RPPR Instruction Guide
rppr_instruction_guide revised feb2020.docx
PHS Research Performance Progress Report and Other Post-award Reporting (OD)
RPPR Instruction Guide
OMB: 0925-0002
NIH
RPPR Instruction Guide


NIH and Other PHS Agency Research Performance Progress
Report (RPPR) Instructional Guide
November 25, 2019


CONTACT US

Document Comments:
We value your feedback on this document. Please email your comments to [email protected].
For policy-related questions:
Please email [email protected].
Troubleshooting support:
Service Desk Ticketing System!
Log-in with your eRA Commons username and password to access the eRA Service Desk web ticketing system to submit a service desk ticket online, view status of your prior tickets, and update your tickets.
Access the eRA Service Desk web ticketing system with your eRA Commons user name and password.
Having trouble logging in?
Click here to submit an online request if you are not able to log in or do not have an eRA Commons account.
NIH Staff/Agency Partner Staff, click here to access the eRA Service Desk web ticketing system.
For information, see the flyer on the new Service Desk Ticketing System (PDF - 212 KB).
Or to contact the eRA Service Desk directly:
Web: http://grants.nih.gov/support (Preferred method of contact)
Toll-free: 1-866-504-9552
Phone: 301-402-7469
Email: [email protected]v (for System-to-System support)
Hours: Mon-Fri, 7:00 a.m. to 8:00 p.m. Eastern Time, except for Federal Holidays
DISCLAIMER STATEMENT

No data shown in illustrations represents any real account, project, or individual. Any resemblance to actual accounts, projects, or individuals is purely coincidental.
DOCUMENT HISTORY

-
Date
Commons System Version
Document Version
Description of Change
Author
4/25/2012
3.3.3.1
1.0.0
Develop initial draft of document
eRA Documentation Team
1/31/2014
3.11.0.5
7.0.0
Updated for AHRQ and multiyear funded awards
eRA Communications
4/25/2014
3.12.0.7
7.1.0
Corrected text and screen prints
eRA Communications
7/18/2014
3.14.0.10
8.0.0
Updated PA PRAM feature; inclusion forms
eRA Communications
10/17/2014
3.16.0.10
9.0.0
Updated for Inclusion Enrollment; NCT selection on multi-projects; Agency Requested PRAM changes
eRA Communications
2/5/2015
3.18.0.13
9.2.0
Added Recall for PRAM; Additional Indirect Cost budget form
eRA Communications
3/11/2015
3.18.2.2
9.3.0
Minor text edits
eRA Communications
4/17/2015
3.19.0.0
9.4.0
AHRQ updates
eRA Communications
7/17/2015
3.21.0.0
9.5.0
Modified instructions for question B.4
eRA Communications
11/3/2015
3.22
9.6.0
Modified instructions in Chapter 7; replaced policy links throughout
eRA Communications
1/25/2016
3.24
9.7.0
Modified instructions for Section 6.2, questions B.2 and B.6; Section 7.4, question B.2
eRA Communications
-
Date
Commons System Version
Document Version
Description of Change
Author
11/22/2016
3.26.4.1
9.7.1
Updates to Figures 86,87
Update Section 6.3, C.2, C.3, and C.5.a, regarding the new selection menus on the left and updated language regarding selections.
eRA Communications
02/14/2017
3.29.2
9.7.2
Added information regarding the new Section I – Program Outcomes which is used with Final and Interim RPPR
eRA Communications & Documentation
03/02/2017
3.29.6
9.7.3
Updated screenshots for Sections A-H and added I. Outcomes.
Updated hyperlink for sample outcomes for Section I. Outcomes Updated hyperlink for F.3.d – Select Agents
eRA Communications & Documentation
05/22/2017
3.30.07
9.7.4
Updated text in sections B.2, C.1, and C.5.
Updated screen shot for question
C.1 (Figure 85)
eRA Communications & Documentation
11/5/2018
3.41.0
9.7.5
Added note on cover page regarding the updated form approval date and to use the online help until the instructional guide is brought up to date.
eRA Communications & Documentation

 LIST
OF TABLES
LIST
OF TABLES

Figure 1: RPPR Navigation Links from Cover Page 6
Figure 2: Status Screen and List of Applications/Grants Links 7
Figure 3: RPPR Link on Status Result – List of Applications/Grants 8
Figure 4: Multi-Year Award RPPR Link 8
Figure 5: Manage RPPR List of Grant Applications 9
Figure 6: Multi-Year RPPR Error Message 9
Figure 7: RPPR Menu for Initiating the Report 11
Figure 8: Status Screen and List of Applications/Grants Links 13
Figure 9: RPPR Link on Status Result – List of Applications/Grants 13
Figure 10: Multi-Year Funded Award RPPR Link 14
Figure 11: Manage RPPR List of Grant Applications 14
Figure 12: RPPR Menu Buttons 15
Figure 13: RPPR Menu Options for Multi-Project RPPRs 16
Figure 14: RPPR Menu for Single-Project with Complicated Structure 16
Figure 15: Edit Link for Overall 17
Figure 16: Adding and Editing Individual Components 18
Figure 17: RPPR Cover Page and Section Navigation Links 19
Figure 19: Total Remaining Characters 20
Figure 20: Human Subjects Link in Question G.4. 21
Figure 21: Manage IDRs for Single Project 22
Figure 22: Manage IDRs for Multi-Year Project 22
Figure 23: Entering Cumulative Inclusion Data 24
Figure 24: Edit Planned Inclusion Data–Grantee Updates in Progress 26
Figure 25: No Inclusion Data Records–Single Project 27
Figure 26: No Inclusion Data Records – Multi-Year Funded Award 28
Figure 27: RPPR H. Budget - Questions H.1 Budget Form & H.2 Subaward Budget Form 31
Figure 28: Organizational DUNS on SF 424 Research & Related Budget 31
Figure 29: SF 424 R&R Budget Form - Question F.5 32
Figure 30: Check for Errors Button on RPPR Menu for a Single-Project RPPR 33
Figure 31: RPPR Error Messages (Examples) 33
Figure 32: Check for Errors Links for Multi-Project RPPRs 34
Figure 33: Errors and Warnings for One Component of a Multi-Project RPPR 35
Figure 34: RPPR Menu – Route Button 35
Figure 35: Route RPPR to Next Reviewer 36
Figure 36: PD/PI Assurance Statement 36
Figure 37: RPPR Successfully Routed 37
Figure 38: RPPR Menu – Recall Button 37
Figure 39: RPPR Successfully Recalled 38
Figure 40: Submit Button on RPPR Menu 38
Figure 41: Successful Submission Message 39
Figure 42: Sample of Multi-Project RPPR Error Messages 40
Figure 43: RPPR Menu – View Button 41
Figure 44: RPPR Menu – View Routing History Button 41
Figure 45: RPPR Routing History 41
Figure 46: Status Menu Option 42
Figure 47: Application ID hyperlink 42
Figure 48: Status Information Screen – e-Application Link 43
Figure 49: Status Information Screen for Multi-Year RPPR 43
Figure 50: Public Access PRAM Link. 45
Figure 51: Routing the Public Access PRAM 45
Figure 52: Route Public Access PRAM to Next Reviewer 46
Figure 53: Public Access PRAM PD/PI Assurance Statement 46
Figure 54: Successfully Routed Public Access PRAM 47
Figure 55: Recall Button for Public Access PRAM 48
Figure 56: Public Access PRAM Link for SO 49
Figure 57: Submitting Public Access PRAM 49
Figure 58: SO Certification of Public Access PRAM 50
Figure 59: Public Access PRAM Submitted to Agency 50
Figure 60: Status Results Showing Hyperlink for Application ID 51
Figure 61: MYF Award PRAM Link in Status Information 51
Figure 62: Agency Requested PRAM Link 52
Figure 63: Add Attachment Button for Agency Requested PRAM 53
Figure 64: Upload Attachment Pop-up 53
Figure 65: Routing the Agency Requested PRAM 54
Figure 66: Route Agency Requested PRAM to Next Reviewer 54
Figure 67: Agency Requested PRAM PD/PI Assurance Statement 54
Figure 68: Successfully Routed Agency Requested PRAM 55
Figure 69: Recall Button for Agency Requested PRAM 56
Figure 70: Agency Requested PRAM Link for SO 57
Figure 71: Submitting Agency Requested PRAM 57
Figure 72: SO Certification of PRAM 58
Figure 73: Agency Requested PRAM Submitted to Agency 58
Figure 74: Grant Number Hyperlink on PRAM Screen 59
Figure 75: Application ID Hyperlink on Status Result for PIs 59
Figure 76: Application ID Hyperlink on Status Result for SOs 60
Figure 77: Status Information with PRAM Link 60
Figure 78: PDF of Multiple Submitted PRAM 61
Figure 79: RPPR Section A. Cover Page 63
Figure 80: RPPR Section B. Accomplishments – Question B1 64
Figure 81: RPPR Section B. Accomplishments – Questions B2 & B3 65
Figure 82: RPPR Section B. Accomplishments – Question B4 66
Figure 83: RPPR Section B. Accomplishments – Question B5 67
Figure 84: RPPR Section B. Accomplishments – Question B6 67
Figure 85: RPPR Section C. Products – Question C1 69
Figure 86: RPPR Section C. Products – Questions C2 & C3 70
Figure 87: RPPR Section C. Products – Questions C4 & C5 71
Figure 88: RPPR Section D. Participants – Question D1 74
Figure 89: RPPR Section D. Participants – Questions D2a & D2b 75
Figure 90: RPPR Section D. Participants – Questions D2c – D2e 77
Figure 91: RPPR Section E. Impact – Questions E1 through E4 78
Figure 92: RPPR Section F. Changes – Questions F1 & F2 79
Figure 93: RPPR Section F. Changes – Question F3 80
Figure 94: RPPR Section G. Special Reporting Requirements – Questions G1 through G3 81
Figure 95: Question G.4. for AHRQ Grantees Only 83
Figure 96: RPPR Section G. Special Reporting Requirements – Question G.4 86
Figure 97: RPPR Section G. Special Reporting Requirements – Questions G5 through G7 87
Figure 98: RPPR Section G. Special Reporting Requirements – Question G8 88
Figure 99: RPPR Section G. Special Reporting Requirements – Question G9 89
Figure 100: RPPR Section G. Special Reporting Requirements – Questions G10 through G12.90 Figure 101: Section H.Budget of RPPR for a Non-SNAP Award 91
Figure 102: SF 424 Research & Related Budget Form Opened for Editing 92
Figure 103: SF 424 R&R Budget Form - Question F.5 92
Figure 104: SF 424 R&R Budget Form - Question F.5 96
Figure 105: SF 424 R&R Budget Form - Question F.5 100
Figure 106: SF 424 R&R Budget Form - Question F.5 104
Figure 107: D.1 Specifying the Components for an Added Individual 108
Figure 108: Associating a Component with the NCT Number 109
Figure 109: SF 424 R&R Budget Form - Question F.5 110
Figure 110: Sample of Section A. Cover Page for a Component 111
Figure 111: SF 424 R&R Budget Form - Question F.5 113

