2694ss01
2694ss01.docx
EPA's Vaccination and Testing Status System
OMB: 2030-0052

UNITED STATES ENVIRONMENTAL PROTECTION AGENCY
WASHINGTON, D.C. 20460
MEMORANDUM
SUBJECT: Emergency Information Collection Request for EPA’s Vaccination and Testing Status System (OMB Control Number 2030-NEW; EPA ICR Number 2694.01)
FROM: Daniel Coogan, Acting Director
Office of Resources and Business Operations
THRU: Courtney Kerwin, PRA Clearance Officer
and Director for Regulatory Support Division
Office of Enterprise Information Programs
TO: Danielle Jones, OMB Desk Officer
Office of Information and Regulatory Affairs
Office of Management and Budget
Pursuant to section 3507(j) of the Paperwork Reduction Act (PRA) (44 U.S.C. 3501 et seq.), as implemented in the Office of Management and Budget (OMB) regulations at 5 CFR §1320.13, the Environmental Protection Agency (EPA) is hereby requesting emergency processing of an information collection necessary for vaccination status and testing results of EPA employees, contractors and grantee recipients that work in EPA facilities. EPA has authority to collect this information under the System of Record Notice for the agency’s Public Health Emergency Workplace Response System, EPA-89.
Following the normal clearance procedures for approval of this information collection during the COVID-19 pandemic response will delay the Agency’s ability collect vaccination status and testing results and to adequately protect its workforce. EPA certifies the requirements of 5 CFR 1320.13(a) are met and it is vital for this collection to be implemented immediately, because: (1) this information is necessary to protect EPA’s workforce, (2) public harm is reasonably likely to result if normal clearance procedures are followed, and (3) an unanticipated event has occurred.
Information Collection is Essential to the Mission of the Agency
The Safer Federal Workforce Task Force’s “COVID-19 Workplace Safety: Agency Model Safety Principles,” dated July 29, 2021, requires employers to ask about the vaccination status of Federal employees and onsite contractors. The guidance states that Federal employees and onsite contractors must sign an attestation confirming their vaccination status, or they will be treated as not fully vaccinated for purposes of safety protocols. Federal agencies also must establish a program to test not fully vaccinated Federal employees and onsite contractors weekly or twice-weekly. EPA is including additional information in this request related to test results and certification of vaccination status and test results. While EPA has no intention of collecting the certification of vaccination status or test results at this time, the agency understands that dealing with COVID-19 is a dynamic environment with ever-changing guidance from OMB. EPA aim to establish a forward-looking information collection structure such that should new direction come from OMB, EPA will be prepared to adapt the application it uses to manage this information and will not have to submit another ICR.
Information Collection Activities Involved in this Emergency Request
Because of the substantial risk to life, safety, or health of the workforce and the public, EPA requests an emergency approval to collect the necessary information on vaccination status and testing results from detailees, interns, volunteers, grantee recipients and contractors that perform work in EPA facilities.
Each item of information requested is based on CDC or industry best practice for tracking vaccination. This information is necessary to identify the vaccination status of EPA personnel and testing results of individuals entering EPA facilities. Including contractors, interns, grantees, and volunteers, enables EPA to capture the total workforce and take appropriate action.
The following information will be collected for COVID Contact Testing:
Name;
Vaccination status;
Documentation of vaccination status;
Vaccine brand;
Date of second vaccine shot (or first shot for J&J);
COVID test results; and
Documentation of COVID test results.
EPA Cannot Reasonably Comply with the Normal Clearance Procedures
EPA cannot reasonably comply with the normal clearance procedures because: (1) an unanticipated event has occurred; and (2) public harm is reasonably likely to result if normal clearance procedures are followed.
As the COVID-19 pandemic continues to grow, the full scope of the impact to Americans remains unknown. We do know however that for government offices to safely open, a robust method for tracking vaccination status and testing results must be implemented. Tracking vaccination status and testing results is a critical tool to control the spread of COVID-19. Delay in approval of this information collection will disrupt EPA’s ability to protect the EPA workforce. In order to properly protect its workforce of federal employees, contractors, volunteers, grantees, and interns, EPA must be aware of vaccination status and testing results to prevent the further spread of COVID-19. It would be impracticable and contrary to the public health to delay implementing this collection of information collection until after EPA has completed the normal PRA clearance procedures.
Agency Has Taken All Practicable Steps to Consult with Affected Parties in Order to Minimize Burden
To formulate the data elements to be collected for proper and efficient contact tracing, EPA consulted the Safer Federal Workforce Task Force and EPA Public Health experts.
Requested Time Period for OMB Action
EPA requests that OMB take action on this request by September 8, 2021.
