepaid gift card for the 36 month interview, and were again provided with an additional $10 on their prepaid gift card per interview to offset the cost of use of their personal cell phone minutes. During the Age 5 Extension, respondents received on their prepaid gift card $45 at 42 months, $50 at 48 months, $55 at 54 months, and $60 at 60 months and 72 months, and were, at each time point, provided with an additional $10 to offset the cost of use of their personal cell phone minutes. We remain concerned that the respondents will become fatigued with the burden involved in a longitudinal study, particularly those who are the most mobile and difficult to locate. Slightly increasing the size of the incentive for the final Y9 telephone interview should help keep respondents engaged and feel that their effort is appreciated, and therefore minimize response bias in the study over time. Study participants will receive a $70 gift card at child age 9 years, and will be provided with an additional $10 on that gift card to offset the cost of use of their personal cell phone minutes.2 Participants selected to complete a replicate dietary intake interview within 10 days of the first interview will receive an additional $70 gift card for the second interview, with an additional $10 on that gift card to offset the use of cellphone minutes as applicable. Child H/W measurements are critical outcome measures for the study. Consequently, study participants who are willing to bring their child to the WIC site or a health-care professional’s office for measurements to have a measurement card completed, an activity requiring the burden of both time and travel with the child, will receive an $80 gift card ($70 as an incentive and $10 as a transportation stipend3) at child age 9, a $10 increase of the incentive they received for these activities at 72 months because at age 9 all children are expected to be in school during the week and it will require additional effort to schedule the visits.. Finally, women who do not have a telephone to use to complete the telephone interviews will be given a prepaid cellphone with 200 minutes valued at $65.00 to complete each interview. We will ask participants to return the phone at the completion of the Y9 Extension. Table A9.1 shows the events that involve participant incentives and the associated incentive amounts.
Our longitudinal incentive plan is comparable to that of other longitudinal studies with similar populations or similar data collection requirements, which have provided incremental increases in incentive amounts through the course of the study, though not necessarily at every data collection event. The Study of Mothers and Children in Palm Beach County, a non-federal study sponsored by local government in Palm Beach County, FL, was a 5-year longitudinal survey (2005-2009) of low-income mothers of newborns in Palm Beach County, with yearly in-person interviews. The mothers received $25 on their prepaid gift cards as ab incentive in years 1-3. Response rates for eligible respondents were 94 percent for the baseline interview, 91 percent for Year 2, and 85 percent for Year 3. To help reduce the possibility of response bias, particularly through loss to follow-up of the most mobile participants, the incentive was increased to $35 on their prepaid gift cards for Years 4 and 5. A response rate of 82 percent was achieved for Year 4 and 85 percent for Year 5, counteracting the potential response bias.
The CDC National Health and Nutrition Evaluation Survey (NHANES) is an example of a study that involves parents accompanying children to have physical measurements completed on the children. In the 2013-2014 data collection (OMB no. 0920-0950 National Health and Nutrition Examination Survey, expiration date 11/30/2015), NHANES provided young children and their parents a total incentive of $60 for bringing children to be measured, and an additional $25 - $70 for transportation costs depending on the distance traveled .
The current study is also a longitudinal study, and retaining the participants for an additional year without introducing response bias is essential to the success of the study. The study has offered incrementally larger incentives for each interview between 30 months and 60 months. Were the incentive to be reduced below the level offered at 72 months for the Y9 telephone interview, some groups of participants, particularly those with lower incomes or greater additional demands on their time, may choose not to participate, greatly increasing the risk of response bias. Based on the evidence of similar uses above, FNS feels strongly that the proposed incentives for the WIC participants are necessary to obtain a sufficient number of completed observations and interviews from a diverse group of respondents.
