Ssa_aims_101821
SSA_AIMS_101821.docx
Using Real-time Prescription and Insurance Claims Data to Support the HIV Care Continuum
OMB: 0920-1361
Using Real-time Prescription and Insurance Claims Data to Support the HIV Care Continuum
OMB No. 0920-NEW
Supporting Statement A
October 18, 2021
Kathy Byrd, MD, MPH, Project Officer
Centers of Disease Control and Prevention
National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention
Division
of HIV/ AIDS Prevention- Surveillance and Epidemiology
HIV
Epidemiology Branch
1600 Clifton Rd., MS US8-4
Atlanta, GA 30333
Phone: 404-639-3083
Fax: 404-639-6127
Email: [email protected]
TABLE OF CONTENTS
A. JUSTIFICATION
1. Circumstances Making the Collection of Information Necessary
2. Purpose and Use of Information Collection
3. Use of Improved Information Technology and Burden Reduction
4. Efforts to Identify Duplication and Use of Similar Information
5. Impact on Small Businesses or Other Small Entities
6. Consequences of Collecting the Information Less Frequently
7. Special Circumstances Relating to the Guidelines of 5 CFR 1320.5
8. Comments in Response to the Federal Register Notice and
Efforts to Consult Outside the Agency
9. Explanation of Any Payment or Gift to Respondents
10. Protection of the Privacy and Confidentiality of Information Provided by Respondents
11. Institutional Review Board (IRB) and Justification for Sensitive Questions
12. Estimates of Annualized Burden Hours and Costs
13. Estimates of Other Total Annual Cost Burden to Respondents or Record Keepers
14. Annualized Cost to the Government
15. Explanation for Program Changes or Adjustments
16. Plans for Tabulation and Publication and Project Time Schedule
17. Reason(s) Display of OMB Expiration Date is Inappropriate
18. Exceptions to Certification for Paperwork Reduction Act Submissions
References
Exhibits
Exhibit A2a Summary of comparison groups
Table A12-1 Estimated Annualized Burden Hours
Table A12-2 Estimated Annualized Burden Costs
Table A14 Annualized Cost to Government
Table A16 Project Time Schedule
LIST OF ATTACHMENTS
Attachment 1: Authorizing Legislation
Attachment 2: Technical Appendix
Attachment 3: Virginia Medicaid data abstraction
Attachment 4: Virginia Care Marker data abstraction
Attachment 5a: Introductory letter – participants
Attachment 5b: Introductory letter – provider participants
Attachment 5c: Introductory letter – control participants
Attachment 5d: Courtesy letter to providers of participants
Attachment 6a: Study information sheet – providers of participants
Attachment 6b: Study information sheet – provider participants
Attachment 7a: Contact protocol – participants
Attachment 7b: Contact protocol – provider participants
Attachment 7c: Contact protocol – control participants
Attachment 8a: Verbal consent – participants
Attachment 8b: Verbal consent – provider participants
Attachment 8c: Verbal consent – control participants
Attachment 8d: HIPPA authorization
Attachment 9: Phase I interview
Attachment 10: Phase II interview
Attachment 11: Referral script
Attachment 12a: PositiveLinks verbal consent and enrollment
Attachment 12b: PositiveLinks Program and Service Agreement letter
Attachment 13a: Clinician consultation guide
Attachment 13b: Clinician consultation guide (screenshots)
Attachment 14a: Post-consultation questionnaire
Attachment 14b: Post-consultation questionnaire (screenshots)
Attachment 15: PositiveLinks data elements
Attachment 16: Privacy Impact Assessment
Attachment 17a: Certified letter – participants
Attachment 17b: Certified letter – control participants
Attachment 18: Institutional Review Board approval letter
Attachment 19: 60-Day Federal Register Notice
Goals
of study:
To evaluate the efficacy of using administrative insurance and
prescription claims (billing) data to identify and intervene upon
persons with HIV who fail to fill antiretroviral (ARV)
prescriptions.
Intended
use:
Data collected will be used to: identify and intervene upon persons
with HIV who fail to fill ARV prescriptions; determine appropriate
participant referrals to address participants’ adherence
barriers; and to determine the efficacy of study interventions.
Methods
to be used to collect data:
Cluster-randomized
controlled trial with targeted patient- and provider-level
support. The
subpopulation to be studied:
Virginia Medicaid enrollees with HIV, aged 19 – 63 years, who
have either never filled an ARV prescription or who are > 30 to
< 90 days late filling their ARV prescriptions.
How
data will be analyzed:
Logistic regression models will be used to test the difference in
HIV viral suppression in the intervention arm compared to the
control arm after adjusting for potential confounders. The
differences in the means of secondary outcomes between intervention
and control groups will be tested using a t-test, with a linear
regression model used to evaluate the effect after adjusting for
confounders.
Justification
A1. Circumstances Making the Collection of Information Necessary
The Centers for Disease Control and Prevention, Division of HIV Prevention requests OMB approval for three years for a new information collection for a research study entitled “Antiretroviral Improvement among Medicaid Enrollees” (AIMS). This study will evaluate the efficacy of a Data-to-Care strategy that uses administrative insurance and prescription claims (billing) data to identify and intervene upon persons with HIV who fail to fill antiretroviral (ARV) prescriptions.
