Change Justification
Justification for Change for 0920-1100_DETECT clean_final.docx
Identification of Behavioral and Clinical Predictors of Early HIV Infection (Project DETECT)
Change Justification
OMB: 0920-1100
Justification for Change
Identification of Behavioral and Clinical Predictors of Early HIV Infection
(Project DETECT)
OMB # 0920-1100
Summary of Changes
We are requesting a change in the information collection request (ICR) for the Identification of Behavioral and Clinical Predictors of Early HIV Infection (Project DETECT) OMB # 0920-1100 (exp. 01/31/2022) in support of a Contract (CDC 200-2014-61285) and two Cooperative Agreements (PS20-001 Evaluation of New HIV Testing Technologies in Clinical Settings with High HIV Incidence) to evaluate the performance of HIV tests for use in point-of-care testing sites in the US. The 5- year Project DETECT Contract was awarded to the University of Washington with a period of performance ending on September 29, 2019. The Project DETECT Cooperative Agreements (DETECT 2.0) were awarded to the University of Washington and Johns Hopkins University for a three-year funding period (from 9/30/2020 to 9/29/2023) and will continue work started by the previous contract with University of Washington. The Project DETECT contract with the University of Washington did not meet the estimated burden and we anticipate the burden will decrease for the two Project DETECT Cooperative Agreements. This decrease is due to the reduction in the estimated time it takes to complete the survey (decrease from 60 minutes to 30 minutes per respondent) and the use of one survey by both sites.
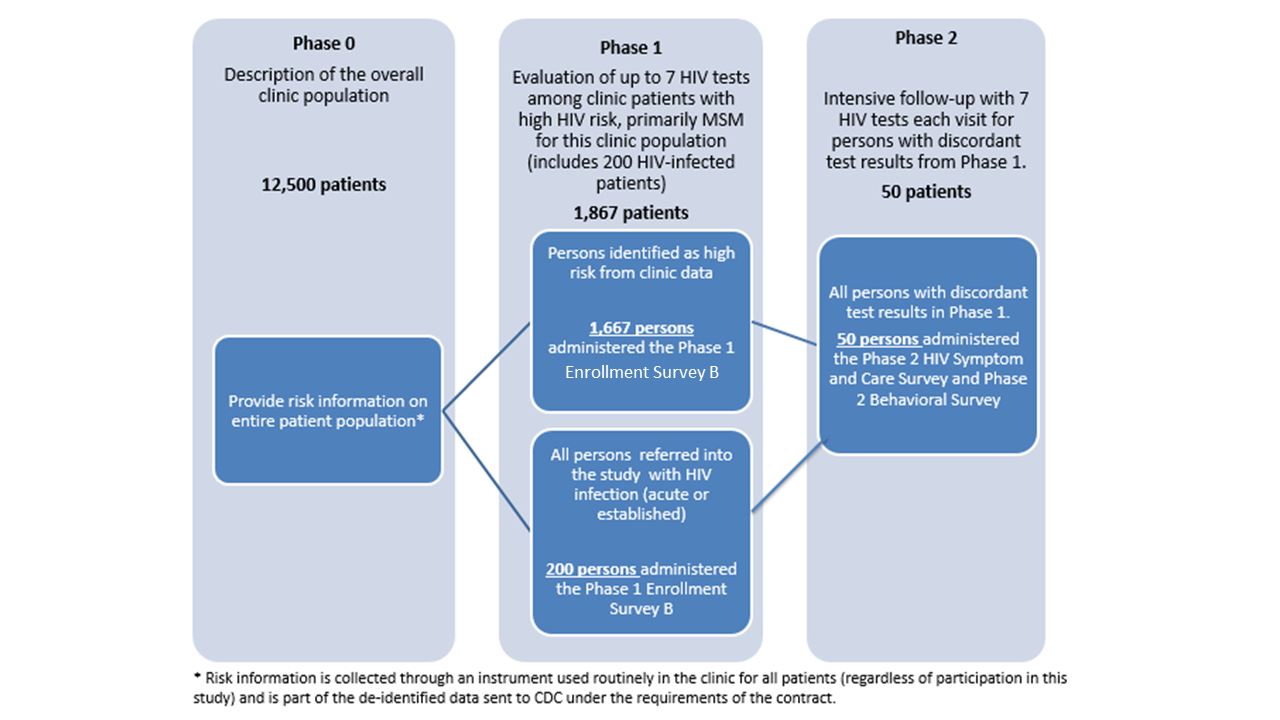
The University of Washington published the findings and protocol for Project DETECT in JMIR Research Protocols (Stekler JD, Violette LR, Clark HA, et al. Prospective Evaluation of HIV Testing Technologies in a Clinical Setting: Protocol for Project DETECT. JMIR Res Protoc 2020;9(1):e16332:1). Between September 2015 and March 2019, there were 14,990 Project DETECT-eligible visits. The UW staff enrolled and tested 1,037 people at risk of HIV, 198 with established HIV infection, and 96 with initial or newly diagnosed infection. Based on the testing conducted among these participants, 27 had discordant tests and were enrolled and followed in Phase 2 (see Figure 1.1). We requested a 3-year extension of this OMB approval to collect data from at least 100 individuals with discordant HIV results. The study implementers recently focused efforts on identifying individuals with early infection in order to reach at least 100 persons with discordant results by the completion of the study in 2022.