Table A9.1. Participant Incentives by Event
Event |
Average Hours per Response |
Incentive Amount |
Incentives for Caregivers |
||
Year 9 (Y9) Telephone Interview |
1 |
$70 |
Personal cellphone minute costs for 9 Year interview |
N/A |
$10 |
Y9 Replicate Dietary Intake Interview (within 10 days) |
.50 |
$70 |
Personal cellphone minute costs for 9 Year replicate dietary intake interview |
N/A |
$10 |
Y9 Child Measurements at WIC Site or Provider’s Office |
1.00 |
$70 |
Transportation costs for travel to WIC site or provider’s office for measurements |
N/A |
$10 |
Grants for WIC sites |
||
Grants to WIC sites to offset costs of measurements at child age 9 years |
0.1667 |
$527 on average per site |
Grants for WIC Agencies |
||
Grants to WIC State Agencies in appreciation of administrative data |
54.7593 |
$1,000.00 |
In addition to the incentives offered to caregivers, we will again offer optional small grants to help WIC sites offset administrative costs associated with weighing and measuring children no longer on WIC at age 9. Such measurements, while not overly burdensome, are outside the regular duties of staff at the WIC sites. Grants will vary in size depending on the number of study participants enrolled at that site when the study participants were still eligible to participate in WIC, and therefore the anticipated administrative burden needing to be offset to weigh and measure the study children. Grant amounts appear in Table A9.2. Sites were grouped by enrollment to approximate number of children projected to need measurements at the site. Based on these estimates, we anticipate the grants will be between $250-$2,000, with an average of approximately $527 per site, the same as that offered for measurements at study child age 6.
Table A9.2. Grant Amounts for WIC Sites
Number of children enrolled |
Grant Amount |
7-28 |
$250 |
29-38 |
$350 |
39-48 |
$450 |
49-60 |
$550 |
61-79 |
$700 |
80-99 |
$800 |
100-200 |
$1,200 |
Over 200 |
$2,000 |
In addition, the State agencies will be offered a monetary grant of $1,000 in appreciation of their effort to provide administrative data on L2FUStudy participants.
A.10 Assurance of Confidentiality Provided to Respondents
Describe any assurance of confidentiality provided to respondents and the basis for the assurance in statute, regulation, or agency policy.
Study participants will be subject to assurances as provided by the Privacy Act of 1974 (5 USC §552a), which requires the safeguarding of individuals against invasion of privacy; these assurances will have been documented in an informed consent form, along with procedures for handling and secure storage of information provided by participants (Appendices A1 and A2). For the Y9 telephone interview (Appendices F1/F2), the replicate dietary intake interview (Appendices F3/F4), and the measurement cards (Appendices H1/H2), personally identifiable information is collected, including names and contact information. For each form, a privacy act statement is included. In addition, all project staff and subcontractors have signed a confidentiality and nondisclosure agreement (Appendix W). We will ensure the privacy and security of electronic data during the data collection and processing period following the system of record notice (SORN) titled FNS-8 USDA/FNS Studies and Reports published in the Federal Register on April 25, 1991 (56 FR 19078).4 Names and phone numbers will not be linked to participants’ responses, study participants will have a unique ID number, and analysis will be conducted on data sets that include only respondent ID numbers. All data will be securely transmitted to the study team via sealed mailings or phone; and will be stored in locked file cabinets or password-protected computers, and accessible only to project staff. If respondents provide any personally identifiable information via text or email, such as their child’s name and date of birth from a health-care professional printout, all personal information will be immediately deleted from the electronic device.5 Names and phone numbers will be destroyed within 12 months after the end of the collection and processing period (approximately 12/2024). As with the previous data collection on WIC ITFPS-2, FNS has contracted with Westat to complete this study. Westat’s Institutional Review Board (IRB) is the organization of record overseeing all human subjects’ activities for the study. A copy of the IRB approval letter is in Appendix X. This information collection request has been reviewed by Wilson J. Moorer, a privacy officer in the FNS Privace Office and was approved with no comments.
A.11 Justification for Sensitive Questions
Provide additional justification for any questions of a sensitive nature, such as sexual behavior or attitudes, religious beliefs, and other matters that are commonly considered private. This justification should include the reasons why the agency considers the questions necessary, the specific uses to be made of the information, the explanation to be given to persons from whom the information is requested, and any steps to be taken to obtain their consent.