Thank you for your assistance in processing this request. Should any questions arise, please contact me at 202-564-1862 or Courtney Kerwin at 202-566-1669.
ATTACHMENT 1: Estimated Annual Burden and Costs for This Information Collection Activity
Type of Respondent |
Form Name |
Number of Respondents |
Number of Responses per Respondent |
Number of Responses (Total) |
Average Burden per Response |
Total Annual Burden (hours) |
Contractors, Interns, Grantees, and Volunteers |
Vaccination and Testing Status System |
20,000 |
2 |
40,000 |
.05 |
2,000 |
Bottom Line Burden
Respondents: 20,000
Responses: 40,000
Hours: 2,000
Capital/O&M Costs: $0
ATTACHMENT 2: Information Collection Instrument
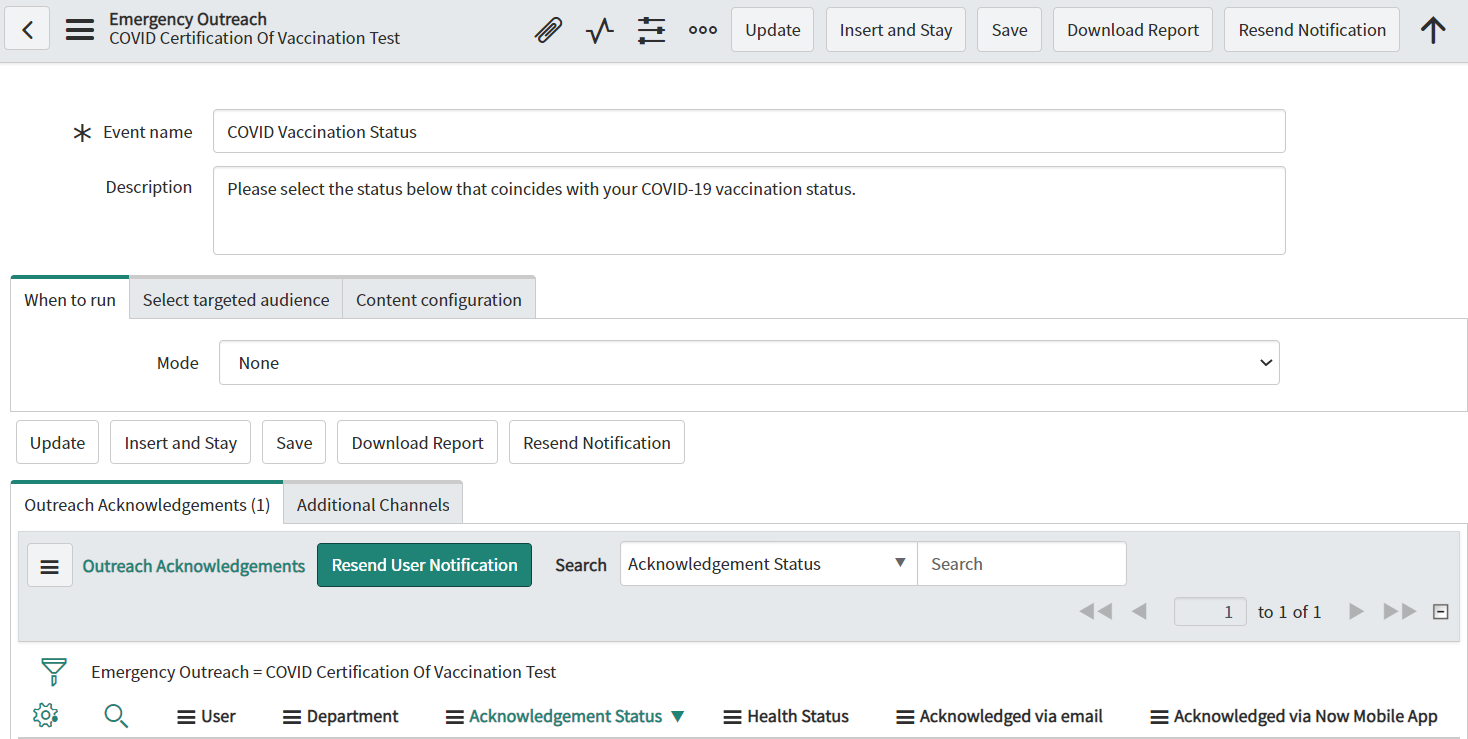
ATTACHMENT
3: Email to Employees
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | Kerwin, Courtney |
| File Modified | 0000-00-00 |
| File Created | 2021-09-06 |
© 2026 OMB.report | Privacy Policy