Ssb_aims_092921
SSB_AIMS_092921.docx
Using Real-time Prescription and Insurance Claims Data to Support the HIV Care Continuum
OMB: 0920-1361
Using Real-time Prescription and Insurance Claims Data to Support the HIV Care Continuum
OMB No. 0920-NEW
Supporting Statement B
September 29, 2021
Kathy Byrd, MD, MPH, Project Officer
Centers of Disease Control and Prevention
National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention
Division
of HIV/ AIDS Prevention- Surveillance and Epidemiology
HIV
Epidemiology Branch
1600 Clifton Rd., MS E-45
Atlanta, GA 30333
Phone: 404-639-3083
Fax: 404-639-6127
Email: [email protected]
TABLE OF CONTENTS
B. COLLECTIONS OF INFORMATION EMPLOYING STATISTICAL METHODS
1. Respondent Universe and Sampling Methods
2. Procedures for the Collection of Information
3. Methods to Maximize Response Rates and Deal with Nonresponse
4. Tests of Procedures or Methods to be Undertaken
5. Individuals Consulted on Statistical Aspects and Individuals Collecting and/or Analyzing Data
EXHIBITS
Exhibit B1: Anticipated Eligible Sample
Exhibit B2-1: Cluster-randomized design: power analysis for comparison of two proportions using large-sample approximation and two-sided un-pooled Z-test
Exhibit B2-2: Cluster-randomized design: study power by number of clusters per study arm and by ICC
Exhibit B3: Statistical consultants
REFERENCES
B. Collections of Information employing statistical methods
1. Respondent Universe and Sampling Methods
The respondent universe is persons with HIV who are enrolled in Virginia Medicaid and who have either never filled a prescription for antiretroviral (ARV) medication or who have failed to fill an ARV prescription within > 30 to < 90 days of the expected fill date.
A validated HIV case identification algorithm will be applied to the Virginia Department of Medical Assistance Services (DMAS—Virginia Medicaid) database to identify persons with HIV who have either never filled an ARV prescription or have not filled an ARV prescription within >30 to < 90 days of the expected fill date. These individuals will be considered potential study participants. Deterministic and probabilistic methods will be used to link the list of potential participants within the Virginia Medicaid database to the Virginia Department of Health (VDH) Care Markers database (an extract of the VDH HIV surveillance database). Individuals that are matched across the two databases (indicating that the persons are both enrolled in Medicaid and confirmed HIV positive) are eligible for study participation.
Additional eligibility criteria
Continuous enrollment in Virginia Medicaid for the preceding 12 months
Age 19 – 63 years
Exclusion criteria
Dual eligibility for Medicare
Other third-party health insurance coverage
Prescription claims indicates a within-class ART switch due to medication toxicity (i.e., there is a new claim for a within-ARV class prescription, during eligibility period).
Antiretroviral resistance as indicated by a claim for resistance testing and a new prescription indicating an ARV class switch, during eligibility period.
Service address of most frequent provider in HIV-related claims for a healthcare facility known to be offering PositiveLinks
Non-English speaking
The study will exclude persons with healthcare providers from clinics that currently offer PositiveLinks because dual availability of the app could bias intervention effects. The study will also exclude non-English speaking individuals because the PositiveLinks platform has not yet been validated in non-English languages.
Cluster randomization will occur at the healthcare provider level and will be based on relevant provider characteristics (e.g., HIV patient volume) and providers’ patient characteristics (i.e., distribution of providers’ patients by age, sex, race/ethnicity, and urbanicity). Each healthcare provider of eligible study participants represents a cluster. Patients with HIV who are served by the same provider represent members of the cluster. Healthcare providers will be randomized 1:1 to either the intervention arm or usual care (i.e., no intervention or control) arm so that their respective patients will, as a group, receive either the intervention or usual care. Randomization at the healthcare provider level prevents contamination across the two study arms; participants whose providers receive the provider-level intervention will not be included in the usual care/control arm.
Study participants are the patients of the randomized healthcare providers. Participants in the intervention arm will be delegated to either a patient-level or provider-level intervention, depending on need; participants who are > 30 to < 90 days late filling their ARV prescription(s) will receive the patient-level intervention and participants who have never filled an ARV prescription will be delegated to the provider-level intervention.
