Tox Survey Questions
OMB Request_NFLIS-Tox Survey Questions 04-29-20.docx
The National Forensic Laboratory Information System Collection of Analysis Data
Tox Survey Questions
OMB: 1117-0034
Change request for the National Forensic Laboratory Information System (NFLIS) 2020 Toxicology Survey:
OMB Control No: 1117-0034
The Drug Enforcement Administration (DEA), Diversion Control Division, is requesting approval of changes made to the previously approved National Forensic Laboratory Information System (NFLIS) 2020 Toxicology (NFLIS-Tox) Survey. DEA had shared the survey instrument with OMB in June 2019 with its formal package request.
This document summarizes the instrument modifications and provides reasons for their importance. The changes were made based on:
ongoing project needs to ensure toxicology laboratory frame integrity;
an internal review of the data from the NFLIS-Tox 2017 Survey, which included an assessment of the data quality and how those data were subsequently used;
a four-member expert panel that reviewed the survey at the Annual Meeting of the Society of Forensic Toxicologists in October 2019; and
cognitive interviews with five participants conducted during March and April 2020.
Following is a summary of the changes:
Seven questions from the NFLIS-Tox 2017 Survey were eliminated from the 2020 survey because the data received were not useful, had low item response, and/or did not serve the project goals. The eliminated questions are documented in Table 1 in the appendix.
Sixteen questions were modified slightly based on the 2017 data assessment and feedback from DEA, the expert panel, cognitive interviews, or editorial review. The changes to these questions are summarized in Table 2 in the appendix, reflect mostly modest edits to the question stem and/or response options, and were made to enhance clarity and data quality and/or to reduce burden.
Ten new questions were added, and two substantive changes were made to existing questions. The following narrative summarizes each change.
On the basis of the expert panel and recent cognitive interviews, the survey is still estimated to take between 30 to 60 minutes to complete.
New Questions and Substantive Changes
The NFLIS team added the following five new questions to ensure toxicology laboratory frame integrity:
New Question 5 asks whether the laboratory conducts only alcohol toxicology testing services. DEA is not interested in alcohol-only toxicology laboratories, so these laboratories are ineligible for the NFLIS-Tox Survey. Respondents indicating that their laboratories conduct only alcohol toxicology testing will be skipped to the end of the survey and thanked for their responses. Capturing ineligibility status of these laboratories is important to ensure frame integrity for NFLIS-Tox. Because many ineligible laboratories in 2020 might be privately owned and operated, they can therefore change service offerings based on client needs. Moving forward, it will be important for DEA to stay abreast of their eligibility status in future surveys.

New Questions 8, 9, 10, and 26 were added regarding reference laboratories. Since the last survey administration, we have identified a handful of new private laboratories that serve the forensic and clinical communities. Questions 8–10 were added to ask about reference laboratories, which are used when a laboratory does not have the capability or capacity to test for specific substances. Question 8 is a yes/no question that asks respondents whether they use reference laboratories. If they do not, they skip to Question 10, which asks if their laboratory serves as a reference laboratory to others. Question 9 asks respondents to identify the laboratory names, cities, and States of reference laboratories they use. The data from this question series will help ensure frame integrity because the analytic team will be able to cross-check responses with the toxicology laboratory universe.

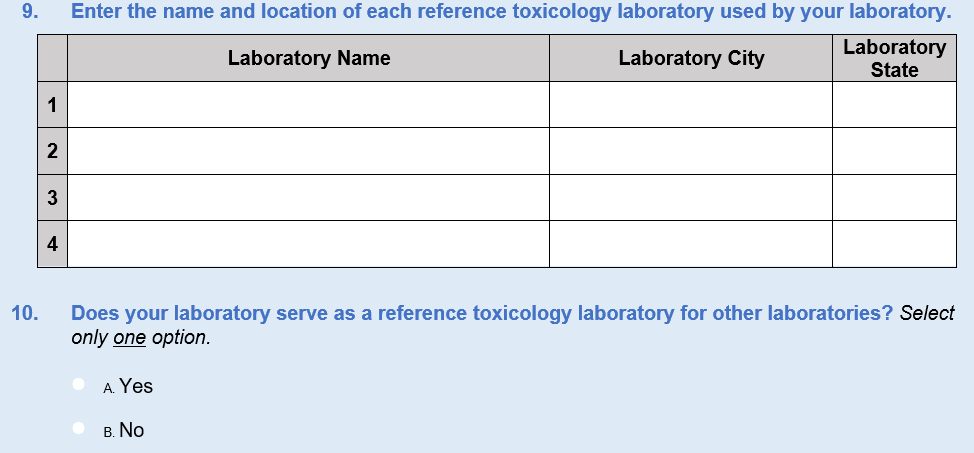
We added Question 26 to inquire about whether the results of samples sent to these laboratories are incorporated into the information management systems. The response options for this new question are yes, no, and “we do not send samples to a reference laboratory.” This information will provide a secondary check on the use of reference laboratories in general and give DEA valuable information about the accessibility and availability of these toxicological data for future reporting.