The purpose of this document is to provide those preparing the Research Performance Progress Report (RPPR) with an explanation of the RPPR module in the eRA Commons and the information required in the report. This document also provides the steps for accessing and completing the report in eRA Commons, as well as navigating, validating, routing, and submitting the RPPR to the awarding agency.
The RPPR is required for all annual non-competing (Type 5) NIH awards and final progress reports (Final-RPPR), including SBIR/STTR awards. NIH requires the submission of an “Interim-RPPR” if the recipient organization has submitted a renewal application (Type 2) on or before the due date by which a Final-RPPR would be required for the current competitive segment. Annual, interim, and final progress reports submitted in any format other than the RPPR will not be processed by the NIH and will require resubmission through the RPPR. Refer to notices NOT-OD-17-022, NOT-OD-17-037, NOT-OD-17-085, NOT-OD-13-035, NOT-OD-14-026 and NOT-OD-14-092 and NOT-OD-15-014 for more information.
Fast-Track Phase II applications (SBIR/STTR Fast-Track Phase I final progress reports), follow the instructions in the Non-Competing Continuation Progress Report PHS 2590 (http://grants.nih.gov/grants/funding/2590/2590.htm).
NIH continues development of the RPPR for administrative extensions (Type 4s). NIH will continue to update the community as progress is made.
All Agency for Healthcare Research and Quality (AHRQ) grantees, exclusive of recipients of multi-year funded awards, are required to use the eRA Commons RPPR module, effective January 1, 2015 (NOT-HS-15-001). Multi-year funded awards (awards in which the budget and project periods are the same and are longer than 12 months) are required to submit a paper PHS 2590.
The RPPR includes numerous references to the NIH Grants Policy Statement, 8.1.2 requirement that significant changes in objectives and scope require prior approval of the agency; for AHRQ awardees the analogous requirement is in the HHS Grants Policy Statement under PriorApproval Requirements.

The NIH Research Performance Progress Report (RPPR) implements the uniform reporting format for interim research progress reporting developed under the auspices of the National Science and Technology Council, through the Committee on Science and the Research Business Models Subcommittee, and established by the Office of Management and Budget for use by agencies that support research and research-related activities.
For NIH, all progress reports must be submitted using the RPPR. For the AHRQ, all annual progress reports, exclusive of those for multi-year funded awards, must be submitted using the RPPR effective January 1, 2015. Other PHS agencies that may eventually utilize the NIH RPPR are the Food and Drug Administration, and Centers for Disease Control and Prevention.
NonNIH agencies may have requirements that differ from those for NIH grantees; refer to the Notice of Award (NoA) or contact the Grants Management Specialist named in the NoA.
Progress reports are required to continue support of a PHS grant for each budget year within a competitive segment. NIH has implemented the Final and Interim-RPPR for final progress reporting. For AHRQ recipients instructions for submitting a Final Progress Report are at http://www.ahrq.gov/funding/grant-mgmt/reptemp.html.
PHS estimates that it will take approximately 15 hours to complete this progress report. An agency may not conduct or sponsor, and a person is not required to respond to, a collection of information unless it displays a currently valid OMB control number. If you have comments regarding the burden estimate or other aspect of the collection of information, including suggestions for reducing the burden, send comments to: NIH, Project Clearance Office, 6705 Rockledge Drive MSC 7974, Bethesda, MD 20892-7974, ATTN: PRA (0925-0002). Do not send progress reports to this address.
Background and Paperwork Burden

Grantees can determine which progress reports are due through the website located at: https://public.era.nih.gov/chl/public/search/index.jsp and should periodically check the site, which is updated on or around the 30th of each month. Progress report due dates are also available in the eRA Commons Status system. In addition, automatic e-mail notifications are sent to the PD/PI prior to due date.
NIH
SNAP: If an award is issued under the SNAP (Streamlined Noncompeting Award Process) provisions, the progress report is due the 15th of the month preceding the month in which the budget period ends (e.g., if the budget period ends 11/30, the due date is 10/15). If the 15th falls on a weekend or Federal holiday, the due date is automatically extended to the next business day. Grantees should consult the NoA to determine when SNAP procedures apply.
Non-SNAP: If an award is not issued under the SNAP provisions, the progress report is due the first of the month preceding the month in which the budget period ends (e.g., if the budget period ends 11/30, the due date is 10/1).
Fellowships: For Fellowships, the progress report is due two months before the beginning date of the next budget period. Occasionally, the Notice of Award (NoA) will indicate a different due date which will supersede these dates.
MYF: Progress Reports for MYF awards are due annually on or before the anniversary of the budget/project period start date of the award. The reporting period for a MYF progress report is the calendar year preceding the anniversary date of the award.
Final RPPR: Final progress reports are due within 120 days of the end of the period of performance.
Interim RPPR: Interim progress reports are due within 120 days of the end of the period of performance. Interim progress reports are only required when a renewal application (Type 2) has been submitted to the NIH for consideration on or before the due date by which a F-RPPR would be due for a competitive segment. If the Type 2 is not funded, the Interim-RPPR will serve as the Final-RPPR for the project. If the Type 2 is funded, the Interim-RPPR will serve as the annual RPPR for the final year of the previous competitive segment.
All AHRQ progress reports due in FY 2015 (10/1/14 – 9/30/15) and beyond are due 3 months before the anniversary of the award. For example, for an FY2014 award issued with a start date of 2/1/14, the annual progress report is due 11/1/14 (i.e., three months before the FY2015 budget period start date (i.e. anniversary date) of 2/1/15). If the due date falls on a weekend or federal holiday, the due date is automatically extended to the next business day.
Please note that AHRQ does not participate in the SNAP (Streamlined Noncompeting Award Process) initiative. AHRQ grantees are to submit detailed budgets for the parent organization and for each consortium involved in the project.