Participants of the provider-level intervention will not receive direct intervention. Instead, the providers of these patients (henceforth referred to as “provider participants”) will receive the provider-level intervention. Providers assigned to the provider-level intervention must be the provider who is most frequently associated with the participants’ Medicaid claims and must be of one of the following specialties: infectious disease, internal medicine, family medicine, or OB/GYN.
The study has a fixed maximum sample size of 1,353 people. The maximum sample size is based on the following: there are 9,022 Medicaid enrollees with HIV in Virginia of whom approximately 75% (6,766) are enrolled continuously in Medicaid and have no additional insurance coverage (e.g., no Medicare or commercial insurance). An estimated 20% of these individuals (1,353) have no prescriptions for ARV medications. (1) Thus, the maximum sample size is 1,353 people. However, in previous Data-to-Care projects implemented by VDH, approximately 20% of individuals identified as being out of care could not be contacted which, if holds true for this study, brings the maximum sample size down to 1,082. As such, we anticipate enrolling a total of 1,000 participants: 500 into the intervention arm and 500 into the control arm. Based on preliminary analysis of the Virginia Medicaid Analytic eXtract we estimate 40 provider participants will receive the provider-level intervention. All analyses will be done at the patient level such that 40 patients of the provider participants and 460 participants of the patient-level intervention will comprise the intervention sample.
There are 40 provider participants respondents who will receive the clinician consultation but whose data will not be analyzed as part of the outcome analyses (thus they are not included in the sample size considerations)—all outcome analyses will occur at the patient level (i.e., the patients of the provider participants will be included in the outcome analyses).
Exhibit B1: Anticipated Eligible Sample
Medicaid enrollees with HIV in Virginia |
9,022 |
Continuously enrolled in Medicaid* |
6,766 |
With no ARV prescriptions† |
1,353 |
Estimated 20% unable to contact ‡ |
1,082 |
* Estimate based on 2012 Virginia Medicaid Analytic eXtract; assumes 12 months of continuous Medicaid enrollment, no dual eligibility for Medicare and no third-party insurance.
† Estimate from Iqbal et al. AIDS Care. 2018 Sep; 30(9): 1128-1134 [1]; includes never filled ARV prescription or late filling ARV prescriptions.
‡ In previous VDH Data-to-Care projects, 20% of individuals identified as being out of care could not be contacted.
Participant eligibility will be determined during the first week of each month until the end of enrollment. After the potential participant identification process and randomization, active recruitment for participants of the patient-level intervention and for provider participants will occur. Enrollment is anticipated to take 6 months.
We will defer recruitment until after the intervention arm follow-up is complete for: 1) eligible potential participants of the provider-level intervention (i.e., patients of the providers who receive the provider-level intervention) and 2) eligible potential participants in the usual care arm (i.e., controls). Neither of the abovementioned groups will receive direct intervention. We will defer recruitment until after intervention follow-up to minimize response bias, which could alter study outcomes. (2)
2. Procedures for the Collection of Information
Data collection methods
Data will be collected from the following: 1) Virginia Medicaid database 2) Virginia Care Marker database (an extract of the VDH HIV surveillance database) 3) Phase I and Phase II patient-level interviews 4) Peer-to-peer clinician consultation and 5) PositiveLinks mobile application (“app”) abstraction.
The grantee, Virginia Commonwealth University (VCU), will be given DMAS and VDH affiliate status which allows VCU to access the Virginia Medicaid and Virginia Care Marker databases on the DMAS and VDH servers, respectively. Data necessary for the study will be placed in study specific files on the secure DMAS and VDH servers by DMAS and VDH personnel. A VCU Data Analyst will abstract data from the Virginia Medicaid and Virginia Care Marker databases (Att 3 and Att 4) monthly until 500 participants of the patient-level intervention and provider participants are enrolled and 500 controls are identified. After enrollment, data will be abstracted for participants of the patient-level intervention, quarterly for 12 months. Additionally, a one-time data abstraction will occur at the end of the intervention follow-up period for the controls and participants of the provider-level intervention; this abstraction will contain 12 months of data retrospective to the date of consent. These data will be used to: 1) determine study eligibility 2) conduct the patient- and provider-level interventions and 3) determine study outcomes.