Despite Department of Health and Human Services’ recommendations that all people with HIV receive antiretroviral therapy (ART), nearly 1 in 5 of the approximately 700,000 people diagnosed and in HIV care in the United States do not receive ART. [1,2] Adherence to ART leads to HIV viral suppression which in turn leads to reduced morbidity, reduced mortality, and decreased risk of HIV transmission. An essential element of the President’s 2019 Ending the HIV Epidemic: A Plan for America initiative is to treat people with HIV rapidly and effectively to reach sustained viral suppression.[3] Effective treatment is one of the initiative’s four key strategies because people with HIV who are adherent to appropriate ART and maintain an undetectable HIV viral load can live long healthy lives with effectively no risk of transmitting HIV to uninfected sexual partners.[4-6] However, among persons with diagnosed HIV, only 57% are in regular HIV medical care and 60% are virally suppressed.[7] The substantial proportion of persons not retained in care has important public health implications; over 40% of new HIV infections are transmitted from persons diagnosed with HIV who are not fully retained in medical care.[8] Additionally, 11% of persons with HIV who are in care but not virally suppress account for another 20% of new infections.[8]
Re-engaging out-of-care persons with HIV back into care confers important individual-level health benefits and population-level prevention benefits. Use of HIV surveillance data to identify out-of-care persons is one strategy for identifying and re-engaging out-of-care persons and is called Data-to-Care or “D2C.” [9] Data-to-Care uses laboratory reports (i.e., CD4 and HIV viral load test results) received by a health department’s HIV surveillance program as markers of HIV care. In this D2C strategy, there is a delay in the identification of out-of-care persons due to the time interval between recommended CD4 and viral load tests (i.e., every 3 to 6 months) and the subsequent reporting of these tests to surveillance. Thus, the current D2C strategy identifies persons already out of care rather than identifying persons at risk for dropping out of care; the current strategy cannot intervene in the short time interval between the start of a gap in care (typically 6 months from the last visit) and the point at which a person is declared out of care. More real-time data are needed to identify persons at risk of dropping out of care and to intervene prior to a gap in care or loss to care.
Administrative prescription claims data are a source to identify HIV-infected persons who have stopped filling ARV medications and who are at risk for becoming out of care. Because most ARVs are prescribed as a 30-day supply of medication, prescription claims data can be used to identify persons who are not filling their medications on a monthly basis.
The purpose of the AIMS study is to develop, implement and evaluate a D2C strategy that uses insurance and real-time prescription claims data to identify 1) persons with HIV who have never been prescribed ART and 2) persons with HIV who fail to pick up prescribed ART medications in a timely manner. These individuals will be targeted either for a provider peer-to-peer counseling intervention or for progressive patient-level adherence and retention interventions. Given the substantial number of persons with HIV who are not on ART (either because ART was never prescribed or because they fail to fill ARV prescriptions) and because HIV viral rebound can occur within weeks of stopping ART, using real time prescription data to identify persons who fail to fill ARV prescriptions and to intervene, could have a significant impact on adherence and viral suppression. Additionally, the strategy could potentially influence retention in care by reducing the timeline to identify persons at risk for failing out of care (as indicated by failing to fill ARV prescriptions).
The AIMS study will advance the understanding of how to use existing, administrative data, in combination with surveillance data, for HIV prevention. The study is aligned with the following operational strategies of the President’s Ending the HIV Epidemic: A Plan for America initiative:[3]
Treat
Prevent
The study is also aligned with the following national HIV prevention goals from the National HIV/AIDS Strategy:[10]
1.B.1 Design and evaluate innovative prevention strategies and combination approaches for preventing HIV infection in high-risk populations and communities and prioritize and promote research to fill gaps in HIV prevention science among the highest risk populations and communities.
1.B.4 Expand prevention with persons living with HIV.
2.A.2 Ensure linkage to HIV medical care and improve retention in care for people living with HIV
2.A.4 Prioritize and promote research to fill gaps in knowledge along the care continuum.
The following section of the U.S. Federal Code is relevant to this data collection: Section 301 of the Public Health Service Act (42 U.S.C.241) which authorizes conduct of “research, investigations, experiments, demonstrations, and studies relating to the causes, diagnosis, treatment, control, and prevention of physical and mental diseases and impairments of man.” (Att 1)
A.2 Purpose and Use of the Information Collected
The AIMS study is a cluster-randomized controlled Data-to-Care education and referral intervention with targeted provider- and patient-level support. The targeted population is persons with HIV who are enrolled in Virginia Medicaid and who have either never filled an ARV prescription or who are > 30 to < 90 days late filling their ARV prescription(s).
We will assess the following outcomes of interest for patients in the intervention group (n=500) and patients in the control group (n=500):
HIV viral suppression (HIV RNA < 200 copies/mL)
Initiation, re-initiation, persistence and adherence to ART, and retention in care.
Because an HIV healthcare provider may provide care to multiple patients, and one component of the intervention is tailored to healthcare providers, the study design relies on cluster randomization at the healthcare provider level. This ensures that the provider intervention is appropriately targeted and that the effects of the provider intervention can be isolated during data analysis. Providers will be randomized to either the intervention arm or to the usual care (i.e., no intervention or control) arm. Study participants are the patients of the randomized healthcare providers. Cluster randomization will be conducted concurrently with the initial screening of potential participants. Participants in the intervention arm will be delegated to either a patient-level or provider-level intervention, depending on need.
Participants who are > 30 to < 90 days late filling their ARV prescription(s) will receive the patient-level intervention (est. N=460). In Phase I, all participants in this group will be interviewed by a study Linkage Coordinator to identify their barriers to ART adherence and then referred to appropriate resources. In Phase II, a more in-depth interview and referral process will be conducted for participants who fail to fill their ARV prescriptions within 30 days of the Phase I interview. Participants in the Phase II interview will also be offered access to PositiveLinks, an evidence-informed mobile application (“app”) which is designed to support ART adherence and retention in care (https://www.positivelinks4ric.com/).
Participants who have never filled an ARV prescription (est. N=40) will be delegated to the provider-level intervention. Participants of the provider-level intervention will not receive direct intervention. Instead, the healthcare providers of these patients (henceforth referred to as “provider participants”) will receive the provider-level intervention. This intervention consists of a peer-to-peer consultation delivered by a clinician from the VDH’s Advisory Committee to the Virginia Medication Assistance Program, or by another HIV clinical expert. Consultations will provide resources and guidance relevant to the needs of the provider and the provider’s patients.
The control group consists of clusters of patients who receive usual care. The clusters will be matched 1:1 with clusters of patients in the intervention group.