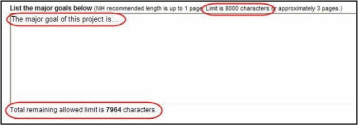
Most text entry boxes have an 8,000 character limit (~3 pages); this limit is standardized across federal agencies implementing the RPPR and entry of more than 8,000 characters is prevented by the system. In an effort to reduce grantee burden and encourage concise responses NIH has stated the recommended length of the response for some questions and, for agency-specific questions has limited the length of the response with text boxes with a limit of less than 8,000 characters. AHRQ grantees should follow NIH recommended lengths for text entries.

Warning: Text exceeding 8,000 characters is cut to 8,000 when using the cut and paste feature.
PDF file uploads (attachments) do not have page limits, but may not be more than 6 megabytes (6MB). PDF attachments are utilized when there may be a need for a grantee to provide considerable detail (e.g., change in human subject protocols that requires a new or revised Protection of Human Subjects section as described in Part II of the competing application instructions). Even when developing PDF responses, grantees are encouraged to be concise and avoid unnecessary detail.
Grantees should generate text attachments using any word processing software and then convert those files to PDF before attaching the files to the appropriate section in the progress report.
The PDF format is used to preserve document formatting. All PDF attachments must be submitted as individual files. Although some software packages allow bundling of multiple PDFs into a single file, eRA systems cannot support “Bundling” or “Portfolio” features at this time. Use of these features may result in delays in agency acceptance of the progress report. Paginated PDF files are also discouraged since they can interfere with system pagination of the entire RPPR document upon submission to the agency. File names will be used and displayed in the assembled PDF submitted to the agency.
Save all files with descriptive file names of 50 characters or less and be sure to only use standard characters in file names: A through Z, a through z, 0 through 9, and underscore (_). Do not use any special characters (example: &, -, *, %, /, and #) or spacing in the file name, and for word separation use an underscore (e.g., My_Attached_File.pdf).
Use an Arial, Helvetica, Palatino Linotype, or Georgia typeface, a black font color, and a font size of 11 points or larger. (A Symbol font may be used to insert Greek letters or special characters; the font size requirement still applies.) Type density, including characters and spaces, must be no more than 15 characters per inch. Type may be no more than six lines per inch.

Use standard paper size (8 ½" x 11). Use at least one-half inch margins (top, bottom, left, and right) for all pages. No information should appear in the margins, including the PI’s name and page numbers.
Use English and avoid jargon. Abbreviations and language that may not be known to the broader scientific community should be avoided unless clearly defined. Internet Web site addresses (URLs) should not be used unless provided under C.2.

The RPPR is completed using the eRA Commons system. The report in Commons consists of separate screens for each of the sections listed below:
Cover Page
Accomplishments
Products
Participants
Impact
Changes
Special Reporting Requirements
Budget
Outcomes
Users may work on various sections in any order, however, it is important to click the Save button in the navigation bar before leaving a screen in order to retain data entered on that screen. Upon submission to the awarding agency, the system will generate a PDF of the progress report, which may be viewed from the RPPR Menu screen using the View button.
Once submitted, the final RPPR, in PDF format, is accessible in Commons via the Status Information screen. Refer to the section titled Viewing the Final RPPR in Commons for detailed steps.
Note that a link to a site outside the RPPR (e.g., U.S. Select Agency Registry in F.3.d, or the NIH human embryonic stem cell Registry in G.6) opens a site in a new browser window. You must close that window to return to the RPPR. Do not close the browser or use the browser’s back button.

Figure 1: RPPR Navigation Links from Cover Page
Only the PD/PI or the PD/PI delegate may initiate an RPPR. When there are multiple PIs (MPI), only the Contact PI or the PD/PI delegate of the Contact PI may initiate the report. To initiate, the user can choose from one of two ways to access the RPPR functionality:
Access RPPR from Status:
Select the Status tab from the Commons menu options.
Select the List of Applications/Grants link from the Status screen or from the menu options.
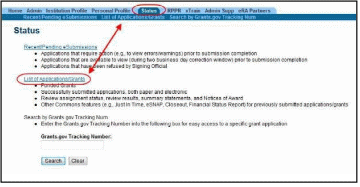
Figure 2: Status Screen and List of Applications/Grants Links
From the Status Result – List of Applications/Grants screen, locate the grant and select the RPPR or Interim RPPR link from the Action column for the specific grant. The RPPR link for the current reporting period is available once the Notice of Award for the prior year has been issued. This link remains available until the RPPR for the current reporting year has been submitted.
For Final RPPRs, select the Requires Closeout link from the status search results, and the Process Final RPPR link from the Closeout Status screen.
For multi-year funded awards, the link will display as RPPR Year <X>, the <X> representing the reporting year. The link for a multi-year funded award is available two months prior to the RPPR due date for the current reporting period and remains available until the RPPR is submitted. Note that AHRQ has not yet implemented the RPPR for multi-year funded awards.

NOTE: While RPPR Year <X> links for multiple years may appear at the same time in Status, you are prevented from initiating a reporting year’s progress report until the progress report(s) of the previous year(s) has been submitted.

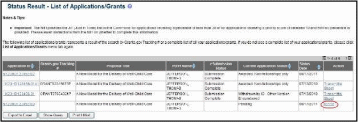
Figure 3: RPPR Link on Status Result – List of Applications/Grants
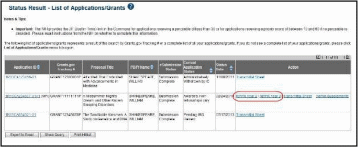
 –OR–
–OR–
Access RPPR from RPPR tab:
Select the RPPR tab from the Commons menu options.
The Manage RPPR screen displays. Manage RPPR is used to view the progress reports to which the user has access and allows the user to select a progress report in order to perform various actions. PD/PIs or users delegated PD/PI updating authority uses the Manage RPPR screen to view their own progress reports. SOs and AOs use the screen to search for grants from their institutions and/or for grants routed to them for review.
Select the specific grant by clicking the hyperlink in the Grant Number

column on the Manage RPPR screen.

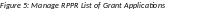 If
an RPPR exists already, Commons displays the report for editing.
If
an RPPR exists already, Commons displays the report for editing.
The RPPR Menu screen displays. The options for the uninitiated report are Initiate and Cancel. Once an RPPR is in progress, the buttons for other options are enabled. These options are discussed later, following the steps for initiation.

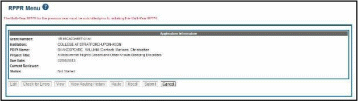
NOTE: For multi-year funded awards, the following message displays when attempting to initiate an RPPR if the previous year’s report has not been submitted:
The Multi-Year RPPR for the previous year must be submitted prior to initiating this Multi- Year RPPR.

 The
RPPR Menu screen includes the following fields:
The
RPPR Menu screen includes the following fields:
Grant Number
This is the complete number of the grant
Grantee Institution
This field contains the name of the applicant’s institution
PD/PI Name
The PD/PI of the grant award for which the progress report is being prepared. In the case of MPIs, a list of PD/PI names displays with the Contact PD/PI indicated by the word Contact.

