Unhealthy Alcohol Use_OMB-SSA_5.28.20_final
Unhealthy Alcohol Use_OMB-SSA_5.28.20_final.docx
AHRQ Addressing Unhealthy Alcohol Use in Primary Care Initiative
OMB: 0935-0254
Supporting Statement
Part A
AHRQ Addressing Unhealthy Alcohol Use in Primary Care Initiative
Agency for Healthcare Research and Quality (AHRQ)
Version: May 28, 2020
A1. Circumstances That Make the Collection of Information Necessary 3
A2. Purpose and Use of Information 6
A3. Improved Information Technology to Reduce Burden 8
A4. Efforts to Identify Duplication 8
A5. Involvement of Small Organizations 8
A6. Consequences of Less Frequent Data Collection 8
A8. Federal Register Notice and Consultation 9
A10. Assurance of Confidentiality 11
A12. Estimation of Information Collection Burden 11
A13. Cost Burden to Respondents or Record Keepers 12
A14. Estimate of Cost to the Federal Government 12
A16. Plan and Time Schedule for Information Collection, Tabulation and Publication 12
A17. Reasons Not to Display OMB Expiration Date 13
A18. Exceptions to Certification for Paperwork Reduction Act Submissions 13
A. Justification
A1. Circumstances That Make the Collection of Information Necessary
The mission of the Agency for Healthcare Research and Quality (AHRQ) set out in its authorizing legislation, The Healthcare Research and Quality Act of 1999 (see http://www.ahrq.gov/hrqa99.pdf), is to enhance the quality, appropriateness, and effectiveness of health services, and access to such services, through the establishment of a broad base of scientific research and through the promotion of improvements in clinical and health systems practices, including the prevention of diseases and other health conditions. AHRQ shall promote health care quality improvement by conducting and supporting:
Research that develops and presents scientific evidence regarding all aspects of health care;
The synthesis and dissemination of available scientific evidence for use by patients, consumers, practitioners, providers, purchasers, policy makers, and educators; and
Initiatives to advance private and public efforts to improve health care quality.
Background for this Collection
In 2010, Congress established the Patient-Centered Outcomes Research Trust Fund and authorized AHRQ to disperse funds to disseminate and implement findings from the outcomes research into day-to-day practice settings. AHRQ’s patient-centered outcomes research initiative identifies research findings that could significantly improve patient outcomes through broader implementation in clinical practice. Under this initiative, in 2019 AHRQ launched a new initiative, Addressing Unhealthy Alcohol Use in Primary Care, in order to promote the uptake of evidence-based practices for unhealthy alcohol use. As part of this initiative, AHRQ selected six grantees to collectively work with more than 700 primary care practices over three years to implement and evaluate strategies to increase the use of evidence-based interventions such as screening for unhealthy alcohol use, brief interventions for adult patients who drink too much, and medication-assisted therapy for patients with an alcohol use disorder. A key component of this initiative is to conduct a multisite, mixed methods formative, summative, and impact evaluation.