Additional information about the study procedures and cluster randomization process is provided in the Technical Appendix (Att 2).
Exhibit A.2.a: Summary of comparison groups
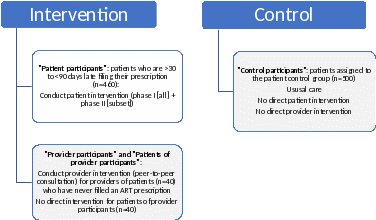

Retrospective data collection (12 months)
Prospective data collection (12 months)
Participants will be followed for 12 months, either prospectively (for the participants of the patient-level intervention) or retrospectively (for the controls and participants of the provider-level intervention). To minimize response bias, which could alter study outcomes (12), consent procedures will be deferred until after the follow-up period for the intervention arm is complete for the following groups: 1) eligible potential participants of the provider-level intervention (i.e., patients of the providers who receive the provider-level intervention) and 2) eligible potential participants in the usual care arm (i.e., control participants).
The AIMS study will use information from five sources: 1) Virginia Medicaid claims abstraction 2) Virginia Care Marker data abstraction 3) Phase I and Phase II patient-level interviews 4) Peer-to-peer clinician consultation and 5) PositiveLinks mobile app abstraction. The purpose and use of each information collection is described below.
Data source: Virginia Medicaid database
The Virginia Medicaid data are existing data that are not collected for the purpose of this study but are routinely collected by DMAS for payment of administrative insurance claims. The Virginia Medicaid database contains demographics and medical diagnosis, procedure and pharmacy claims for Virginia Medicaid enrollees. The database also includes identifying information such as name, contact information, and prescriber information. Secondary data from the Virginia Medicaid database will be abstracted. The purpose of the data abstraction is three-fold: 1) to determine study eligibility 2) to conduct the patient- and provider-level interventions and 3) to determine study outcomes.
A VCU Data Analyst will abstract data elements listed in Att 3 to determine study eligibility. These data will be abstracted monthly until 500 participants of the patient-level intervention and provider participants are enrolled and 500 controls are identified; enrollment is estimated to take six months to complete. These data will be securely transferred to the VDH server where they will be matched to the Virginia Care Marker data; the match will determine study eligibility. Although these database queries will abstract personally identifiable information (PII) no PII will be accessed outside of the DMAS servers (which routinely contain these data) and none will be sent to CDC. The PII collected will also be used to conduct the patient- and provider-level interventions (e.g., PII will be used to contact potential participants and enrollees).
After enrollment, data will be abstracted for participants of the patient-level intervention, quarterly for 12 months. Additionally, a one-time abstraction will occur at the end of the intervention follow-up period for the controls and participants of the provider-level intervention; this abstraction will contain 12 months of data retrospective to the date of consent. A VCU Data Analyst will abstract these data. De-identified data elements listed in Att 3 will be sent to CDC, quarterly, for participants of the patient-level intervention, and one time at the end of the intervention follow-up period for the controls and participants of the provider-level intervention. These data will be analyzed to determine study outcomes (e.g., viral suppression).
Data source: Virginia Care Marker database
The Virginia Care Markers data are existing data that are not collected for the purpose of this study but are routinely collected by VDH for HIV surveillance. The Virginia Care Markers database contains information on all people with HIV in Virginia and includes HIV surveillance data (e.g., HIV viral load, CD4 counts), care reports for persons receiving ADAP benefits, vital status, demographics and some care utilization data for people receiving care within the Ryan White HIV care program (e.g., dates of medical visit, antiretroviral therapy prescriptions). Secondary data from the Virginia Care Markers database will be abstracted. The purpose of the data abstraction is three-fold: 1) to determine study eligibility and 2) to conduct the patient- and provider-level interventions and 3) to determine study outcomes.
A VCU Data Analyst will abstract data elements listed in Att 4 to determine study eligibility. These data will be abstracted monthly until 500 participants of the patient-level intervention and provider participants are enrolled and 500 controls identified; enrollment is estimated to take six months to complete. These data will be matched to the Medicaid data; the match will determine study eligibility. Although these database queries will abstract personally identifiable information (PII) no PII will be accessed outside of the VDH servers (which routinely contain these data) and none will be sent to CDC. The PII collected will also be used to conduct the patient- and provider-level interventions (i.e., PII will be used to contact potential participants and enrollees)
After enrollment, data will be abstracted for participants of the patient-level intervention, quarterly for 12 months. Additionally, a one-time abstraction will occur at the end of the intervention follow-up period for the controls and for participants of the provider-level intervention; this abstraction will contain 12 months of data retrospective to the date of consent. A VCU Data Analyst will abstract these data. De-identified data elements listed in Att 4 will be sent to CDC, quarterly, for participants of the patient-level intervention, and one time at the end of the intervention follow-up period for the controls and participants of the provider-level intervention. These data will be analyzed to determine study outcomes (e.g., viral suppression).
Data source: Patient-level semi-structured interviews
A one-time Phase I semi-structured interview (Att 9) will be administered by a study Linkage Coordinator for participants identified as > 30 to < 60 days late filling ARV prescriptions and a one-time Phase II semi-structured interview (Att 10) will be administered by a study Linkage Coordinator for participants identified as > 60 to < 90 days late filling ARV prescriptions. In Phase II, the participant will also be offered PositiveLinks, an evidence-informed mobile app which is designed to support ART adherence and retention in care (https://www.positivelinks4ric.com/).[11] The purpose of the Phase I and II interviews is to identify participants’ adherence barriers; this information will be used to refer participants to appropriate resources to assist their adherence to ART. The Linkage Coordinator will enter all data from the Phase I and II interviews directly into a REDCap database during the interviews. De-identified data elements will be sent quarterly to CDC for analysis to determine study outcomes.