Project Title
The project title of the grant
Due Date
NIH
The due date of the progress report for awards issued under the SNAP (Streamlined Noncompeting Award Process) provisions is the 15th of the month preceding the month in which the budget period ends (e.g., if the budget period ends 11/30, the due date is 10/15). If the award is not issued under SNAP provisions, the progress report is due the first of the month preceding the month in which the budget period ends (e.g., if the budget period ends 11/30, the due date is 10/1). If the due date falls on a weekend or federal holiday, the due date is automatically extended to the next business day. Progress reports for Fellowships are due two months before the beginning date of the next budget period. Occasionally the Notice of Award (NoA) will indicate a different due date which will supersede these dates. Grantees should consult the NoA to determine when SNAP procedures apply.
AHRQ
All AHRQ progress reports due in FY 2015 (10/1/14 – 9/30/15) and beyond are due 3 months before the anniversary of the award. For example, for an FY2014 award issued with a start date of 2/1/14, the annual progress report is due 11/1/14 (i.e., three months before the FY2015 budget period start date (i.e. anniversary date) of 2/1/15). If the due date falls on a weekend or federal holiday, the due date is automatically extended to the next business day.
Current Reviewer
The name of the current reviewer or organization (e.g., PD/PI name, NIH). This value is blank before the RPPR is initiated.
Status
The current state of the progress report. Possible values are as follows: Not Started, PD/PI Work in Progress, Reviewer Work in Progress, and Submitted to Agency.
Buttons
The displayed and enabled buttons vary depending on the status of the RPPR and/or the limitations of the current user’s role. The possible available actions include the following:
Initiate: Begins the RPPR process. Available for grants with a status of Not Started. Access is granted to PD/PIs and PD/PI delegates. An RPPR can be initiated even if required information in the Personal Profile and Institution Profile sections is missing. If any of this information is incorrect or missing, a prompt will appear to correct/complete the information after initiating the report. Processing may continue on the RPPR without making the corrections; however, the RPPR will not pass validations for submission to the agency until the errors are corrected.
Edit: Opens the RPPR for edits. Available for progress reports with a status of Work in Progress (WIP). Access is granted to PD/PIs or PD/PI delegates when the PD/PI is the current reviewer, AOs when the AO is the current reviewer, and SOs when the SO is the current reviewer. The Edit button allows the user to view and edit RPPR information.
View: Opens the RPPR report in PDF format, as it will be seen by the agency. Available for progress reports with a status of Work in Progress (WIP) or Submitted to Agency. Access is granted to PD/PIs, PD/PI delegates, and reviewers. Until the RPPR is

submitted to agency, the PDF report shows a status of Draft and a blank submission date.
Check for Errors: Checks the RPPR for any errors or warnings. Available for progress reports with a status of Work in Progress (WIP). Access is granted to any user with access to the grant. The RPPR can be validated at any time while in the status of WIP and can be validated multiple times.
View Routing History: Opens a page that displays a routing history table. Available for progress reports with a status of Work in Progress (WIP) or Submitted to Agency. Access is granted to PD/PIs, PD/PI delegates, and reviewers.
Route: Routes the RPPR to the next reviewer for further review or corrections. Available for progress reports with a status of Work in Progress (WIP). Access is granted to the current reviewer. A PD/PI delegate cannot route an RPPR to the next reviewer.
Recall: Recalls RPPRs that have been forwarded to another reviewer and resets the user as the current reviewer. Available for reports with a status of Work in Progress (WIP). Access is granted to the last reviewer (who recalls the report from the current reviewer). Signing Officials and PD/PIs can recall an RPPR even if they are not the last reviewer whenever it has a status of Reviewer Work in Progress. This is useful in situations when a RPPR has been routed to the wrong person or to someone who is unavailable.
Submit: Submits the RPPR to the Agency. Available for reports with a status of Work in Progress (WIP). Access is granted to the SO when the SO is the current reviewer and to the PD/PI when the PD/PI has been delegated Progress Report authority.

NOTE: A PD/PI with Progress Report authority cannot submit a non-SNAP or F RPPR.

Cancel: Closes the RPPR Menu screen and returns the user to the previous screen.
Select the Initiate button to begin the RPPR.
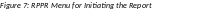 Once
initiated, Commons creates the report in a PD/PI Work in Progress
status and sets the current reviewer. A message displays as
follows:
Once
initiated, Commons creates the report in a PD/PI Work in Progress
status and sets the current reviewer. A message displays as
follows:
The RPPR has been successfully initiated.

NOTE: If at any time initiation fails due to business rules validations, error or warning messages display on the screen.

Once initiated, the editing process can begin. The RPPR is accessed for editing via the RPPR Menu screen. The editing feature for single-project RPPRs is different from those of multiproject RPPRs. The steps for accessing each type of RPPR are outlined in the sections that follow. Refer to Accessing a Single-Project RPPR for Editing or Accessing a Multi-Project and Single-Project with Complicated Structure RPPR for Editing as appropriate.
Once an RPPR is initiated, its status becomes PD/PI Work in Progress and it becomes available for editing. The PD/PI or delegate uses the Edit option for viewing and completing the report. Additionally, this option is available to the SO or AO when that user is the current reviewer of the report.

NOTE: For RPPRs with multiple PD/PIs (MPI awards), only the Contact PD/PI has access to the Edit feature unless the Contact PD/PI has granted progress report authority to other PD/PIs. Without this authority, MPIs can only view the RPPR PDF and its routing history.

There are two means of accessing the progress report for editing. These are similar methods used for initiating the report and are as follows:
Access RPPR from Status:
Select the Status tab from the Commons menu options.
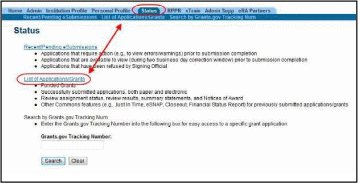
Figure 8: Status Screen and List of Applications/Grants Links
From the Status Result – List of Applications/Grants screen, locate the grant and select the RPPR link from the Action column for the specific grant. For multi-year funded awards, the link will display as RPPR Year <X>, the <X> representing the reporting year.
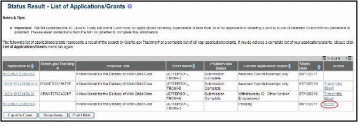
Figure 9: RPPR Link on Status Result – List of Applications/Grants
Access RPPR from RPPR tab:
Select the RPPR tab from the Commons menu options.
Select the specific grant by clicking the hyperlink in the Grant Number column on the Manage RPPR screen. SOs/AOs must perform a query first.
 The
appropriate RPPR Menu screen – either for single-project
or multi-project RPPRs – displays with editing options.
The
appropriate RPPR Menu screen – either for single-project
or multi-project RPPRs – displays with editing options.
Accessing a Single-Project RPPR for Editing
For single-project awards, the RPPR Menu screen displays with buttons for the following available options:
Edit
Check for Errors View
View Routing History Route
Cancel

NOTE: Once an RPPR has been routed for review, the Recall and Submit buttons are enabled. These functions are covered in subsequent chapters.

 Select
the Edit button to open the RPPR for
editing.
Select
the Edit button to open the RPPR for
editing.
Refer to the section of this document titled Editing the RPPR Forms for more information on editing the forms.
Accessing a Multi-Project and Single-Project with Complicated Structure RPPR for Editing
A multi-project RPPR is a progress report submitted for a funded program (activity code) which has multiple, interrelated components sharing a common focus or objective.
A component (for the purposes of applications and progress reports) is a distinct, reviewable part of the multi-project application or progress report for which there is a business need to gather detailed information identified in the funding opportunity announcement (FOA).
Components typically include general information (component organization, project periods, project title, etc.), performance sites, personnel, and budget. The FOA defines the construction and naming convention for the application; the funded application defines the construction and naming convention for the progress report.
For multi-project awards, the RPPR Menu screen displays with buttons for the following available options found within the Application Information section of the screen:
View
View Routing History
Route Cancel

NOTE: Once an RPPR has been routed for review, the Recall and Submit buttons are enabled. These functions are covered in subsequent chapters.