A study Linkage Coordinator will administer a one-time Phase I and one-time Phase II semi-structured interview (Att 9 and Att 10) with participants of the patient-level intervention. The purpose of the interviews is to determine participants’ adherence barriers and to then refer participants to appropriate resources to address the barrier(s). The study Linkage Coordinator will have previous training in motivational interviewing. The Linkage Coordinator will enter all data from the Phase I and Phase II interviews directly into a Research Electronic Data Capture (REDCap) database, a secure web application for building and managing online surveys and databases. REDCap will be prepopulated with basic participant information (e.g., name, contact information) from the Medicaid and Care Marker databases and programmed with appropriate skip patterns and branching logic.
Providers of study eligible participants who have never filled an ARV prescription will receive the provider-level intervention. During the provider-level intervention, a member of VDH’s Advisory Committee to the Virginia Medication Assistance Program or another HIV clinical expert will conduct a peer-to-peer clinician consultation with the provider participant. The clinician consultant will use a guided prompt to elicit information to inform the consultation. (Att 13a and Att 13b) After the consultation, the clinician consultant will document the provider participant’s barriers to ART prescribing and recommended resources in a brief post-consultation questionnaire. (Att 14a) The questionnaire will be directly entered into a REDCap database, via a secure link provided by the study Linkage Coordinator. (Att 14b)
Lastly, a study Linkage Coordinator will download PositiveLinks data (Att 15) from the administrative web portal of the mobile app. Additionally, app launches will be determined using Google Analytics. For all participants, the Linkage Coordinator will monitor the community message board daily for misinformation and inflammatory comments. The Linkage Coordinator will monitor direct messages daily to respond to participants’ inquiries.
Data Transmittal
No identifiable individual-level data will be stored at VCU; these data will remain on the DMAS and VDH servers (which routinely contain this information). Virginia Commonwealth University will construct de-identified analytic datasets. Only de-identified analytic datasets will be transferred and downloaded onto VCU servers. All study data will be de-identified and all PII elements will be removed from the original data, and a new de-identified analytic dataset will be created in accordance with HIPAA regulations and 45 CFR 164.514. Only de-identified analytic datasets will be sent to CDC. Neither CDC nor VCU will be able to re-identify participants in the de-identified analytic dataset. The de-identified analytic dataset will be electronically transmitted to CDC through the CDC Secure Data Network. All data transmissions are automatically encrypted by the software that generates the transfer files. Security certificates are used to control access to the Secure Data Network. De-identified data elements from participants of the patient-level intervention and from the post-consultation questionnaire will be sent quarterly to CDC. Data from participants of the provider-level intervention and from the controls will be sent one time at the end of the intervention arm follow-up. These data will be used to determine study outcomes (e.g., viral suppression).
Analysis plan summary
Analysis of study outcomes will occur at the patient level (i.e., proportions virally suppressed among participants of the patient-level intervention and patients of the healthcare providers who received the provider-level intervention). We will perform an intent-to-treat analysis to evaluate the effects of the intervention. The binomial study outcomes of HIV viral suppression (primary outcome) and secondary outcomes (initiation, re-initiation, persistence and adherence to ART, and retention in care) will be evaluated by comparing the proportions between each intervention arm and the usual care, or control, arm. Chi-squared tests will be used to evaluate the statistical significance of the effects of treatments on proportions. Logistic regression will also be used to test for the intervention effects after adjusting for potential confounders. The differences in the means of persistence between the intervention and control groups will be tested using a t-test and a linear regression model will be used to evaluate the effect after adjusting for confounders.
Success of randomization will be assessed by summarizing the baseline variables by study arm. Continuous variables will be characterized by their mean value, standard deviation, and range, or by median and interquartile range. Categorical variables will be summarized by counts and proportions.