In general, questions on the WIC participant questionnaires, and measures of child height and weight, are not considered to be sensitive. Participants can choose to skip any question, or to discontinue participation in the study. The majority of questions required for the interviews were cognitively tested for the Base Study, and no participants expressed unwillingness to answer the questions, nor have study participants expressed concerns about the questions as they have answered them through the 72-month interview. The remaining questions were based on or drawn from established studies with similar populations and have undergone expert review for comprehensibility, and simulation testing for flow and timing. All documents in this information collection request were reviewed by Wilson J. Moorer, a privacy officer in the FNS Privace Office and approved with no comments.
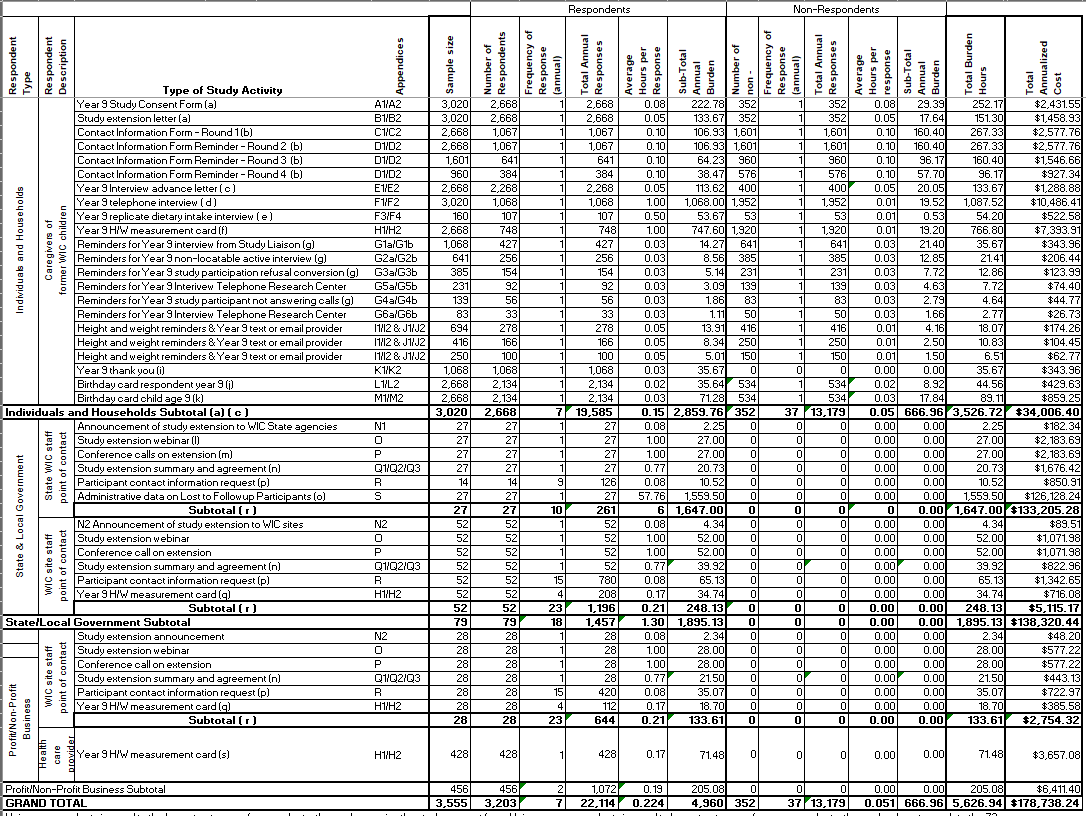
A.12 Estimates of Respondent Burden Including Annualized Hourly Cost
Provide estimates of the hour burden of the collection of information. The statement should:
A. Indicate the number of respondents, frequency of response, annual hour burden, and an explanation of how the burden was estimated. If this request for approval covers more than one form, provide separate hour burden estimates for each form and aggregate the hour burdens in Item 13 of OMB Form 83-I.
This is a revision of a currently approved information collection. With this revision, FNS estimates that this collection will have 3,203 respondents, 352 non-respondents, 35,293 responses, and 5,627 burden hours. The target participant is the child. We will collect information about the child from the child’s caregiver (typically the child’s mother) and from WIC State agency and local site staff. The burden table (Appendix Y) presents the number of respondents, frequency of response, and annual hour burden to collect these data. The assumptions used to estimate burden are based on the study team’s professional experience, survey expert simulation testing for timing of the interviews, and input from Public Health Foundation Enterprise for WIC (PHFE WIC).