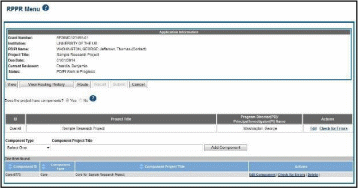
Figure 13: RPPR Menu Options for Multi-Project RPPRs
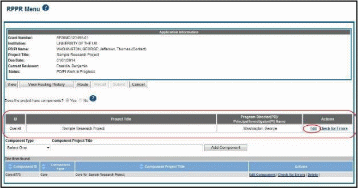
Overall
Below the Application Information is a table showing the Overall ID, Project Title, Program Director/Principal Investigator (PD/PI) Name, and an Actions column with links.
The RPPR Menu for a multi-project RPPR without components does not include the component table. Additionally, the No radio button on the Does the project have components? Field is selected.
Refer to the figure below for an example of a single-project with complicated structure RPPR.
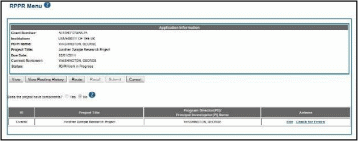
Figure 14: RPPR Menu for Single-Project with Complicated Structure
To edit the RPPR for the Overall, select the Edit link from the Actions column.
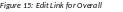 Individual
Components
Individual
Components
If the award has individual components (e.g., a multi-project award), each component must be reported in the RPPR. To add components, select the Yes radio button next to the question Does the project have components? Selecting Yes displays the Add Component feature for the individual components.

NOTE: Any individual components previously added will already be displayed in a table beneath this feature. In this scenario, the “Does this project have components?” option is disabled. This includes components which were part of a previously submitted progress report for the grant.

To add an individual component:
Select the correct option from the Component Type drop-down list.
Enter the Component Project Title.
Select the Add Component button.
Added individual components display in a table beneath the Overall, showing the Component ID, Component Type, Component Project Title, and available links in the Actions column.
Select the Edit Component link in the Actions column for the component to edit its RPPR.
Refer to the section of this document titled Editing the RPPR Forms for more information on editing the RPPR forms.
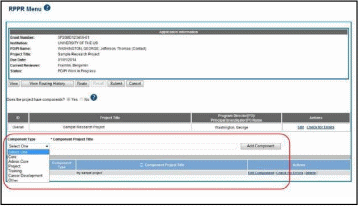
Figure 16: Adding and Editing Individual Components
Individual components can be removed from the RPPR by selecting the corresponding Delete link from the Actions column of the specific component, followed by the OK button on the confirmation pop-up message. The delete option is not available for Overall.

IMPORTANT: If you choose to delete a component, all data related to this component – including all budget data – will be lost. This data cannot be recovered once it has been deleted.
Editing the RPPR Forms
After selecting the appropriate editing option, the RPPR section A. Cover Page displays. The Cover Page includes information about the grant, PD/PI, signing and administrative officials, organization, and project/reporting/budget periods. Some of this information may be auto- populated. For more information on the Cover Page, refer to the section of this document titled Section A – Cover Page located in the Instructions for RPPR Sections A–I.
Update the information as necessary and select the Save button.
The Cover Page includes tabs at the top and links at the bottom of the page for navigating to the other sections (e.g., Accomplishments, Participants), which may be completed in any order.
Before navigating to and from any of these sections, it is always necessary to select the Save button to save all changes on the current page. Navigating away from any page on the RPPR without selecting Save results in the loss of any information entered prior to the last save.
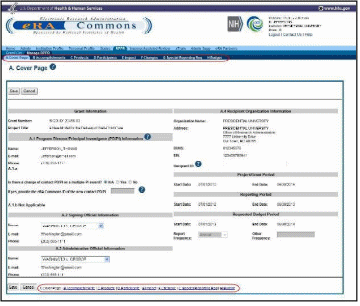
Figure 17: RPPR Cover Page and Section Navigation Links
Sections can be completed in any order. To navigate and populate the other sections of the RPPR, select the appropriate link from the top or bottom of the page.
The same navigational links appear on each section of the RPPR. For information on the specific fields in each section refer to Chapter 6 Instructions for RPPR Sections A–I.
Complete the appropriate fields of the report.
Details for completing each section are discussed later in this document. Many of the fields on these pages, however, behave in a similar manner and are discussed below.
Add/New
To use the Add/New feature, enter or select data into the appropriate fields. Select the Add/New
button to add the data to a table.
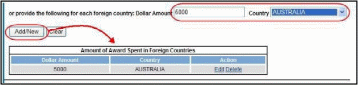
 Items
can be edited or deleted from the table using the Action links.
Items
can be edited or deleted from the table using the Action links.
Text Box
All text boxes on the RPPR have character limits. The number of characters available is reflected beneath each text box as characters are entered.
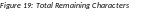 Changing
Saved Responses
Changing
Saved Responses
While in WIP status, answers may be changed. A warning message displays as follows:
The entered/uploaded response will be deleted. Do you wish to continue?
The user editing the information can choose to Continue or Cancel the action. Choosing Continue deletes the previous response, removes any attachments, and disables the relevant fields associated with the question. Choosing Cancel cancels the change.
Select the Save button before navigating to the next page.
When an RPPR is ready for review and submission, it is routed to the next reviewer. Refer to the section of this document titled Route the RPPR for steps on routing to the next reviewer.
Editing Inclusion Enrollment Data
The following chapter discusses inclusion data in the Human Subjects System (HSS) as accessed and processed via your RPPR. For more information on HSS, please refer to the HSS Online Help or the HSS Training website.
To update inclusion enrollment data, select the Human Subjects link from question G.4 of section G. Special Reporting Requirements. For additional information on inclusion procedures in the RPPR, please review Chapter 6.7 Section G–Special Reporting Requirements.
IMPORTANT:
Before
selecting the
Human Subjects link,
select the Save
button on the RPPR
to save all of your work in Section
G. Failure to do
so will result in a loss of data on your report.


Figure 20: Human Subjects Link in Question G.4
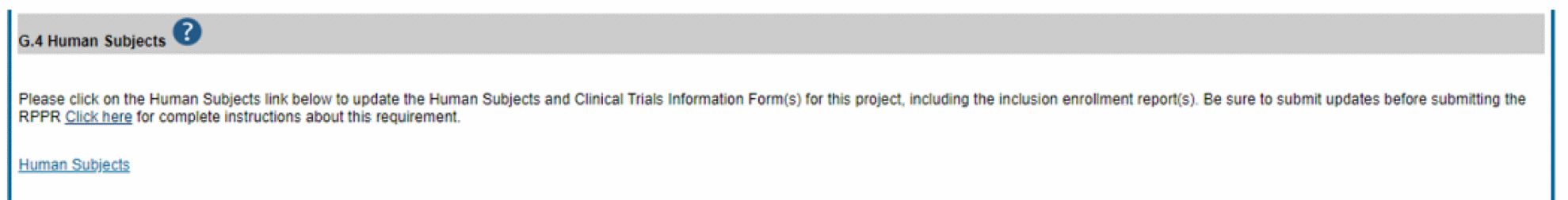
The Human Subjects link will take you to the Application Information screen. Click on the HSCT Post Submission tab. This will take you to the Study Record(s) screen that displays all the study records and delayed onset records for the grant. You may view the information within a study record, including the last inclusion enrollment report (IER) by clicking on the View button under the Action column within the list of study records.
To edit the inclusion information, click the Edit button at the top of the screen. You will now see an Edit button under the Action column within the list of study records. Click on this Edit button to make changes to the study record information, including the inclusion enrollment data.