Unhealthy alcohol use, defined as behaviors ranging from risky drinking to alcohol use disorders, is estimated to be the third leading cause of preventable death in the United States.1 Between 2006 and 2010, nearly one in ten deaths were alcohol-related.2 In addition to early mortality, unhealthy alcohol use is associated with a host of adverse outcomes, including unintentional injuries and the development or exacerbation of a range of physical and behavioral health conditions.3 The Centers for Disease Control and Prevention estimates suggest that excessive alcohol consumption costs the United States $249 billion annually.4
Despite increasing awareness of harmful consequences, unhealthy alcohol use remains relatively common. In 2018, approximately 24.5 percent of individuals 12 years and older reported current binge drinking (≥5 drinks for men, or ≥4 drinks for women, on the same occasion on ≥1 days in the previous month), and 6.1 percent reported current heavy alcohol use (≥5 drinks for men, or ≥4 drinks for women, on the same occasion on ≥5 or more days in the previous month).5 Yet, health professionals often miss opportunities to discuss these issues because they are not aware of patients’ alcohol use. Only one in six adults reports talking with a doctor or nurse about their drinking.6 Even among those whose alcohol use meets diagnostic criteria for an alcohol use disorder, treatment remains uncommon. Of the 14.1 million adults (aged 18 and older) with an alcohol use disorder in 2017,7 only 6.5 percent received any form of treatment.8
In 2018 the United States Preventive Services Task Force updated its 2013 recommendation on screening and behavioral counseling interventions to address unhealthy alcohol use in adolescents and adults. The Task Force recommends “screening for unhealthy alcohol use in primary care settings in adults 18 years or older” and recommends “providing persons engaged in risky or hazardous drinking with brief behavioral counseling interventions to reduce unhealthy alcohol use.”9 The Task Force found that brief screening instruments for unhealthy alcohol use used for adult populations commonly resulted in high sensitivity and specificity (between 0.70 and 0.85), and that across all populations studied, brief counseling interventions were associated with positive changes in behavior (i.e., a decrease in drinks per week, a lower proportion of patients exceeding recommended drinking limits, and a lower proportion of patients reporting a heavy use episode).10 The Task Force found no evidence of harmful effects for screening or interventions. In its 2019 annual report to Congress, the Task Force highlighted the critical importance of screening and brief intervention for unhealthy alcohol use and recommended a greater emphasis on research to improve the delivery of these services for adults.11
When individuals are screened in primary care practices and found to exhibit high-risk drinking or alcohol use disorder, medication-assisted therapy (the combination of medications and behavioral therapies) using United States Food and Drug Administration-approved medications has been found to be effective for managing withdrawal, reducing or eliminating use, and preventing relapse.12 Evidence has found that medications (alone or with medical advice/specialty care) are efficacious in decreasing heavy drinking and health risks associated with alcohol use.13,14
Under the AHRQ initiative, six grantees will work to improve the management of unhealthy alcohol use in primary care by disseminating and implementing evidence-based practices for screening, brief intervention, referral to treatment, and medication-assisted therapy. As a part of their own evaluations, grantees will collect data from the 750 practices participating in the initiative. This data will be shared with the evaluator, NORC at the University of Chicago. NORC’s multisite, mixed methods evaluation will include primary data collection, as well as this secondary data collected by the six grantee teams. Collectively the data will allow the evaluator to assess the implementation and impact of the six grants.
The project goals, as laid out in the AHRQ request for applications include:
Success of recruitment and retention strategies across all six grantees to engage primary care practices for implementation of screening, brief intervention, referral to treatment, and medication-assisted therapy, across the initiative;
Effectiveness of the grantees’ collective dissemination and implementation strategies, and the factors associated with the success and/or failure of the strategies as it relates to populations, settings and the influence of contextual factors;
Success at the practice level in increasing the number of patients screened, identified, and treated; and
Overall impact on changes in processes or outcomes that can be attributed to the initiative.
To achieve the goals of the multisite evaluation AHRQ is requesting OMB approval for three years for new data collection by the evaluator. The evaluator’s primary data collection is requested to achieve the goals of the multisite evaluation and includes the following data collection activities:
Semi-Structured Qualitative Interviews will take place in-person and/or by telephone with key staff from each grantee team (i.e., principal investigator, co-investigator, evaluation lead, practice facilitation/implementation lead, and project manager) and with clinicians and staff at one primary care practice working with each grantee. Interviews will be conducted annually beginning at the end of Year 1, for a total of three time points per grantee. During Years 1 and 3 the interviews will be conducted by phone; Year 2 interviews will be collected in-person. The interviews for both grantee teams and primary care practice staff will cover domains such as understanding the practice implementation and changes overtime, methods of supporting practices, barriers and facilitators to implementation, strategies to overcome barriers, and the number and type of staff implementing screening, brief intervention, referral to treatment, and medication-assisted therapy.