Data source: Peer-to-peer clinician consultation
A one-time peer-to-peer clinician consultation will be administered by a member of VDH’s Advisory Committee to the Virginia Medication Assistance Program or by another HIV clinical expert. The clinician consultant will use a guided prompt to elicit information which can be used to inform the consultation. (Att 13a and 13b) After the consultation, the clinician consultant will document the provider’s barriers to ART prescribing and recommended resources in a brief post-consultation questionnaire. (Att 14a) The questionnaire will be directly entered into a REDCap database, via a secure link provided by the Linkage Coordinator. (Att 14b) De-identified data elements will be sent quarterly to CDC for analysis to determine study outcomes.
Data source: PositiveLinks mobile app
A Linkage Coordinator will download app data through the app’s administrative web portal. These data will be collected quarterly and include response rates for daily queries about medication, mood and stress, response rates for weekly quizzes, posts to the community message board and messages to the study Linkage Coordinator. (Att 15) The Linkage Coordinator will use Google Analytics to measure app launches. For all participants, the Linkage Coordinator will monitor the community message board daily for misinformation and inflammatory comments (which will be removed). The Linkage Coordinator will monitor direct messages daily to respond to participants’ inquiries. De-identified data elements will be sent quarterly to CDC for analysis to determine study outcomes (Att 15).
Findings from the study will inform strategies for ensuring that time-critical ARV therapy is provided to persons with HIV.
A.3 Use of Improved Information Technology and Burden Reduction
No data will be collected on paper forms. Data will be downloaded directly from the Virginia Medicaid and Virginia Care Marker databases. Because these data will be downloaded from existing data sources, the burden of collection is minimal. Data from the PositiveLinks app will be directly downloaded from the app’s administrative web portal making the burden of collection minimal. Data collected from the Phase I and II interviews and the peer-to-peer clinician consultation will be directly (or through a secure link) entered into a REDCap database. Although both the Phase I and Phase II interviews are mostly designed to be open-ended conversations, anticipated response categories will be programmed into REDCap, and skip patterns and branching logic will be used to reduce the burden of data entry.
A.4 Efforts to Identify Duplication and Use of Similar Information
CDC personnel have conducted extensive computerized searches of PubMED and MEDLINE. While there is available literature detailing traditional D2C programs (i.e., D2C programs that use HIV surveillance data) we could find no literature on D2C programs that used insurance or prescription claims to implement a D2C strategy. In addition, no literature describing use of D2C programs to improve adherence to antiretroviral therapy were identified.
A.5. Impact on Small Businesses or Other Small Entities
The provider-level intervention will involve healthcare providers of HIV patients who have never filled ARV prescriptions. Some of these providers may come from small medical practices. To minimize the intervention time burden on these providers, only one 30-minute peer-to-peer clinician consultation will be conducted, per provider participant. The patient-level intervention will not impact small businesses or other small entities.
A.6 Consequences of Collecting the Information Less Frequently
The purpose of the intervention is to identify persons who fail to fill ARV prescriptions, within 30 – 90 days of the expected fill date, and to quickly intervene. As such, the Virginia Medicaid and Virginia Care Marker data will be abstracted monthly throughout enrollment of participants of the patient-level intervention and of provider participants, until the study has reached the enrollment threshold. Collecting the data less frequently disables the identification of (and subsequent intervention upon) individuals who are between 30 and 90 days late filling ARV prescriptions. Failure to intervene upon these individuals could result in HIV viral rebound and increased transmission risk. After enrollment into the patient-level intervention is complete, data from the Virginia Medicaid and Virginia Care Marker databases will be abstracted quarterly. Although data will not be downloaded more than quarterly from the PositiveLinks app, the study Linkage Coordinator will monitor the direct messaging and the community message board daily. Daily monitoring of direct messaging is necessary for the Linkage Coordinator to respond to participants in a timely manner. Similarly, daily monitoring of the community message board is necessary to remove posted misinformation. The Linkage Coordinator will also monitor app launches at one, two, four and twelve weeks to address any usability and accessibility issues participants might be experiencing.
A.7. Special Circumstances Relating to the Guidelines of 5 CFR 1320.5
This request fully complies with the regulation 5 CFR 1320.5.
A.8. Comments in Response to the Federal Register Notice and Efforts to Consult Outside the Agency
A 60-day Federal Register Notice was published in the Federal Register on 07/12/2021, Volume 86, Number 130] Pages 36550 – 36552. (Att 19) There were no public comments.
The development of study data procedures was a collaborative effort between CDC, Virginia Commonwealth University, Virginia Department of Health, Virginia Department of Medical Assistance Services, University of Virginia, and the National Institute of Mental Health. The following persons have reviewed the data procedures for content, clarity, frequency of collection and necessity. Each individual was consulted in 2019, 2020 or 2021 and each is either a subject matter expert on HIV, HIV surveillance, HIV programs, Medicaid services and data, or epidemiology/statistics.
April Kimmel, Principal Investigator Virginia Commonwealth University 800 East Leigh St, Suite 3200 Richmond, VA 23298 804.628.6273 |
Kate Gilmore, Data Manager and Project Coordinator Virginia Department of Health 109 Governor St. Richmond, VA 23219
|
Bassam Dahman, Statistician Virginia Commonwealth University 800 East Leigh St, Suite 3200 Richmond, VA 23298 804-628-3443 |
Lauryn Walker, Senior Advisor, Chief Deputy and Chief Health Economist Virginia Department of Medical Assistance Services 600 E Broad St. Richmond, VA 23219
|
Chelsea Canan, Lead HIV Epidemiologist Virginia Department of Health 109 Governor St. Richmond, VA 23219 |
Rebecca Dilingham University of Virginia |
Hunter Robertson, Program Analyst Virginia Department of Health 109 Governor St. Richmond, VA 23219 |
Michael Stirratt, Project Officer National Institute of Mental Health 6001 Executive Blvd Bethesda, MD 20892 240.627.3875 |
A.9. Explanation of Any Payment or Gift to Respondents
No payment or gifts will be provided to participants for any data collection activity.