Individuals and Households - Caregivers of former WIC children. The sample size of the study participants, who are caregivers of former WIC children, is 3,020 (from the Base sample cohort of live births). We estimate that 2,668 study participants will respond to one study extension consent (0.0835 hours per response) and one study extension letter (0.0501 hours per response). They will respond up to four requests to update their contact information (0.1002 hours per response). As needed depending on individual response patterns, study participants will receive communications reminding them to participate. At a maximum, participants will receive one reminder about the interview from study liaisons (0.0334 hours per response), one reminder about the interview for those who have no phone number (0.0334 hours per response), one refusal conversion letter for those who refuse the interview (0.0334 hours per response), one reminder about the interview from those who do not answer calls (0.0334 hours per response), two interview reminders from the Telephone Research Center (0.0334 hours per response), three reminders about child height and weight measurements (0.0501 hours per response). An estimated 2,668 study participants will respond to one advance letter for the Y9 telephone interview (0.0501 hours per response), approximately 1,068 study participants will respond to one Y9 interview (1 hour per response), and 107 will respond to one Y9 replicate dietary intake interview (0.50 hours per response). An estimated 748 participants will take their children to be weighed and measured once at age 9 (1.0 hours per response). An estimated 1,068 study participants will read a thank you note from the study (0.0334 hours per response), and 2,134 will read birthday cards for the caregivers (0.0167 hours per response) and birthday cards for the children (0.0334 hours per response). Estimates of burden for study communications are based on professional experience with similar materials, and simulation testing of timing for reading the materials. Estimates of burden for the Y9 telephone interview and replicate dietary intake interview are based on a combination of extensive records of burden from previous administrations of the dietary intake interview on this study, and expert simulation testing of timing of non-intake questions. Estimates of burden for child measurements are based on professional knowledge from PHFE WIC.
State and Local Government – State WIC staff and WIC site staff. We expect that all of the WIC State Agencies and WIC sites that have been collaborating with the study will continue to do so. A total of 27 WIC State Agency staff and 80 WIC site staff will read one study extension announcement (0.0835 hours per response), attend one webinar briefing (1.00 hours per response), participate in one conference call about the study extension (1.00 hours per response), and read one study extension summary and agreement along with two addenda to the study agreement summarizing study participation for for their affiliated study sites and the administrative data request on the L2FU Study participants (a total of 0. 7677 hours) . Eighty WIC staff will read the study extension summary and agreement and addenda (0.7677 per response). A total of 14 State Agencies will respond to requests for assistance with participant contact information (0.0835 hours per response, 9 annual responses). A total of 80 WIC site staff will conduct measurements on study children who visit the clinic to be weighed and measured (0.1667 hours per response, 4 annual responses), and 80 staff members will respond to requests for assistance with participant contact information (0.0835 hours per response, 15 annual responses). A total of 27 WIC Stage Agencies will respond to the data request for administrative data on study participants who left the study during the first five years (54.7593 hours per response, 1 annual response). Estimates of burden for the communication materials is based on professional experience with similar materials, and simulation testing of timing for reading the materials. Estimates of timing for the webinar and conference calls are based on actual timing for similar activities in the past on WIC ITFPS-2. Estimates of burden for child measurements are based on professional knowledge from PHFE WIC. Estimates of burden for the administrative data request is based on the assumption of manual retrieval of archived data for inactive participants.
Profit/Non-Profit Business – Health care professional. We expect that 428 study participants will take their children to a health care professional to be weighed and measured around age 9. Therefore, a total of 428 health-care professionals will conduct measurements on study children who visit their office to be weighed and measured (0.1670 hours per response).
B. Provide estimates of annualized cost to respondents for the hour burdens for collections of information, identifying and using appropriate wage rate categories.