A two-sided significance level alpha = 0.05 (Type I error) will be used throughout the analyses to perform significance testing and 95% confidence intervals will be constructed. Heterogeneous variables at both provider and patient level will be probed as potential confounders and adjusted for in multilevel modeling. Incomplete records and missing data will be summarized by study arms; graphical and modeling approaches will be used to assess the patterns of missing data, followed by application of an appropriate imputation approach.
Sample Size Justification
The primary endpoint of the study is the binomial proportion of persons virally suppressed (HIV RNA < 200 copies/mL). For the purposes of the sample size and power analysis, the expected effect of intervention is expressed as the difference in post-intervention proportions of persons virally suppressed (%VS) between the intervention and control arms.
By denoting %VS in the intervention and control arms as P1 and P2 respectively, the primary null (H0) and alternative (H1) hypotheses of the study can be formulated as follows:
H0: P1 – P2 = 0 versus two-sided alternative H1: P1 – P2 ≠ 0
This project has a fixed maximum sample size of approximately 500 participants per arm. For the sample size of 500 per study arm, we have > 80% power to reject the null hypothesis of no difference between study groups when the absolute difference is at least 10%.
In Exhibit B2-1, the power of the study is presented as a function of the difference to be detected, cluster size, number of clusters, and intracluster correlation coefficient (ICC). The difference to be detected, which takes on values 0.10 and 0.15, represents the anticipated effect of the intervention to be detected with high probability. It is computed as the difference of proportions of virally suppressed patients between the intervention and control arms under the alternative hypothesis (D = P1 – P2 | H1). The cluster size and number of clusters represent the number of patients served by the same provider and the count of providers, respectively; both numbers pertain to one study arm and are assumed to be the same across the arms. The sample size per study arm is equal to the number of patients per cluster times the number of clusters. Finally, the ICC denotes the correlation between any two patients in the same cluster and is represented by a series of typical values from 0.01 to 0.06.
Exhibit B2-1: Cluster-randomized design: power analysis for comparison of two proportions using large-sample approximation and two-sided un-pooled Z-test (assumptions: Type I error (alpha) = 0.05, and percent of viral suppression in control arm P2 = 0.5)
Difference (D)* to be detected |
Cluster size (# patients) |
# Clusters (providers) per study arm |
Intraclass Correlation Coefficient (ρ) |
|||||
0.01 |
0.02 |
0.03 |
0.04 |
0.05 |
0.06 |
|||
10% |
7 |
20 |
37.8% |
35.9% |
34.4% |
33.0% |
31.8% |
30.5% |
30 |
52.2% |
50.0% |
47.9% |
46.1% |
44.4% |
42.8% |
||
40 |
64.3% |
61.8% |
59.6% |
57.4% |
55.6% |
53.6% |
||
50 |
73.9% |
71.5% |
69.2% |
67.1% |
65.1% |
63.1% |
||
60 |
81.2% |
79.0% |
76.9% |
74.9% |
73.0% |
70.9% |
||
70 |
86.7% |
84.9% |
83.0% |
81.1% |
79.2% |
77.5% |
||
80 |
90.8% |
89.2% |
87.6% |
85.9% |
84.3% |
82.6% |
||
15% |
7 |
20 |
70.7% |
68.0% |
65.8% |
63.6% |
61.6% |
59.6% |
30 |
86.5% |
84.5% |
82.5% |
80.8% |
78.9% |
77.1% |
||
40 |
94.2% |
93.0% |
91.8% |
90.4% |
89.1% |
87.6% |
||
50 |
97.7% |
97.0% |
96.3% |
95.5% |
94.6% |
93.7% |
||
60 |
99.1% |
98.8% |
98.4% |
97.9% |
97.4% |
96.8% |
||
70 |
99.7% |
99.5% |
99.3% |
99.1% |
98.8% |
98.5% |
||
80 |
99.9% |
99.8% |
99.7% |
99.6% |
99.5% |
99.3% |
||
10% |
5 |
20 |
29.1% |
28.1% |
27.3% |
26.6% |
25.8% |
25.1% |
30 |
40.5% |
39.2% |
38.0% |
37.0% |
35.9% |
34.9% |
||
40 |
51.0% |
49.5% |
48.1% |
46.8% |
45.5% |
44.4% |