The secondary data required by AHRQ’s RFA-HS-18-002 to be collected by grantees and analyzed by the evaluator is listed in Attachment A. Secondary data will include:
Primary care practices will submit to grantees aggregate process measure data that will be used to assess changes in the number of patients receiving screening, brief intervention, referral to treatment, and/or medication-assisted therapy at the practice level. Grantees will submit data on practice characteristics to determine the relationship between those characteristics and the number of patients receiving screening, brief intervention, referral to treatment, and/or medication-assisted therapy. Grantees will provide the evaluator data to analyze the relationship between the type and frequency of practice interactions and the number of patients screened, receiving brief intervention, medication-assisted therapy, and/or treated for unhealthy alcohol use. Grantees will track changes in practices over time in order for the evaluator to assess what activities the practice is conducting to identify and manage unhealthy alcohol use.
The AHRQ Addressing Unhealthy Alcohol Use in Primary Care Initiative is being undertaken pursuant to AHRQ’s mission to enhance the quality, appropriateness, and effectiveness of health services, and access to such services, through the establishment of a broad base of scientific research and through the promotion of improvements in clinical and health systems practices, including the prevention of diseases and other health conditions. 42 U.S.C. 299.
A2. Purpose and Use of Information
The multisite evaluation approach includes three components: 1) a formative evaluation, 2) summative evaluation, and 3) an impact evaluation. The formative evaluation will focus on grantees’ dissemination and implementation of screening, brief intervention, referral to treatment, and medication-assisted therapy in primary care practices. The summative evaluation will assess the strategies grantees implement and whether grantees are increasing the identification and management of unhealthy alcohol use in primary care, while the impact evaluation will analyze secondary grantee data and evaluate the sustainability of the initiative. The evaluator’s qualitative data collection described below and analysis of grantees’ secondary data will support this evaluation.
The multisite evaluation will examine the relationship between contextual factors, such as practice staff and characteristics and type/frequency of engagement in order to illuminate which factors impact screening, brief intervention, referral to treatment, and medication-assisted therapy implementation. The evaluator will qualitatively examine the sustainability of the changes introduced during the initiative to better understand grantees’ approaches to implementing patient-centered outcomes findings and barriers and facilitators to improving the management of unhealthy alcohol use in primary care.
Semi-Structured Qualitative Key Informant Interviews
Semi-structured qualitative interviews with grantees and practice staff will include telephone and in-person interviews and practice site visits. The interviews will provide qualitative data on grantees’ approaches to disseminating and implementing patient-centered outcomes findings and barriers and facilitators to improving the management of unhealthy alcohol use in primary care. The interviews will highlight the differences in grantee strategies and how those differences impact the implementation of screening, brief intervention, referral to treatment, and medication-assisted therapy into primary care practices. Qualitative key informant interviews provide a deep dive and a more thorough understanding of grantee and primary care practices’ experiences throughout the initiative. Identifying best practices and lessons learned provides critical data for future unhealthy alcohol use implementation. The Key Informant Interview Guide for Grantees is contained in Attachment B. The Key Informant Interview Guide for Practices is contained in Attachment C. The practice interview guide covers similar domains as the grantee version, but is tailored to primary care practice personnel. In order to test the validity and reliability of the interview guides, the evaluator conducted pilot testing with technical experts and project consultants. The evaluator conducted mock interviews of both practice and grantee interview guides to gain feedback on question comprehension and wording.
Telephone Interviews with Grantees and Practices: Key Informant Interviews will be conducted with key staff from each grantee team (i.e., principal investigator, evaluation lead, practice facilitator/implementation lead, project manager) and with key staff from one practice affiliated with each grantee (i.e., clinician champion, frontline clinician, practice manager, non-clinical staff such as front/back office staff or medical assistant) at the end of Year 1 and again in Year 3. The discussions will cover domains such as understanding methods of supporting practices, grantee and practice barriers and facilitators to implementation, strategies to overcome barriers, and the number and type of staff involved. Using the same semi-structured interview guides at different points in time will allow the evaluator to track changes in what is salient to the grantees and what is salient to practices over the course of the initiative.