A.10. Protection of the Privacy and Confidentiality of Information Provided by Respondents
Virginia Commonwealth University will be given DMAS and VDH affiliate status which allows them to access the Virginia Medicaid and Virginia Care Marker databases on the DMAS and VDH servers, respectively. Data necessary for the study will be placed in study specific files on the secure DMAS and VDH servers by DMAS and VDH personnel. VCU will only have access to the study files. VCU will have access to these data through study-specific amendments to existing cross-agency and cross-institutional data use agreements.
The CDC National Center for HIV/AIDS, Viral Hepatitis, STD and TB Prevention (NCHHSTP) Government Information Specialist in the Privacy Unit, Office of the Chief Information Officer reviewed this submission and determined that the Privacy Act applies to this activity: Privacy Act System of Records Notice (SORN) 09-20-0136 (Epidemiologic Studies and Surveillance of Disease Problems). (Att 16) However, no personally identifiable data collected during the study will be transmitted to CDC.
Consistent with Section 301(d) of the Public Health Service Act, a Certificate of Confidentiality (CoC) applies to this research because this research is funded or supported by CDC and the following are true: the research involves Human Subjects as defined by 45 CFR Part 46; the research involves information about an individual for which there is at least a very small risk, that some combination of the information, a request for the information, and other available data sources could be used to deduce the identity of an individual. The Certificate of Confidentiality protects the privacy of subjects by limiting the disclosure of identifiable, sensitive information; the research team cannot be forced (e.g., court subpoena) to disclose identifying information from study participants for any civil, criminal, administrative, legislative, or other proceeding, whether at the federal, state, or local level.
Two databases used in this study (Virginia Medicaid and Virginia Care Marker) routinely contain personally identifiable information. These existing data are not collected for the purpose of this study but are routinely collected by the Virginia Department of Medical Assistance Services (Virginia Medicaid) for payment of administrative insurance claims and the Virginia Department of Health for HIV surveillance. The study will use identifiable information to identify persons for intervention (e.g., identifiable data will be used to determine persons late filling ARV prescriptions and to link the Virginia Medicaid and Virginia Care Marker datasets) and to facilitate the collection of response data (e.g., names and telephone numbers will be used to contact participants for patient- and provider-level interventions, name of participants’ Medicaid Managed Care Organization will be used to refer participants to appropriate resources). These identifiable data will remain on the DMAS and VDH servers (which routinely contain this information)—no identifiable data will be downloaded to VCU servers and none will be sent to CDC.
The following is a summary of procedures used to keep participant information secure:
Virginia Department of Medical Assistance Services (DMAS – Virginia Medicaid)
There will be no paper data files kept at DMAS for this study. All data will be electronic. All DMAS study data will be stored and encrypted on DMAS servers. Data security for DMAS is managed by DMAS and the Virginia Information Technology Agency (VITA). Access to DMAS servers requires either a VITA-secure laptop, or secure software token with VITA controls. Only authorized project personnel will have access to the data for study purposes. Minimum standards for data storage and handling include the following: confidential and protected data are only stored on centrally managed network servers; sensitive data are only stored on centrally managed network servers which are backed up according to agency standards and encrypted with industry-approved algorithms; point-to-point encryption is used when accessing confidential and protected data; remote access to servers is encrypted using agency-approved tools; offsite computers used for remote access to servers must meet agency-approved Workstation Security Standards.
Study data transferred across Virginia state agencies (i.e., between DMAS and VDH) will be uploaded and downloaded from a secure File Transfer Protocol (FTP) server managed according to HIPAA physical, administrative, and technical security safeguards by DMAS and Virginia Commonwealth University; the data transfer will comply with all US regulations regarding the security of identifiable research information.
Virginia Department of Health
There will be no paper data files kept at VDH for this study. All data will be electronic. All VDH computers containing electronic surveillance data are inside locked rooms and floors. Authorized VDH staff have access to the common work areas; however, only authorized staff have access to electronic data systems and/or file rooms containing hard copy records.
Managers within the Division of Disease Prevention (DDP) approve all network access, for Division staff, via Active Directory Security Groups. These Security Groups control access to specific data and files. Security Groups are also used to provide access to non-web-based data systems. Maintenance of network accounts is performed by VITA. Staff must follow the Procedures for Establishing and Managing Network Domain and DDP Database Access Accounts to have Commonwealth of Virginia (COV) account access, remote email, and database/data system access. Confidential databases used by the Division are maintained solely by Division staff. Database access is structured with rights limited to staff whose job requires such access. User accounts and rights are set up and maintained by the applicable Data Manager or designated back up. Any changes to user accounts must be approved by Division management.
VDH contracts with VITA for all IT network operations. All confidential databases are maintained within the COV network; however, user rights to specific servers, files and databases are controlled by the Active Directory Security Groups. All network users have unique passwords that require forced changes at defined periods. Standard security configurations, including network access and password management, are maintained by VITA and follow Information Technology Resource Management standard policies and procedures prepared by VDH.
Code key
A unique randomly created 9-digit linking code will be created for each participant in the study. Participants will be assigned the code, during screening for eligible potential participants, on the DMAS servers, to minimize the electronic transfer of personally identifiable information between VDH and DMAS. The code key will allow re-identification of participants in the de-identified analytic dataset. Re-identification of participants cannot occur without both the linking code and a code key dataset. The key dataset, that includes the link between the original identifiers and the newly created code for each participant, will be saved on the VDH server in a secured password protected folder separate from both the original data and from the de-identified analytic dataset. Only a VDH Data Security Manager will have access to the key data. The code key will be destroyed at the end of the study once the minimum time required for data retention has been met as per VCU Data Retention Policy.
University of Virginia
Data collected via the PositiveLinks app will be stored on the PositiveLinks secure server, a password-protected and encrypted virtual web platform accessible only to study team members. Data collected from the app, along with PositiveLinks usernames, will be securely transmitted electronically to secure University of Virginia servers. These servers comply with University of Virginia policies and standards including the Data Protection of University Information policy, Institutional Data Protection Standards, and Security of Network- Connected Devices Standard (https://security.virginia.edu/data-protection).Data retained at University of Virginia will not include name or date of birth or other identifiers. For final documentation purposes, University of Virginia will transfer a complete copy of PositiveLinks data to VCU using a secure FTP transfer after the 12-month intervention follow-up period is complete.