The Inclusion Enrollment Report (IER) is housed within the Study Record and can be found in Section 2- Study Population Characteristics. Here, you can add a new inclusion enrollment report, or edit a report that was previously created.
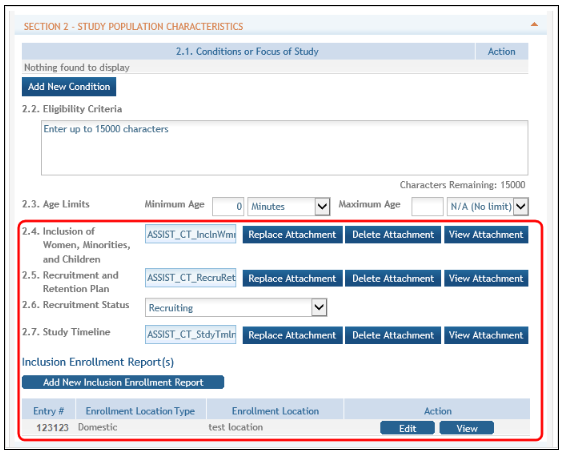
Figure 22: Adding/Editing an Inclusion Enrollment Report
Select the Edit button in the Action column to edit the Inclusion Enrollment Report.
The Inclusion Enrollment Report screen contains the following information:
Inclusion Enrollment Report Fields
Using an Existing Dataset or Resource
Displays Yes/No as indicated by radio buttons
Enrollment Location Type
Displays Domestic/Foreign as indicated by radio buttons
Enrollment Country(ies)
Displays the country of enrollment as identified from a dropdown list
Enrollment Location(s)
Displays a location entered by the user.
Comments
An optional text field for entering cumulative enrollment comments. If any comments for cumulative form were entered before, this field is pre-populated when editing an existing IER.
See the application guide instructions for more information about Inclusion Enrollment report fields.
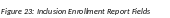
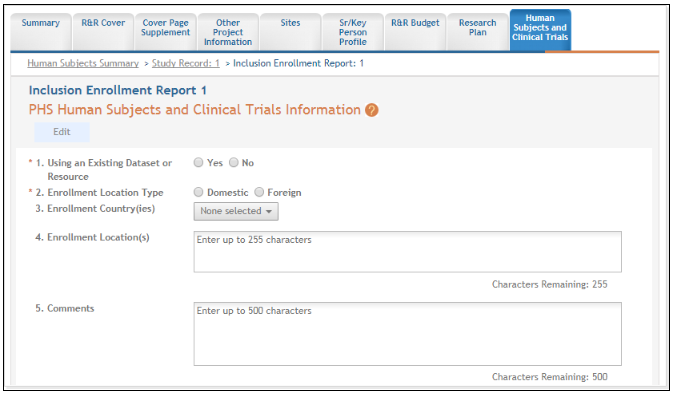
The cumulative enrollment table includes racial categories along the left side the of the table and ethnic categories, divided by sex/gender, along the top of the table. The individual enrollment count cells are editable and set to zero by default, when populating a new IER. When editing an existing form, these values are pre-populated with any other value previously entered. The total fields are calculated by HSS and sum up as rows and columns accordingly. The total values are not editable fields.
NOTE: The cumulative inclusion table includes fields for entering Unknown/Not Reported race, ethnicity, and sex/gender data.
There are two ways to edit the existing Inclusion Enrollment Report (IER) data for Cumulative (Actual) counts:
You can update the cells online in the existing report itself.
You can download a template for entering participant-level data by clicking on the Download Participant Level Data Template button.
This will download a spreadsheet file in the proper CSV format to be used by the system.
Fill the template out with data, save the changes, and then upload the spreadsheet by clicking on the Upload Participant Level Data Attachment button. This uploaded data will populate the cells in the report.
You can click on the Download Current Participant Level Data button to download the file containing the data for your own records.
If you need to clear the current records, use the Remove Current Participant Level Data button.
Update the values in the individual enrollment count cells as necessary, and select the Save and Release Lock button. To leave the form without saving any changes, select the Cancel and Release Lock button instead. Saving and canceling both return you to the Study Record(s) screen.
POLICY: Individual-level participant data on sex/gender, race, ethnicity and age at enrollment is required in progress reports for competitive applications submitted for due dates on or after January 25, 2019 (See NIH Guide Notice NOT-OD-18-116). Inclusion data for subsequent RPPRs must be provided using the file upload option.
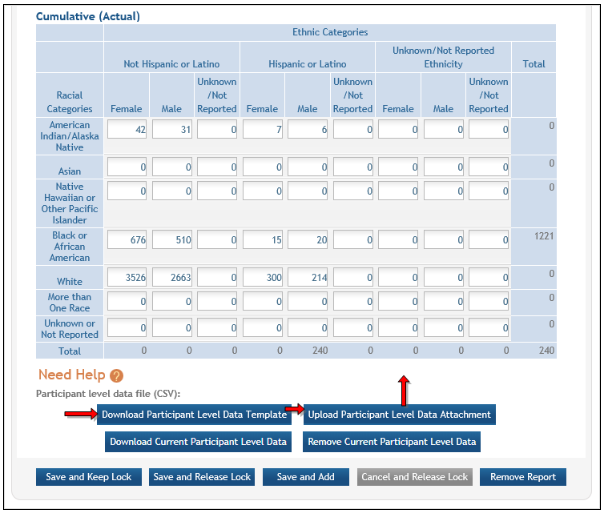
Figure 24: Cumulative (Actual) Enrollment Table
Changes to Planned Enrollment
If there are changes from the planned enrollment originally approved for funding, contact the program officer to discuss updating/revising the planned enrollment. See Chapter 6.7 Section G– Special Reporting Requirements of this guide for more information.
From the Study Record(s) screen, select the Edit link in the Action column of the Inclusion Enrollment Report section to edit the Inclusion Enrollment Report.
The planned enrollment table includes racial categories along the left side of the table and ethnic categories, divided by sex/gender, along the top of the table. The individual enrollment count cells are editable and set to zero by default, when populating a new IER. When editing an existing form, these values are pre-populated with any other value previously entered. The total fields are calculated by HSS and sum up as rows and columns accordingly. The total values are not editable fields.
POLICY: For additional information on racial and ethnic categories or inclusion policy, refer to the OER inclusion web page and FAQs.
Update the values in the individual enrollment count cells as necessary, and select the Save and Release Lock button. To leave the form without saving any changes, select the Cancel and Release Lock button instead. Saving and cancelling both return you to the Study Record(s) screen.
When inclusion monitoring is required and no IERs exist, RPPR system will NOT allow the submission of the progress report without IER(s). For the current FY it will display an error message and require that you either submit a new enrollment record.
This is true for the current FY of a multi-year award as well. For the past FYs (when the progress report is late), a standard message is displayed in lieu of the error message as follows:
NIH policy requires inclusion to be monitored, but no inclusion data record(s) (IERs) have been provided.
This standard message will appear on both the screen and the PDF version of the progress report.
From the Study Records(s) screen, select the Add New Inclusion Enrollment Report to create a new IER. If no study records exist, first add a study record using instructions in the online help.
Upon a successful save of a new IER, attributes (Foreign/domestic indicator/planned comments), Planned Inclusion Data (as entered), and Cumulative Inclusion Data (as zeroes) are also created; the new IER is assigned a unique IER #.
Budget Form (H.1)
To add a budget, choose an option from the drop-down list and select the Add Budget button. The added budget type appears in the first table. Use the Edit link in the Action column to open the form for editing. Select the Save button before exiting the form. Most awards now use the SF424 R&R budget form. However, training awards may use the SF424 and/or the PHS 398 training budget. The PHS Additional Indirect Costs budget form is available as an optional form for the Overall component of a multi-project award with multiple institutions/organizations.
Please contact the Grants Management Specialist assigned to your grant if you have questions on the appropriate form to use.
Budget types include:
SF 424 Research & Related Budget form
PHS 398 Training Budget
PHS Additional Indirect Costs form

NOTE: Budget types can be deleted by selecting the Delete link from the Action column for the specific budget type. After deleting a form, be sure to save the RPPR before navigating away from the H.Budget tab. Save buttons are located at the top and bottom of the screen.

Subaward Budget Form (H.2)
To add a subaward budget, choose an option from the drop-down list and select the Add Subaward button. The added budget type appears in the second table. Use the Edit link in the Action column to open the form for editing. Select the Save button before exiting the form.
Subaward budget types include:
SF 424 Research & Related Subaward Budget form
PHS 398 Subaward Training Budget
The grantee may select up to 30 subaward budgets.

NOTE: Subaward budget types can be deleted by selecting the Delete link from the Action column for the specific subaward.

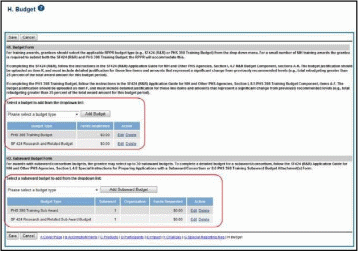
Figure 27: RPPR H. Budget - Questions H.1 Budget Form & H.2 Subaward Budget Form

NOTE: Remember to save the information before exiting the form by selecting one of the Save
buttons located at the top and bottom of the form.