The estimated annualized cost for this collection is $178,738,24. This includes $34,006.40 for Individual/Household at $7.25 per hour for Caregivers of former WIC children (average national minimum wage); $138,320.44 for State & Local Government at $60.81 per hour for state and local WIC administrators (job category “Management Occupations” code #11-0000) and at $15.50 per hour for WIC site staff (job category “Healthcare Support Occupations” code #31-0000); and $6,411.40 for Profit/Non-Profit Business at $38.47 per hour for Health care professionals (job category “Registered Nurses” code 29-1141) and at $15.50 per hour for WIC site staff (job category “Healthcare Support Occupations” code #31-0000). In all estimates, an additional 33% of the estimated base annual respondent cost was added to the estimates to represnent fully loaded wages (Appendix Y). The estimate of costs to state and local government is based on the burden estimates and utilizes the U.S. Department of Labor, Bureau of Labor Statistics, 2020National Occupational Employment and Wage Statistics (https://www.bls.gov/oes/current/oes110000.htm). The estimate of costs to Profit/Non-Profit Business is based on the burden estimates and utilizes the U.S. Department of Labor, Bureau of Labor Statistics, 2020 National Occupational Employment and Wage Statistics, (https://www.bls.gov/oes/current/oes291141.htm). No respondents will be asked to keep records of data; therefore no burden hours have been estimated for recordkeeping. Table A12-1 shows the hour burden for different types of respondents.
Table A12-1 Burden by Respondent Type
A.13 Estimates of Other Total Annualized Cost Burden
Provide estimates of the total annual cost burden to respondents or record keepers resulting from the collection of information, (do not include the cost of any hour burden shown in items 12 and 14). The cost estimates should be split into two components: (a) a total capital and start-up cost component annualized over its expected useful life; and (b) a total operation and maintenance and purchase of services component.
There are no capital/start-up or ongoing operation/maintenance costs associated with this information collection.
A.14 Annualized Cost to the Federal Government
Provide estimates of annualized cost to the Federal government. Also, provide a description of the method used to estimate cost and any other expense that would not have been incurred without this collection of information.
Total annual cost to the federal government is $3,878,523. Contractor costs associated with this study total $3,817,072 over 5 years, with an estimated $763,414 annual cost to the federal government. This is based on an estimate of 33,850 labor hours, with a salary range of $29.17– $399.65 per hour, and includes instrument development; data collection and retention; analysis; reporting; and overhead costs, including computing, copying, supplies, postage, shipping, incentives, and other miscellaneous items.
The cost of the FNS employee, Social Science Research Analyst, involved in project oversight with the study is estimated at GS-13, step 1 at $49.68 per hour. We anticipate this person will work 832 hours per year for 4.67 years for a combined total of 3,885 hours. The annual cost for the FNS employee is $54,972, this includes an additional 33% of the estimated base annual cost to represent fully loaded wages. Additionally, the Branch Chief who provides oversight for work conducted by the Research Analyst is estimated at GS-14, step 1 at $58.71 per hour. We anticipate this person will work 83 hours per year for 4.67 years for a total of 387.61 hours. The annual cost for this employee is $6,479 (this figure also represents fully loaded wages by adding an additional 33% of estimated base annual cost). Federal employee pay rates are based on the General Schedule of the Office of Personnel Management (OPM) for 2021 for the Washington DC locality (source for the federal hourly wage rates:https://www.opm.gov/policy-data-oversight/pay-leave/salaries-wages/salary-tables/pdf/2021/DCB_h.pdf).
A.15 Explanation for Program Changes or Adjustments
Explain the reasons for any program changes or adjustments reported in Items 13 or 14 of the OMB Form 83-1.
This is a revision of a currently approved data collection. This information collection is currently approved with 5,564 burden hours and 52,076 responses. As a result of this revision, FNS estimates that the annual burden hours for this collection will increase by 63, due to a program change with the addition of the administrative data collection on the L2FU Study participants. The reason for the 16,783 decrease in burden responses is primarily due to no longer needing as many contact information form reminders and birthday greetings that were collected as part of Year 6 of the ITFSP-2. With this renewal, FNS estimates that this information collection will have 5,627 burden hours and 35,293 responses.
A.16 Plans for Tabulation and Publication and Project Time Schedule
For collections of information whose results are planned to be published, outline plans for tabulation and publication.
The data collection and reporting for WIC ITFPS-2 Y9 Extension will begin after OMB approval and when the cohort of study children reach their 9th birthdays, in March 2022, and extend until Spring 2025.