Site Visits with Grantees and Practices: In Year 2, interviews will occur in-person at grantee locations. The same Key Informant Interview Guide for Grantees will be used but the in-person meetings will facilitate more active engagement with grantee teams. As possible, during the grantee site visits, practice site visits will also be conducted, using the Key Informant Interview Guide for Practices. Practice discussions will be conducted with clinicians and staff at a small number of primary care practices affiliated with each grantee. Practice site visits will cover methods of screening, brief intervention, referral to treatment, and medication-assisted therapy implementation into practice flow, barriers and facilitators to implementation, strategies to overcome barriers, and the number and type of clinical and non-clinical staff involved.
A3. Improved Information Technology to Reduce Burden
Rather than conducting all key informant interviews in-person, interviews will be conducted by telephone in Years 1 and 3, in order to minimize burden to grantees and practices. No information technology burdens exist for this qualitative data collection.
A4. Efforts to Identify Duplication
AHRQ is not aware of any other national efforts to improve management of unhealthy alcohol use in primary care practices that covers multiple geographic regions and aligns with the United States Preventive Services Task Force’s 2018 recommendations. While independent and individual health system/practice screening, brief intervention, referral to treatment, and medication-assisted therapy quality improvement efforts may be underway in local environments, the data collected under this national initiative does not currently exist, making this data collection necessary.
A5. Involvement of Small Organizations
Primary care practices are the only small organizations eligible for inclusion in the multisite evaluation. For this project, only data collection activities that provide critical information for conducting the evaluation have been included and the information being requested has been held to the minimum required for the intended use.re practice
A6. Consequences of Less Frequent Data Collection
This data collection effort will be part of the multisite evaluation to assess the adoption of screening, brief intervention, referral to treatment, and medication-assisted therapy in primary care practice settings, evaluate the characteristics of primary care practices associated with increases in adoption, and to understand the impact and sustainability of the initiative within primary care practices. The planned frequency of data collection is necessary to accurately assess the adoption and effectiveness of the implementation. If the data collection were less frequent, the evaluation could not be completed.
The semi-structured Key Informant Interviews will be conducted annually beginning at the end of Year 1, for a total of three time points per grantee to collect qualitative information on grantees’ approaches to disseminating and implementing patient-centered outcomes research findings and barriers and facilitators to improving the management of unhealthy alcohol use in primary care. Key Informant Interviews will also be conducted with practices in order to collect qualitative information on the processes practices have adopted in order to disseminate and implement patient-centered outcomes research findings and barriers and facilitators to improving the management of unhealthy alcohol use in primary care.
A7. Special Circumstances
This request is consistent with the general information collection guidelines of 5 CFR 1320.5(d)(2). No special circumstances apply.
A8. Federal Register Notice and Consultation
Federal Register Notice and Comments
In accordance with the Paperwork Reduction Act of 1995 (Pub. L. 104-13) and Office of Management and Budget (OMB) regulations at 5 CFR Part 1320 (60 FR 44978, August 29, 1995), AHRQ published a notice in the Federal Register announcing the agency’s intention to request an OMB review of this information collection activity. This notice was published on March 30, 2020, Volume 85, Number 61, page 17580, and provided a sixty-day period for public comment. A copy of this notice is attached as Attachment D. During the notice and comment period, the government received no requests for information or substantive comments.