Three new questions and two substantial revisions were included in the 2020 instrument to gather enhanced information about the organizational arrangement and services offered because public and private toxicology laboratories have many types of hierarchies and offer a wide range of forensic and clinical services and to collect more precise workload measures.
Question 6 from the 2017 survey asked respondents, “Which of the following services does your laboratory provide?” with a “select all that apply” response option that included the following items:
□ A. Laboratory provides many services (e.g., other forensics); toxicology is one service
□ B. Laboratory provides only toxicology services
□ C. Laboratory provides postmortem toxicology testing
□ D. Laboratory provides toxicology testing in driving cases
□ E. Laboratory provides clinical toxicology testing (e.g., pain management)
□ F. Other (please specify)
When we examined the 2017 responses for Question 6, more specific information was clearly needed with respect to toxicology testing to ensure clarity and data quality. Thus, a new Question 6 was added to ask whether the laboratory provides only toxicology services or more than toxicology services. New Question 6 is followed by a revised Question 7—a modified version of the original Question 6 from 2020—which asks respondents about the specific types of toxicology testing conducted. Questions 6 and 7 tested well during the cognitive interviews.

Question 7 from the 2017 instrument was double barreled, designed to obtain the laboratory’s organizational hierarchy and the networking capacity if the laboratory was in a system. This question proved difficult for the 2020 cognitive interview participants, who suggested splitting it into separate questions to ease understanding and to be more inclusive, given the diversity of organizational arrangements and networking capacity across the private and public sectors. Thus, the following changes were made to the 2020 instrument:
We revised the original question (now revised Question 11) to ask respondents to describe their laboratory’s organizational structure (i.e., a standalone facility with no other relationship to other laboratories, a central laboratory in a network, or a satellite laboratory in a network), with a skip pattern. For respondents indicating in Question 11 that their laboratory is part of a network, new Question 12 is a follow-up yes/no question that asks whether those laboratories can share data.
New Question 13 follows the skip pattern for networked laboratories to inquire how many laboratories are included in the network. These data will further ensure frame integrity.

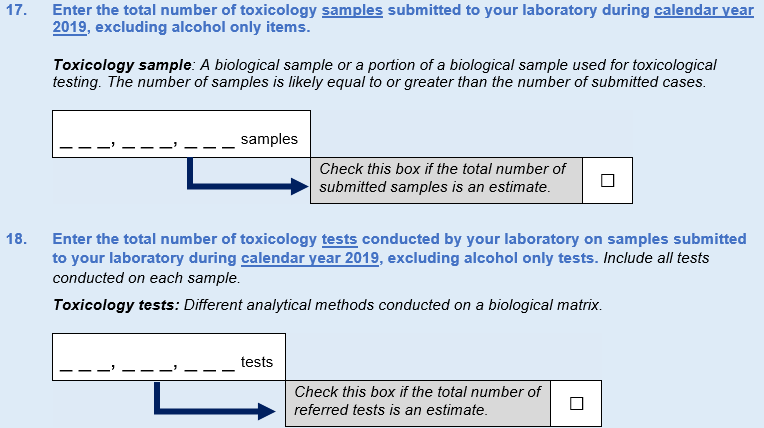
Finally, in the 2017 instrument, DEA asked about the number of toxicology cases referred to the laboratory. Expert panel participants pointed out that this question provides only a gross measure of workload, because they often receive several samples in a given case, which may be counted differently across laboratories. Thus, it was recommended that DEA inquire about the number of toxicology samples submitted to the laboratory with a follow-up question about the number of toxicology tests conducted on those samples. This resulted in new Questions 17 and 18. Estimate box options were included for both new questions to ease respondent burden.
We welcome any comments or questions from OMB regarding these changes.
Appendix
Table 1. NFLIS-Tox 2017 Survey Questions That Were Eliminated for the NFLIS-Tox 2020 Survey Administration
Question No. |
2017 Questions That Were Eliminated |
5 |
What type of organization is most directly responsible for your laboratory? Select only one option. |
14 |
Does your toxicology laboratory report unconfirmed screening results? |
15 |
Are there instances where your laboratory performs toxicology testing for specific drugs that would be contrary to laboratory standard operating procedures (e.g., only based on client specifications)? |
21 |
Does your information management system have the ability to export customized files? Select all that apply. |
22 |
Does your laboratory have the ability to electronically transfer exported files? Select all that apply. |
25 |
Of the types of assistance that you specified in Question 24 that would ease your participation in NFLIS, which one is the most important? Select all that apply. |
26 |
Does your laboratory participate in any other drug-related data collection efforts? Select all that apply. |
Table 2. Summary of Minor Changes to NFLIS-Tox Survey Questions
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | Smiley-McDonald, Hope |
| File Modified | 0000-00-00 |
| File Created | 2021-11-16 |
© 2026 OMB.report | Privacy Policy