Table A16.1. Data Collection and Reporting Schedule
Activity |
Schedule |
Participant Notification of Study Extension |
2 weeks following OMB approval |
Data Collection |
March 2022 – November 2023 |
Data Analysis |
December 2023 – June 2024 |
Draft Report |
June 2024 |
Final Report |
December 2024 |
Publish Final Cleared Report |
Spring 2025 |
FNS expects to release one report to the public for the findings of the Y9 Extension, in spring 2025. The report will be posted on the FNS web site. The study team will conduct descriptive analyses, cross-sectional analyses looking at associations between key variables, and longitudinal analyses that fully leverage the study data. Table A16.2 presents an overview of the research questions that will be addressed in the report, and data collection activities that contribute to them. These analyses will aid FNS to understand and plan improvements to the WIC program, its technical assistance, and future research. Findings will be also be published in professional journals and publications intended for general audiences such as nutrition educators.
Table A16.2. Research Questions and Principal Data Sources for Year 9 Report
Research question |
Main source of data |
Objective 1: Examine the dietary behaviors and health outcomes of study children around age 9 years. Provide longitudinal comparisons to examine changes associated with WIC participation patterns. |
|
|
Caregiver Interview |
|
Caregiver Interview |
|
Caregiver Interview Child Measurements |
|
Caregiver Interview |
|
Caregiver Interview |
|
Caregiver Interview |
|
Caregiver Interview |
|
Caregiver Interview |
|
Caregiver Interview Child Measurements |
|
Caregiver Interview Child Measurements |
|
Caregiver Interview Child Measurements |
Research question |
Main source of data |
|
Caregiver Interview Child Measurements |
|
Caregiver Interview |
Objective 2: Describe the food security status of the children and their households. |
|
|
Caregiver Interview |
|
Caregiver Interview |
|
Caregiver Interview |
Objective 3: Describe caregivers’ feeding practices. |
|
|
Caregiver Interview |
|
Caregiver Interview |
|
Caregiver Interview |
|
Caregiver Interview |
Objective 4: Describe the children’s food and health-related environmental characteristics. |
|
|
Caregiver Interview |
|
Caregiver Interview |
|
Caregiver Interview |
|
Caregiver Interview |
A.17 Reason Display of OMB Expiration Date is Inappropriate
If seeking approval to not display the expiration date for OMB approval of the information collection, explain the reasons that display would be inappropriate.
All data collection instruments will display the OMB control number and expiration date.
A.18 Exceptions to Certification for Paperwork Reduction Act Submissions
Explain each exception to the certification statement identified in Item 19 “Certification for Paperwork Reduction Act.”
There are no exceptions to the certification statement.
1FNCS Corporate Priorities FY 2010 Guide (April 2010). USDA Food, Nutrition, and Consumer Services. Available at: http://www.fns.usda.gov/ora/menu/gpra/FY2010PrioritiesGuide.pdf. Accessed on: 5/13/2011.
2$10 for personal cell phone use is based on costs for pay-as-you-go plans popular with lower income users who cannot always afford contracts. Specifically, TracFone, widely available at discount department stores, charges $39.99 for 200 minutes of talk time. A 60-minute interview would therefore cost participants approximately $10 for personal cell phone minutes.
3 In 2018, average public transportation cost in the US for a single segment of a trip was $1.60, and historically costs have risen approximately $0.06/year (US Department of Transportation, Bureau of Transportation Statistics, https://www.bts.gov/content/fares-all-transit-modes-unlinked-trip-1990-2018). We therefore anticipate the average public transportation cost for one adult and the study child, for a round trip, will be approximately $7.00. Because this is an average, however, and many of our study participants live in more expensive areas for transportation or may need to bring more than one child to avoid child care costs, we round the total amount up to $10.
4Published in the Federal Register on April 25, 1991 (56 FR 19078).
5 Respondents are instructed to cover their child’s name and date of birth prior to sending a picture of the health care record to the study liaison.
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | Crystal MacAllum |
| File Modified | 0000-00-00 |
| File Created | 2022-01-11 |
© 2026 OMB.report | Privacy Policy