Consultation with Experts Outside of the Study
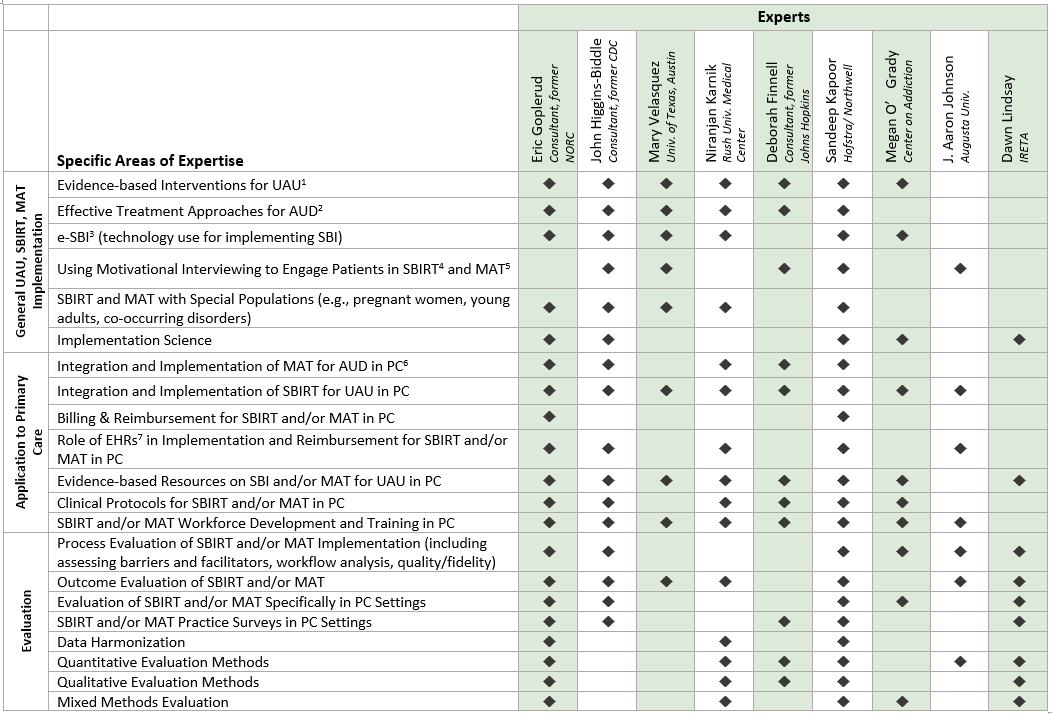
AHRQ has and will continue to engage experts and key stakeholders for this initiative and evaluation. AHRQ will engage individuals and organizations, including the subject matter experts comprising a technical expert panel and project consultants. Each of these groups have provided guidance on developing the project plan and design, and developing the evaluation plan and data collection tools. The panel consists of individuals with knowledge and expertise in unhealthy alcohol use, screening, brief intervention, referral to treatment, and medication-assisted therapy implementation, applications in primary care, and evaluation (see Exhibit A.1 for list of technical expert panel members). Consultants provide knowledge and expertise in substance use prevention, early intervention, and treatment. Panel members and consultants are tasked with providing critical feedback on all aspects of this program. This includes reviewing data collection processes and documents, sharing their expertise on effective screening and treatment implementation and engagement processes for practices, and providing feedback on evaluation strategies. Panel members have reviewed and provided feedback on the multisite evaluation plan and engaged in planning and development discussions. The first virtual technical expert panel meeting occurred on November 21, 2019 and the second on February 18th, 2020.
AHRQ did not consult with other federal partners for this project.
Exhibit A.1 Addressing Unhealthy Alcohol Use: Technical Expert Panel Expertise Matrix
A9. Incentives for Respondents
No incentives are planned for grantees or practices contributing data to AHRQ.
A10. Assurance of Confidentiality
Individuals will be assured of the confidentiality of their responses under Section 944(c) of the Public Health Service Act. 42 U.S.C. 299c-3(c). This law mandates that information gathered for research conducted or supported by AHRQ that identifies individuals or establishments be used only for the purpose for which it was supplied. Information that can be directly identified, such as patient name and/or social security number will not be collected. All collected data will be submitted securely to AHRQ’s contractor, NORC at the University of Chicago. All data will be stored on NORC’s secure servers.