For single-project RPPRs, the DUNS number will automatically populate the DUNS number of the grantee organization on the budget form.
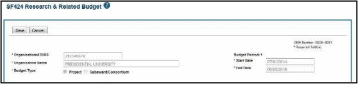
Figure 28: Organizational DUNS on SF 424 Research & Related Budget
For multi-component RPPRs the grantee must enter the DUNS and Organization Name fields, as the DUNS number will not automatically populate the DUNS number.
To add the DUNS number:
Enter the DUNS number into the Organizational DUNS field or select the magnifying glass icon to search for and select the DUNS number. You can search using a secondary DUNS number, however, the form will reflect the primary DUNS after you select the organization.
The Organizational DUNS field updates with the information and the Enter Name of Organization field updates to reflect the new DUNS.
To add the organization name:
Enter the organization name into the Organization Name field or select the magnifying glass icon to search for and select the new organization name.
The Organization Name field updates with the information and the Organizational DUNS
field updates to reflect the new organization.

NOTE: If subaward budgets are completed, the system will not calculate the budget line item
F.5 for the main budget (see figure below). Total consortium costs for the main budget MUST be computed and entered manually into budget line item F.5.

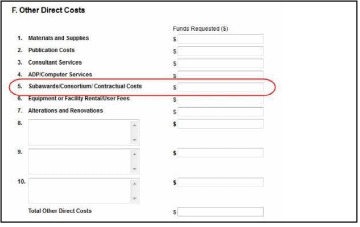
At any time before an RPPR is submitted to agency, an error check can be performed to verify that the report passes the business rules and system validations in place. Any user who has access to the RPPR may perform the error check.
Checking for Errors on Single-Project RPPRs
To perform an error check on the RPPR for single-project RPPRs, select the Check for Errors
button from the RPPR Menu screen.
Figure 30: Check for Errors Button on RPPR Menu for a Single-Project RPPR
If errors or a warning exist, the appropriate error or warning message displays for each failed occurrence. All errors must be corrected prior to submission; the system will prevent submission of an RPPR containing errors. However, the system will not prevent submission of an RPPR when a warning message is displayed.

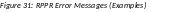 If
all validations pass, a message displays indicating: No
errors found on validation.
If
all validations pass, a message displays indicating: No
errors found on validation.
Checking for Errors on a Multi-Project RPPR
To perform an error check on the Overall or individual component of a multi-project RPPR, select the Check for Errors link from the Actions column of the RPPR Menu screen for the Overall or individual component being validated.

NOTE: Refer to section 5.3.2 Accessing a Multi-Project and Single-Project with Complicated Structure RPPR for Editing for information on adding individual components to the RPPR.

Figure 32: Check for Errors Links for Multi-Project RPPRs
If errors or a warning exist for the chosen component, the appropriate error or warning message displays for each failed occurrence. Select the Check for Errors button of the other components to perform a check against them.

All
errors must be corrected prior to submission;
the system will prevent submission of an RPPR containing errors.
However, the system will not prevent submission of an RPPR when a
warning message is displayed.
Figure 33: Errors and Warnings for One Component of a Multi-Project RPPR
If all validations pass, a message displays indicating: No errors found on validation.
Progress reports in Work in Progress (WIP) status can be routed to others for review or corrections by the current reviewer of the report. The routing feature is found on the RPPR Menu screen.

NOTE: A PD/PI delegate cannot route an RPPR to the next reviewer.

To route an RPPR to the next reviewer:
Select the Route button from the RPPR Menu screen.

NOTE: The figure above shows the RPPR Menu for a single-project RPPR, however, multiproject RPPRs have a similar Route button on their own RPPR Menu screen.

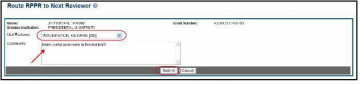
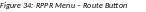 The
Route RPPR to Next Reviewer screen displays. From this screen,
the next reviewer can be chosen from a list of reviewers, and
comments can be added.
The
Route RPPR to Next Reviewer screen displays. From this screen,
the next reviewer can be chosen from a list of reviewers, and
comments can be added.
Select a reviewer from the Next Reviewer drop-down list.
Optional: Enter comments in the Comments text box to provide information to the next reviewer.

 5.
When routed by the
PD/PI only: The
PD/PI Assurance statement displays. Select the I
Agree button to
continue.
5.
When routed by the
PD/PI only: The
PD/PI Assurance statement displays. Select the I
Agree button to
continue.

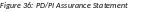 The
RPPR Menu displays once again. If the routing is successful,
the message on the screen reads as follows:
The
RPPR Menu displays once again. If the routing is successful,
the message on the screen reads as follows:
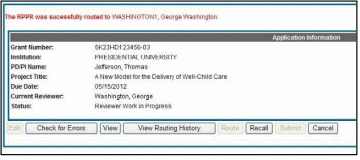
The RPPR was successfully routed to [Selected Reviewer User ID], [Selected Reviewer Name].
The person who routed the RPPR can no longer edit the report (Edit button becomes disabled). The editing feature is now available only to the new reviewer. The RPPR status is updated to Reviewer Work in Progress.
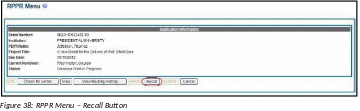
RPPRs that have been routed to a reviewer can be recalled by the person who performed the routing action. This is useful in situations when the report was routed to the wrong person or the reviewer is unavailable. The last reviewer of the report is able to recall it; however, Signing Officials at the Institution and the Contact PD/PI who are not the last reviewer can also recall the report when it is in a status of Reviewer Work in Progress.

NOTE: A PD/PI delegate does not have the ability to recall the RPPR.

To recall an RPPR, select the Recall button from the RPPR Menu screen.
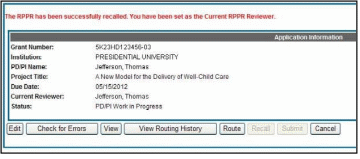
A message displays on the screen indicating: The RPPR has been successfully recalled. You have been set as the Current RPPR Reviewer.
The status of the RPPR is updated to PD/PI Work in Progress or Reviewer Work in Progress, the reviewer from whom the RPPR is recalled receives an email informing him of the action, and the RPPR routing audit history is updated to reflect the action.
Additionally, the Edit and Route buttons are enabled, providing the new reviewer with the ability to continue editing the RPPR or to route it to another reviewer.
Grantees are strongly encouraged to view the RPPR prior to submission to ensure that the correct information and attachments are provided (see 5.7 View the RPPR).
Completed and validated RPPRs in a status of Work in Progress can be submitted to the Agency for acceptance. This act is performed by the Signing Official (SO) when the SO is the current reviewer of the report. For SNAP awards only, PD/PIs may also submit the report if they have been delegated submit authority by the SO.

NOTE: A PD/PI with Progress Report authority cannot submit a non-SNAP or F RPPR.

To submit the RPPR to agency:
Select the Submit button from the RPPR Menu, Interim RPPR Menu, or Final RPPR Menu screen.
Figure 40: Submit Button on RPPR Menu
The Submit RPPR screen displays a certification statement as follows:
In submitting this RPPR [Interim-RPPR or Final RPPR], the SO (or PD/PI with delegated authority), certifies to the best of his/her knowledge that the grantee organization is in compliance with the terms and conditions specified in the Notice of Award and Grants Policy Statement, and verifies the accuracy and validity of all administrative, fiscal, and scientific information in the progress report. The SO (or PD/PI with delegated authority) further certifies that the grantee organization will be accountable for the appropriate use of any funds awarded and for the performance of the grant- supported project or activities resulting from the progress report. Deliberate withholding, falsification, or misrepresentation of information could result in administrative actions such as withdrawal of a progress report, suspension and/or termination of an award, debarment of individuals, as well as possible criminal penalties. The grantee institution may be liable for the reimbursement of funds associated with any inappropriate or fraudulent conduct of the project activity.
Select the I Agree button to sign off on the certification.
The RPPR [Interim-RPPR or Final RPPR] is validated for systemic and business rules. If there are any validation failures, they are indicated by error messages on the RPPR Menu screen. Errors must be corrected in order to submit the RPPR [Interim-RPPR or Final RPPR].
If warnings exist, they are displayed on the RPPR Menu screen. Although the RPPR can be submitted with warnings present, the warning messages should be reviewed to determine if an issue should be addressed.
If Warnings Exist: To address issues associated with warnings, select the Cancel button, correct the issue, and resubmit the RPPR again. To continue with submission despite the warnings, select the OK button.
If all validations pass, the RPPR Menu screen displays the following message: The RPPR [Interim-RPPR or Final RPPR] has been successfully submitted to PHS.
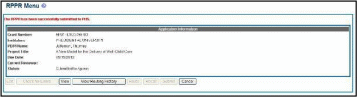
 The
current reviewer is updated to the awarding agency, the RPPR status
is updated to Submitted to Agency, and the RPPR Submission
date is recorded. The routing history is updated to reflect the
submission to Agency.
The
current reviewer is updated to the awarding agency, the RPPR status
is updated to Submitted to Agency, and the RPPR Submission
date is recorded. The routing history is updated to reflect the
submission to Agency.
Any citations associated with the RPPR in C.1. Publications are officially associated with the award in MyNCBI.
When an RPPR is submitted to Agency, email notification is sent to the PD/PI (Contact PI) on the grant and the SO and AO assigned to the RPPR.
5.6.1 Submission Errors and Warnings for Multi-Project RPPRs
Upon submission, multi-project RPPRs are validated for systemic and business rules just as are single-project RPPRs. However, for multi-projects, the errors and warnings are displayed differently. When errors and/or warnings are found on multi-project RPPRs, the RPPR Menu screen displays the Overall messages followed by the messages of the other components.
All errors must be corrected in order to submit the RPPR.
If warnings exist, they are displayed on the RPPR Menu screen under the errors. Although the RPPR can be submitted with warnings present, the warning messages should be reviewed to determine if an issue should be addressed.