In the new Cooperative Agreements, the purposes of data collection will remain unchanged and the overall content remains unchanged. The data collection instrument will move from a QDS to a REDCap data platform. Compared to QDS, the current data collection instrument, REDCap is less costly, easier to program, and easier for data collection and management. Additionally, the annualized burden hours have decreased to account for the data obtained during the DETECT 1.0 project which showed that the burden time was less than initially estimated (from an estimated 60 minutes to 30 minutes per respondent for the survey). Furthermore, only one survey (Attachment 7a and 7b) will be used by both sites with all respondents completing the same survey (Survey A in Attachment 6a and 6b has been removed). The data collection will serve three primary purposes: 1) Compare the performance characteristics of new point of care (POC) HIV tests for detection of early infection, 2) ascertain whether a questionnaire administered at clinic intake can identify persons at highest risk of infection (most likely to have early infection) accurately enough to target the use of POC tests for early infection, and 3) describe the potential impact of earlier diagnosis of infected persons for curtailing HIV transmission, as defined by incidence of specific sexual behaviors and activities.
Data will be collected in three concurrent phases as described in the following Figure:
Figure 1.1. Description of Study Phases
Changes in Estimates of Annualized Burden Hours
We anticipate a decrease in the annualized burden hours based on updated estimates for completing Phase 1 Enrollment Survey B, obtained during the original project DETECT.
Exhibit A12A. Estimate of Annualized Burden Hours |
|||||
Type of Respondent |
Form Name |
Number of Respondents |
Number of Responses per Respondent |
Average Minutes Per Response |
Total Response Burden (Hours) |
Persons eligible for study |
Phase 1 Consent |
2,334 |
1 |
15/60 |
584 |
Enrolled participants
|
Phase 1 Enrollment Survey B |
1,867 |
1 |
30/60 |
934 |
Phase 2 Consent |
50 |
1 |
15/60 |
13 |
|
Phase 2 HIV Symptom and Care survey |
50 |
9 |
5/60 |
38 |
|
Phase 2 Behavioral Survey |
50 |
1 |
30/60 |
25 |
|
Total |
|
|
|
|
1,594 |
TABLE WITH SURVEY CHANGES ATTACHED
Page
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| File Title | ACRF Changes from 2003 form to proposed 2010 form |
| Author | znt1 |
| File Modified | 0000-00-00 |
| File Created | 2021-12-08 |
© 2025 OMB.report | Privacy Policy