A11. Sensitive Questions
Neither grantees nor primary care practices will be asked questions of a sensitive nature.
A12. Estimation of Information Collection Burden
Exhibit A.2a shows the estimated annualized burden hours for the respondents’ time to complete the semi-structured Key Informant Interviews. For the three-year clearance period, the estimated annualized burden hours for the interviews are 60.
Exhibit A.2a Estimated Annualized Burden Hours
Data Collection Activity |
Number of Respondents |
Number of responses per respondent |
Hours per response |
Total Burden hours |
Semi-Structured Interviews |
60 |
1 |
1.0 |
60 |
Total |
60 |
|
|
60 |
Exhibit A.2b shows the estimated annualized cost burden based on the respondents’ time to complete the Key Informant Interviews. The total annualized cost burden is estimated to be $6,109.
Exhibit A.2b Estimated Annualized Cost Burden
Form Name |
Number of Respondents |
Total Burden hours |
Average Hourly Wage Rate* |
Total Cost Burden |
Semi-Structured Interviews |
60 |
60 |
$101.82a |
$6,109 |
Total |
60 |
60 |
|
$6,109 |
*National Compensation Survey: Occupational wages in the United States May 2018“U.S. Department of Labor, Bureau of Labor Statistics:” https://www.bls.gov/oes/current/oes_stru.htm
a Based on the mean wages for 29-1062 Family and General Practitioners
A13. Cost Burden to Respondents or Record Keepers
Capital and maintenance costs include the purchase of equipment, computers or computer software or services, or storage facilities for records, as a result of complying with this data collection. There are no direct costs to respondents other than their time to participate in the project.
A14. Estimate of Cost to the Federal Government
The total estimated cost to the Federal government is $199,582. This includes the contractor costs for project development, designing and conducting the evaluation, analyzing and reporting results, and disseminating project findings.
Exhibit A.3a Estimated Total and Annualized Cost
Cost Component |
Total Cost |
Annualized Cost |
Project development |
$19,056 |
$6,352 |
Design and conduct evaluation |
$132,816 |
$44,272 |
Analyze and report results |
$20,886 |
$6,962 |
Disseminate project findings |
$26,824 |
$8,941 |
Total |
$199,582 |
$66,527 |
Exhibit A.3b Federal Government Personnel Cost
Activity |
Federal Personnel |
Annual Salary |
% of time |
Cost |
Oversight |
Health Scientist Administrator |
$160,000 |
15 |
$24,000 |
Total |
$24,000 |
|||
A15. Change in Burden
This is a new request under OMB, thus no changes in hour burden are expected or reported here.
A16. Plan and Time Schedule for Information Collection, Tabulation and Publication
Activity |
Month, Year |
Beginning of data collection |
September-November 2020 |
NIH/AcademyHealth Annual Conference on the Science of Dissemination and Implementation in Health |
Fall 2021 |
End of data collection |
June-August 2022 |
Public Webinar |
June-August 2022 |
AHRQ Social Media & Website Updates |
September 2020 – August 2022 |
NIH/AcademyHealth Annual Conference on the Science of Dissemination and Implementation in Health |
Fall 2022 |
Final manuscript submitted (estimated) |
June-August 2023 |
A17. Reasons Not to Display OMB Expiration Date
All instruments will display the expiration date for OMB approval.
A18. Exceptions to Certification for Paperwork Reduction Act Submissions
No exceptions are necessary for this information collection.