Virginia Commonwealth University
Study data will be securely stored on DMAS and VDH servers and will be analyzed by authorized VCU personnel. VCU project personnel will access the DMAS server via a remote, secure web-based system using VITA-secured DMAS laptops or software to access the data. Remote access will occur at individual workstations physically located in an office building with various levels of physical access controls, including security guards, card access, and locking department and office doors which are locked when vacant. Only authorized project personnel will have access to computer output. A data use agreement between VCU and DMAS will incorporate confidentiality standards and limitations on use of the Medicaid data to ensure data security and ethical standards of data use are met.
VCU project personnel will access Virginia Care Marker data via remote, secure Virtual Private Network (VPN). VCU will use VDH-provided and VDH-maintained laptops and will follow policies set by the VDH Security and Confidentiality Procedures. Access to workstations requires an authorized COV account. Dual factor authentication is required to sign into the VDH server via VPN. VCU project personnel’s individual workstations are physically located in an office building with various levels of physical access controls, including security guards, card access, and locking department and office doors which are locked when vacant. Logical access to workstations requires an authorized Active Directory account. A data use agreement between VCU and DMAS will incorporate confidentiality standards and limitations on use of the VDH data to ensure data security and ethical standards of data use are met.
No identifiable individual-level data will be stored at VCU. Only de-identified analytic datasets will be transferred and downloaded onto VCU servers. VCU personnel will not be able to re-identify participants in the de-identified analytic dataset. The dataset will be protected from improper use and disclosure through use of university-wide data security standards as outlined in the VCU Research Data Ownership, Retention, Access, and Security policy. Only authorized persons at VCU will have access to these data.
Centers for Disease Control and Prevention
No identifiable data will be sent to CDC. Virginia Commonwealth University will construct de-identified analytic datasets. All study data will be de-identified and all PII elements will be removed from the original data, and a new de-identified analytic dataset will be created in accordance with HIPAA regulations and 45 CFR 164.514. Participant names, social security numbers, medical record or plan numbers, and Medicaid identification and account numbers will be removed from the analytic datasets. All contact information, such as telephone numbers or street addresses will be removed from the data. Units of geographic analysis smaller than the state level (e.g., county) will be retained; however, street addresses will be removed.
Only de-identified analytic datasets will be sent to CDC. CDC will not be able to re-identify participants in the de-identified analytic dataset. These datasets will be sent to CDC through the CDC Secure Data Network. All data transmissions are automatically encrypted by the software that generates the transfer files. Security certificates are used to control access to the Secure Data Network.
In addition, the following procedures will be used to protect participant records:
Data records, received by CDC, will only be identified by a unique participant ID number.
All study data will be encrypted and stored on a secure CDC server.
Only authorized and authenticated CDC-based study staff will have access to the data at CDC.
CDC study staff will complete the computer-based Collaborative Institutional Training Initiative (CITI) research ethics and compliance training.
CDC study staff will complete the Information Security Awareness Training annually.
Papers and presentations, on study results, will report aggregated information and will not contain any identifying information that can be traced back to a study participant.
Patient-level intervention
The introductory letter sent to potential eligible participants will include safeguards (e.g., including a general description about the nature of the study and general study team contact information) to protect confidentiality and minimize the risk of HIV status disclosure. For example, the letter will state that the research study is about adherence to prescribed medication, versus adherence to prescribed HIV medication. The introductory letter will include limited personal information or study-specific contact information to maintain confidentiality. The letter will include a unique ID to use during identity verification that will occur during the consenting process.
Identity verification procedures will be implemented for the patient-level intervention to ensure the correct person is reached during recruitment and consent (Att 7a). These procedures include having the Linkage Coordinator ask potential participants to provide the unique participant ID that was listed on the eligible participant’s introductory letter, and to provide their name and date of birth before providing any information about the study. Participants who do not wish to confirm their identity may request that they be sent a certified letter. (Att 17a) The certified letter will explain the study and provide contact information for the study Linkage Coordinator. With certified letters, the addressee must sign to accept the letter, decreasing the chance that the letter reaches an unintended participant. Additionally, the Linkage Coordinator will ensure that participants feel they can freely engage in a conversation before beginning to describe the study, beginning the consent process, or beginning the intervention. If a participant feels that they cannot, at the time of the Linkage Coordinator’s contact, freely converse with the Linkage Coordinator, the participant may choose to reschedule the call for when they can speak more privately, move to a more private location for the phone call, or to not participate. Similar procedures will be implemented for the control participants and participants of the provider-level intervention. (Att 7c and 17b)
PositiveLinks mobile application
The mobile application comes with several privacy features. The application is not currently available on the Google Play or the Apple App stores so it cannot be downloaded by the general public. The app’s messaging and calling features are contained entirely within the application so that messages and calls made through the app’s interface will not appear in the phone’s message or call logs. Use of the community message board requires participant selection and use of a username to protect anonymity. Lastly, opening the application requires a password set by the participant.
A.11. Institutional Review Board (IRB) and Justification for Sensitive Questions
IRB protocol
The study protocol has been approved by the Virginia Commonwealth University IRB. (Att 18)
Sensitive Questions
A Linkage Coordinator will administer a Phase I and II semi-structured interview during the patient-level intervention. The interview is meant to be a fluid conversation with the participant to elicit the participant’s adherence barriers. During the interviews, the Linkage Coordinator will probe for adherence barriers; the participant could raise topics that might be considered sensitive (e.g., substance use)—the Linkage Coordinator, however, will not directly ask the participant any sensitive questions. No sensitive questions will be asked of providers during the peer-to-peer consultation (provider-level intervention).