||
50 |
60.2% |
58.6% |
57.1% |
55.6% |
54.2% |
52.8% |
||
60 |
68.0% |
66.4% |
64.8% |
63.3% |
61.8% |
60.4% |
||
70 |
74.6% |
73.1% |
71.5% |
69.9% |
68.5% |
67.1% |
||
80 |
80.0% |
78.6% |
77.1% |
75.6% |
74.2% |
72.8% |
||
15% |
5 |
20 |
57.1% |
55.3% |
54.0% |
52.6% |
51.2% |
49.8% |
30 |
74.3% |
72.6% |
71.0% |
69.7% |
68.0% |
66.6% |
||
40 |
85.4% |
84.2% |
82.8% |
81.5% |
80.1% |
78.9% |
||
50 |
92.1% |
91.1% |
90.1% |
89.1% |
88.1% |
87.0% |
||
60 |
95.8% |
95.2% |
94.5% |
93.7% |
93.0% |
92.2% |
||
70 |
97.9% |
97.5% |
97.0% |
96.5% |
96.0% |
95.5% |
||
80 |
98.9% |
98.7% |
98.4% |
98.1% |
97.8% |
97.4% |
||
*Difference (D) to be detected represents the anticipated effect of intervention to be detected with high probability; it is computed under the alternative hypothesis (H1) by subtracting the proportion of virally suppressed patients (%VS) in the control arm from %VS in the intervention arm: D = P1 – P2 | H1.
For example, sample size of 400 patients in the intervention arm and 400 patients in the control arm, (for a total of 800 patients obtained by recruiting 80 providers (clusters) in each study arm with 5 HIV patients per each provider) will achieve 97.4% to 98.9% power to detect at least 0.15 difference of proportions of viral suppression (%VS) between the intervention and control arms—assumptions: the ICC varies from 0.01 to 0.06; two-sided Type I error (alpha) = 0.05; and %VS in the control group, P2 = 0.50.
In a different scenario, sample size of 490 patients in the intervention arm and 490 patients in the control arm (obtained by recruiting 70 providers in each study arm with 7 patients per provider), with ICC ranging from 0.01 to 0.05 and the same assumptions about the alpha and P2, will achieve 79.2% to 89.7% power to detect at least 10% difference of %VS between the study arms.
Exhibit B2-2 presents another look at the study power as a function of the number of clusters (K) per study arm and ICC, computed using the same un-pooled Z-test. The number of clusters on X axis varies from 20 to 80, and power curves are drawn for the range of ICC from 0.01 to 0.06. Both quantities are assumed to be the same across the study arms. The values of %VS in the intervention and control arms are fixed at P1 = 0.65 and P2 = 0.50 levels, respectively, while the cluster size is assumed to be equal to 5 patients per cluster. As shown on the figure, an increase in ICC leads to a reduction in power, although the power to detect at least 15% difference in %VS stays consistently above 90% when the number of clusters per study arm is at least 60, or the number of patients per study arm is at least 300.
Exhibit B2-2. Cluster-randomized design: study power by number of clusters per study arm and by ICC (assumptions: %VS in intervention group P1 = 0.65, %VS in control group P2 = 0.50, two-sided Type I error (alpha) = 0.05, cluster size M=5)
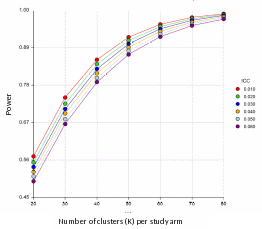
All analyses will be conducted at the patient level. An estimated 40 provider participants (i.e., providers of participants of the provider intervention) will receive the peer-to-peer consultation. Sample size estimates are based on a sample size of 500 patient participants for each arm. Given that the study outcomes will be determined at the patient level, no sample size estimate was calculated for providers receiving peer-to-peer consultations; patient participants of these providers are included in the above sample size calculation and will be analyzed as part of the intervention arm.
3. Methods to Maximize Response Rate and Deal with Nonresponse
The Virginia Medicaid and Virginia Care Marker data are existing data that are not collected for the purpose of this study but are routinely collected by DMAS and VDH for payment of administrative insurance claims and for HIV surveillance, respectively.