List of Attachments
Attachment A: AHRQ RFA-HS-18-002 – Required Data
Attachment B: Key Informant Interview Guides - Grantees
Attachment C: Key Informant Interview Guides - Practices
Attachment D: Federal Register Notice
1 National Institutes of Health (NIH), National Institute on Alcohol Abuse and Alcoholism. Alcohol Facts and Statistics. December 2019. Available at https://www.niaaa.nih.gov/publications/brochures-and-fact-sheets/alcohol-facts-and-statistics
2 White, A. M., Castle, I.-J. P., Hingson, R. W., & Powell, P. A. (2020). Using Death Certificates to Explore Changes in Alcohol-Related Mortality in the United States, 1999 to 2017. Alcoholism: Clinical and Experimental Research, 44(1), 178–187. doi: 10.1111/acer.14239
3 Rehm J, Gmel GE Sr, Gmel G, et al. The relationship between different dimensions of alcohol use and the burden of disease—an update. Addiction. 2017;112(6):968-1001. doi:10.1111/add.13757
4 Sacks JJ, Gonzales KR, Bouchery EE, Tomedi LE, Brewer RD. 2010 national and state costs of excessive alcohol consumption. Am J Prev Med. 2015;49(5):e73–e79. https://doi.org/10.1016/j.amepre.2015.05.031
5 Substance Abuse and Mental Health Services Administration (SAMHSA). Key substance use and mental health indicators in the United States: Results from the 2018 National Survey on Drug Use and Health. (HHS Publication No. PEP19-5068, NSDUH Series H-54). Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration; 2019. Available at: https://www.samhsa.gov/data/ sites/default/files/cbhsq-reports/NSDUHNationalFindingsReport2018/ NSDUHNationalFindingsReport2018.pdf.
6 Centers for Disease Control and Prevention (CDC). Alcohol screening and counseling. Vital Signs. January 2014. Available at https://www.cdc.gov/vitalsigns/alcohol-screening-counseling/index.html.
7 Substance Abuse and Mental Health Services Administration (SAMHSA). 2017 National Survey on Drug Use and Health (NSDUH). Table 5.5A—Alcohol Use Disorder in Past Year Among Persons Aged 12 or Older, by Age Group and Demographic Characteristics: Numbers in Thousands, 2016 and 2017; 2018. Available at: https://www.samhsa. gov/data/sites/default/files/cbhsq-reports/NSDUHDetailedTabs2017/NSDUHDetailedTabs 2017.htm#tab5-5A.
8 National Institute on Alcohol Abuse and Alcoholism (NIAAA). Alcohol Facts and Statistics. Available at: https://www.niaaa.nih.gov/publications/brochures-and-fact-sheets/alcohol-facts-and-statistics
9 US Preventive Services Task Force. Screening and Behavioral Counseling Interventions to Reduce Unhealthy Alcohol Use in Adolescents and Adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;320(18):1899–1909. doi:https://doi.org/10.1001/jama.2018.16789
10 O'Connor EA, Perdue LA, Senger CA, Rushkin M, Patnode CD, Bean SI, Jonas DE. Screening and behavioral counseling interventions to reduce unhealthy alcohol use in adolescents and adults: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;320(18):1910-1928. doi: 10.1001/jama.2018.12086.
11 US Preventive Services Task Force. Ninth Annual Report to Congress on High-Priority Evidence Gaps for Clinical Preventive Services. November 2019. Available at: https://www.uspreventiveservicestaskforce.org/Page/ Name/ninth-annual-report-to-congress-on-high-priority-evidence-gaps-for-clinical-preventive-services.
12 SAMHSA Medication for the Treatment of Alcohol Use Disorder: A Brief Guide. Available at: https://store.samhsa.gov/system/files/sma15-4907.pdf.
13 Jonas DE, Amick HR, Feltner C, et al. Pharmacotherapy for adults with alcohol use disorders in outpatient settings: a systematic review and meta-analysis. JAMA. 2014;311(18):1889-1900.
14 Principles of Drug Addiction Treatment: A Research Guide (Third Edition). NIDA. 2018. https://www.drugabuse.gov/node/pdf/675/principles-of-drug-addiction-treatment-a-research-based-guidethird-edition.
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | Patrick Moulding |
| File Modified | 0000-00-00 |
| File Created | 2022-07-06 |
© 2026 OMB.report | Privacy Policy