A.12. Estimates of Annualized Burden Hours and Costs
A. Estimated Annualized Burden Hours
The following burden estimates are based on the annualized number of respondents.
A one-time study consent for all participants and provider participants will be conducted by a study Linkage Coordinator. The consent process is estimated to take 15 minutes per person. The burden hours for the consent process are: 38 hours for the 153 participants of the patient-level intervention (Att 8a);3 hours for the 13 provider participants of the provider-level intervention (Att 8b); 3 hours for the 13 participants of the provider-level intervention (Att 8c); and 42 hours for the 167 control participants (Att 8c). Obtaining HIPPA authorization is estimated to take 5 minutes per person. The burden hours for obtaining HIPPA authorization are: 13 hours for the 153 participants of the patient-level intervention (Att 8d); 1 hour for the 13 participants of the provider-level intervention (Att 8d); and 14 hours for the 167 control participants (Att 8d). Time for contacting participants is included in the estimated time for consent and HIPPA authorization (Att 7a, Att 7b and Att 7c). The PositiveLinks verbal consent and enrollment process is estimated to take 1 hour to complete. (Att 12a) Thirty-three participants will enroll in PositiveLinks resulting in a total of 33 burden hours.
A one-time Phase I semi-structured interview will be administered, by a study Linkage Coordinator, for approximately 153 participants. (Att 9) The Phase I interview is estimated to take approximately 30 minutes to complete. A total of 77 hours will be spent collecting these data. A Phase II semi-structured interview will be administered, by a study Linkage Coordinator, for approximately 33 participants. (Att 10) The Phase II interview is estimated to take approximately 30 minutes to complete. A total of 17 hours will be spent collecting these data. Time for referral to resources to address participants’ adherence barriers (Att 11) is included in the estimated time to complete the Phase I and Phase II interviews. A peer-to-peer clinician consultation will be conducted by 3 clinician consultants from the Advisory Committee to the Virginia Medication Assistance Program or by another HIV clinical expert, for approximately 13 provider participants. (Att 13a) The clinician consultation is estimated to take approximately 30 minutes to complete for a total of 13 burden hours (6 burden hours for the clinician consultants and 7 burden hours for the provider participants). The clinician consultants will complete a post-consultation questionnaire for each peer-to-peer clinician consultation they complete. (Att 14a) The post-consultation questionnaire is estimated to take 10 minutes to complete. It is anticipated that 3 clinician consultants will each complete 4 post-consultation questionnaires for a total of 2 burden hours.
Table A12-1: Estimated Annualized Burden Hours
Respondents |
Form Name |
Number of respondents |
Number of responses per respondent |
Average burden per response (in hours) |
Total burden hours |
Participants of patient-level intervention |
Verbal consent—participants Att 8a |
153 |
1 |
15/60 |
38 |
Provider participants |
Verbal consent—provider participants Att 8b |
13 |
1 |
15/60 |
3 |
Participants of provider-level intervention |
Verbal consent— control participants Att 8c |
13 |
1 |
15/60 |
3 |
Control participants |
Verbal consent—control participants Att 8c |
167 |
1 |
15/60 |
42 |
Participants of patient-level intervention |
HIPPA authorization Att 8d |
153 |
1 |
5/60 |
13 |
Participants of provider-level intervention |
HIPPA authorization Att 8d |
13 |
1 |
5/60 |
1 |
Control participants |
HIPPA authorization Att 8d |
167 |
1 |
5/60 |
14 |
PositiveLinks participants |
PositiveLinks verbal consent and enrollment Att 12a |
33 |
1 |
60/60 |
33 |
Participants of patient-level intervention |
Phase I interview Att 9 |
153 |
1 |
30/60 |
77 |
Participants of patient-level intervention |
Phase II interview Att 10 |
33 |
1 |
30/60 |
17 |
Advisory Committee to the Virginia Medication Assistance Program member and other HIV clinical experts |
Clinician consultation guide Att 13a |
3 |
4 |
30/60 |
6 |
Provider participants |
Clinician consultation guide Att 13a |
13 |
1 |
30/60 |
7 |
Advisory Committee to the Virginia Medication Assistance Program member and other HIV clinical experts |
Post-consultation questionnaire Att 14a |
3 |
4 |
10/60 |
2 |
Total |
|
|
|
|
256 |
The annualized burden cost is $6,909. The median hourly wage for participants of the patient-level and provider-level interventions and of the control participants is $21.74. Provider participants and clinician consultants, from the Advisory Committee to the Virginia Medication Assistance Program member or other HIV clinical experts, will engage in the peer-to-peer clinician consultation (provider-level intervention). These individuals will generally be physicians with a median hourly wage of $98.90. All estimates of hourly wage rates are based on the May 2020 Bureau of Labor Statistics, State Occupational Employment and Wage Estimates for Virginia.
Table A12-2: Estimated Annualized Burden Costs
Respondents |
Form Name |
Number of respondents |
Number of responses |
Avg. burden per response |
Hourly wage rate |
Total burden cost |
Participants of patient-level intervention |
Verbal consent—participants Att 8a |
153 |
1 |
15/60 |
$21.74 |
$832 |
Provider participants |
Verbal consent— provider participants Att 8b |
13 |
1 |
15/60 |
$98.90 |
$321
|
Participants of provider-level intervention |
Verbal consent— control participants Att 8c |
13 |
1 |
15/60 |
$21.74 |
$71 |
Control participants |
Verbal consent— control participants Att 8c |
167 |
1 |
15/60 |
$21.74 |
$908
|
Participants of patient-level intervention |
HIPPA authorization Att 8d |
153 |
1 |
5/60 |
$21.74 |
$277 |
Participants of provider-level intervention |
HIPPA authorization Att 8d |
13 |
1 |
5/60 |
$21.74 |
$24 |
Control participants |
HIPPA authorization Att 8d |
167 |
1 |
5/60 |
$21.74 |
$303 |
PositiveLinks participants |
PositiveLinks verbal consent and enrollment Att 12a |
33 |
1 |
60/60 |
$21.74 |
$717 |
Participants of patient-level intervention |
Phase I interview Att 9 |
153 |
1 |
30/60 |
$21.74 |
$1,663 |
Participants of patient-level intervention |
Phase II interview Att 10 |
33 |
1 |
30/60 |
$21.74 |
$359 |
Advisory Committee to the Virginia Medication Assistance Program member and other HIV clinical expects |
Clinician consultation guide Att 13a |
3 |
4 |
30/60 |
$98.90 |
$594 |
Provider participants |
Clinician consultation guide Att 13a |
13 |
1 |
30/60 |
$98.90 |
$642 |
Advisory Committee to the Virginia Medication Assistance Program member and other HIV clinical expects |
Post-consultation questionnaire Att 14a |
3 |
4 |
10/60 |
$98.90 |
$198 |
Total |
|
|
|
|
|
$6,909 |
A.13. Estimates of other Total Annual Cost Burden to Respondents or Record Keepers
There are no direct costs to respondents other than their time to participate in the data collection.