For both the patient-level and provider-level intervention three contact attempts will be made by a study Linkage Coordinator to reach a potential participant. If the Linkage Coordinator calls a potential participant and no one answers, the Linkage Coordinator will leave a voice mail with a call back number. If the Linkage Coordinator is unable to reach a potential participant of the patient-level intervention during the Phase I eligibility period (> 30 to < 60 days late filling their ARV prescription) and the individual is subsequently identified as > 60 to < 90 days late filling their ARV prescription (thus making them eligible for the Phase II patient-level intervention), the Linkage Coordinator will try another 3 attempts to reach the individual to enroll them into the Phase II patient-level intervention. Additionally, the Linkage Coordinator will ensure that participants feel they can freely engage in a conversation before beginning to describe the study, beginning the consent process, or beginning the intervention. If a participant feels that they cannot, at the time of the Linkage Coordinator’s contact, freely converse with the Linkage Coordinator, the participant may choose to reschedule the call for when they can speak more privately or to move to a more private location for the phone call.
Lastly, a study Linkage Coordinator will monitor PositiveLinks app launches at one, two, four and twelve weeks and follow up with participants to address any usability and accessibility issues participants might be experiencing.
4. Tests of Procedures or Methods to be Undertaken
The Virginia Medicaid and Virginia Care Marker data are existing data that are not collected for the purpose of this study but are routinely collected by DMAS and VDH for payment of administrative insurance claims and for HIV surveillance, respectively. Data will be abstracted from these databases. Data elements collected from the patient- and provider-level interventions have been reviewed by study team members from Virginia Commonwealth University, Virginia Department of Health, Virginia Department of Medical Assistance Services, University of Virginia, Centers for Disease Control and Prevention, and National Institutes of Health.
5. Individuals Consulted on Statistical Aspects and Individuals Collecting and/or Analyzing Data
Exhibit B3 below lists the study team members who were consulted on the aspects of research design and those who will be analyzing the data. Data will be collected by VCU through a co-operative agreement. Virginia Commonwealth University will sub-contract with DMAS and VDH. Please note: The CDC staff are primarily responsible for: providing technical assistance in the design and implementation of the research; assisting the development of the research protocol and data collection instruments for CDC Project Determination and local IRB reviews; working with investigators to facilitate appropriate research activities; and analyzing data and presenting findings at meetings and in publications. CDC staff will neither interact with nor collect data from study participants. No individual identifiers will be linkable to collected data shared with or accessible by CDC staff, and no personally identifiable information will be shared with or accessible by CDC staff.
Exhibit B3: Statistical consultants
Name |
Title |
Organization |
Phone/email |
Role |
Kathy Byrd |
Medical Epidemiologist |
CDC |
404-639-3083 |
|
Bassam Dahman |
Mathematical Statistician
|
Virginia Commonwealth University
|
804.628.3443 |
|
Yang Yang Deng |
Data Manager |
Virginia Commonwealth University |
|
|
Roman Gvetadze |
Mathematical Statistician
|
CDC |
404.639.3522 |
|
April Kimmel |
Principle Investigator |
Virginia Commonwealth University |
804.628.6273 [email protected] |
|
Tiffany Williams |
Data Manager |
CDC (contractor) |
404.718.8781 [email protected] |
|
CDC personnel responsible for receiving and approving co-operative agreement deliverables:
Kathy Byrd
Medical Epidemiologist
Epidemiology Branch, Division of HIV/AIDS Prevention
1600 Clifton Rd. NE, MS US8-4
Atlanta, GA 30333
T: 404.639.3083
Email: [email protected]
References
Iqbal K, Huang YA, Peters P, Weidle P, Hoover K Antiretroviral treatment among commercially insured persons living with HIV in an era of universal treatment in the United States - 2012-2014. AIDS Care. 2018 Sep; 30 (9): 1128-1134.
McRae AD, Weijer C, Binik A et al. Who is the research subject in cluster randomized trials in health research? Trials 2011; 12: 183.
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | CDC User |
| File Modified | 0000-00-00 |
| File Created | 2021-10-20 |
© 2026 OMB.report | Privacy Policy