A.14. Annualized Cost to the Government
Table A14: Annualized Cost to the Government
|
Federal salary grade |
Salary |
% effort |
Annualized cost |
Co-operative agreement grant |
---- |
---- |
---- |
$549,784 |
CDC Project Officer |
GS 14-10 |
$149,129 |
50% |
$74,566 |
Project Coordinator |
Contractor |
$67,523 |
50% |
$33,762 |
CDC Statistician |
GS 14-10 |
$149,129 |
20% |
$29,826 |
CDC Data manager |
Contractor |
$81,487 |
20% |
$16,297 |
CDC travel |
---- |
---- |
---- |
$5,000 |
Total |
|
|
|
$709,235 |
The annualized cost to the government is $709,235. The information collection described in this request will be funded, coordinated and managed through a cooperative agreement with the grantee, Virginia Commonwealth University. The federal personnel involved in the AIMS study include a Project Officer at the GS 14 equivalent level, a Statistician at the GS-14 level, a Project Coordinator and a Data Manager both of whom are CDC contractors. Travel is for conducting site visits.
Salary estimates were obtained from the US Office of Personnel Management salary scale at https://www.opm.gov/policy-data-oversight/pay-leave/salaries-wages/salary-tables/pdf/2021/ATL.pdf.
A.15. Explanation for Program Changes or Adjustments
This is a new data/information collection.
A.16. Plans for Tabulation and Publication and Project Schedule
Table A16 Project Time Schedule
Activity |
Time Schedule |
OMB approval |
6 months after submission |
Participant enrollment |
1 – 6 months after OMB approval |
Patient-level intervention |
1 – 9 months after OMB approval |
Provider-level intervention |
1 – 9 months after OMB approval |
Data collection |
1 – 21 months after OMB approval |
Analyses of study outcomes |
21 – 33 months after OMB approval |
Dissemination of study outcomes |
27 – 36 months after OMB approval |
A.17. Reason(s) Display of OMB Expiration Date is Inappropriate
No data will be recorded on paper files; all data collection will be electronic.
A.18. Exceptions to Certification for Paperwork Reduction Act Submissions
There are no exceptions to the certification.
References
1. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV. Department of Health and Human Services. Available at http://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf. Accessed January 28, 2020.
2. Iqbal K, Huang YA, Peters P, et al. Antiretroviral treatment among commercially insured persons living with HIV in an era of universal treatment in the United States - 2012-2014. AIDS Care 2018; 30 (9): 1128-1134.
3. Fauci AS, Redfield RR, Sigounas G, et al. Ending the HIV epidemic: A plan for the United States. Jama 2019; 321 (9): 844-845.
4. Cohen MS, Chen YQ, McCauley M, et al. Antiretroviral therapy for the prevention of HIV-1 transmission. N Engl J Med 2016; 375 (9): 830-839.
5. Rodger AJ, Cambiano V, Bruun T, et al. Risk of HIV transmission through condomless sex in serodifferent gay couples with the HIV-positive partner taking suppressive antiretroviral therapy (partner): Final results of a multicentre, prospective, observational study. Lancet 2019; 393 (10189): 2428-2438.
6. Rodger AJ, Cambiano V, Bruun T, et al. Sexual activity without condoms and risk of HIV transmission in serodifferent couples when the HIV-positive partner is using suppressive antiretroviral therapy. JAMA 2016; 316 (2): 171-181.
7. Centers for Disease Control and Prevention. Monitoring selected national HIV prevention and care objectives by using HIV surveillance data—United States and 6 dependent areas, 2016. HIV surveillance supplemental report 2018; 23(no. 4). Http://www.Cdc.Gov/hiv/library/reports/hiv-surveillance.Html. Published June 2018. Accessed [date].
8. Li Z, Purcell DW, Sansom SL, et al. Vital signs: HIV transmission along the continuum of care - United States, 2016. MMWR Morb Mortal Wkly Rep 2019; 68 (11): 267-272.
9. Centers for Disease Control and Prevention. Data to care. Available at: https://effectiveinterventions.cdc.gov/en/2018-design/data-to-care/group-1/data-to-care.
10. Office of National AIDS policy. National HIV/AIDS strategy for the United States. Updated to 2020. Available at: https://files.hiv.gov/s3fs-public/nhas-update.pdf. Accessed October 15, 2019.
11. Centers for Disease Control and Prevention. Compendium of evidence-based interventions and best practices for HIV prevention. Division of HIV/AIDS Prevention, Atlanta, GA. https://www.cdc.gov/hiv/research/interventionresearch/compendium/index.Html. Accessed January 28, 2020.
12. McRae AD, Weijer C, Binik A et al. Who is the research subject in cluster randomized trials in health research? Trials 2011; 12: 183.
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | Byrd, Kathy K. (CDC/DDID/NCHHSTP/DHPSE) |
| File Modified | 0000-00-00 |
| File Created | 2021-10-20 |
© 2026 OMB.report | Privacy Policy