Figure 42: Sample of Multi-Project RPPR Error Messages
As indicated in 5.6, grantees are strongly encouraged to view the RPPR prior to submission to ensure that the correct information and attachments are provided (see 5.6 Submit the RPPR to Agency).
PD/PIs, PD/PI delegates, and reviewers can view a PDF version of an RPPR in Work in
P ro g re ss (WIP) or Submitted to Agency status to see how it will be seen by the Agency. Until the RPPR is submitted to agency, the PDF report shows a status of Draft and a blank submission date.
To view the RPPR form, select the View button from the RPPR Menu screen.
From initiation to submission to Agency, the routing of an RPPR is captured for auditing purposes. PD/PIs, PD/PI delegates, and reviewers can view the routing history for Work in Progress or Submitted to Agency RPPRs at any time, even when they are not the current reviewer.
Select the View Routing History button from the RPPR Menu screen.
Figure 44: RPPR Menu – View Routing History Button
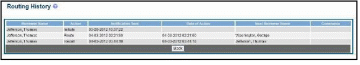
The Routing History screen displays showing the Reviewer Name, Action, Notification Sent
(date and time), Date of Action, Next Reviewer Name, and Comments (when available).
 To
close the screen, select the Back
button.
To
close the screen, select the Back
button.
The completed RPPR, in PDF format, is accessible in Commons within the Status Information screen. To view the final RPPR, perform the following steps:
From Commons, select the Status menu option.
Select the link for List of Applications/Grants.
Figure 47: Application ID hyperlink
The Status Information screen displays with the Other Relevant Documents section in the top right corner.
The progress reports for incrementally-funded and multi-year funded awards are displayed differently in Other Relevant Documents.
For an incrementally-funded RPPR: Select the e-Application link from the
Other Relevant Documents section of the Status Information screen.
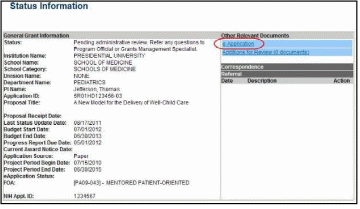
Figure 48: Status Information Screen – e-Application Link
For multi-year funded awards: Select the appropriate year’s link in the Research Performance Progress Report section. Links will appear as follows: RPPR Year <X> <MM/DD/YYYY>
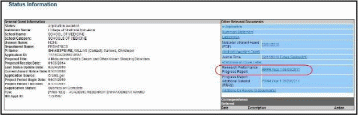
Figure 49: Status Information Screen for Multi-Year RPPR
The PDF version of the RPPR opens in a separate window.

NOTE: The submitted RPPR can also be accessed from the RPPR Menu screen. The View
button opens the PDF version of the RPPR.

The Public Access Progress Report Additional Materials (PRAM) feature provides a means for the grantee to enter, review, and submit information in response to the automated notification sent when an NIH grantee organization submits an RPPR with non-compliant publications. The system sends the automated email to the PD/PI requesting verification that all publications are in compliance with the NIH Public Access Policy. The SO and AO assigned to the RPPR on the cover page (see 6.1 Section A – Cover Page) will receive a copy (cc:) of the email. While an email response to the GMS and PO is acceptable at this time, the grantee is encouraged to respond using the Public Access PRAM feature in eRA Commons. AHRQ does not currently use the PRAM feature for public access compliance notifications.
Using the PRAM feature, grantees can upload and submit a My NCBI PDF report demonstrating that previously non-compliant papers reported on the RPPR are now compliant. Compliant papers have a status of Complete, N/A (not applicable), PMC Journal in Process, or In process at NIHMS. Please see http://publicaccess.nih.gov/include-pmcid-citations.htm for additional information. If unable to provide the verification of compliance, grantees can upload and submit justification for why specific publications cannot be brought into compliance.
As with the RPPR, a PD/PI (or Contact PI in the case of multiple PIs) can enter the Public Access PRAM, but can only submit it if the PD/PI is delegated with Submit Progress Report authority. Otherwise, only the SO can submit the PRAM to Agency.
The following sections cover the steps for initiating and submitting Public Access PRAM.
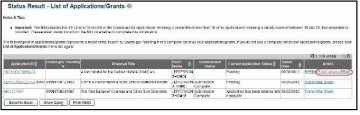
The PD/PI (Contact PI) or PD/PI Delegate can initiate Public Access PRAM by following the steps below:
Access the eRA Commons Status Result – List of Applications/Grants screen.
Select the Public Access PRAM link from the Action column of the appropriate grant.
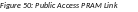 The
Progress Report
Additional Materials (PRAM) screen
displays. Grant
Information including
Grant Number, PD/PI Name, Project Title, Institution, Status, and
Current Reviewer displays at the top of the screen. The Public
Access Compliance section
at the bottom contains guidance for responding to the automated email
requesting evidence of compliance with a field and buttons for
uploading and maintaining attachments.
The
Progress Report
Additional Materials (PRAM) screen
displays. Grant
Information including
Grant Number, PD/PI Name, Project Title, Institution, Status, and
Current Reviewer displays at the top of the screen. The Public
Access Compliance section
at the bottom contains guidance for responding to the automated email
requesting evidence of compliance with a field and buttons for
uploading and maintaining attachments.
Use the Add Attachment button to browse and select the My NCBI PDF or another PDF document providing justification. Note that selecting the Cancel button closes the screen instead.
Select the Route button at the bottom of the screen.


NOTE: The options for Delete Attachment and View Attachment display once an attachment has been uploaded.

Optional: Select the View Attachment button to view the document. Select the Delete Attachment button to remove the document.
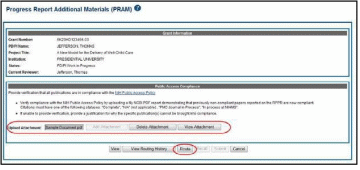
When the Route button is selected, the Route PRAM to Next Reviewer screen displays. A list of all available reviewers exists in the drop-down for Next Reviewer.
Select an SO from the Next Reviewer drop-down list.
Enter text into the Comments field as necessary. This is not a mandatory field.
Select the Submit button to continue.
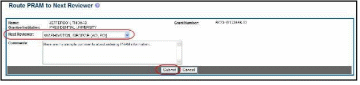
Figure 52: Route Public Access PRAM to Next Reviewer
The Route PRAM to Next Reviewer screen displays the PD/PI Assurance statement.
Read the assurance statement and select the Submit button to agree to the content and continue routing the PRAM to the next reviewer.

Figure 53: Public Access PRAM PD/PI Assurance Statement
The Progress Report Additional Materials (PRAM) screen displays with a message indicating that the PRAM was successfully routed to the selected reviewer. Additionally, the status is updated and shown as Reviewer Work in Progress. At this point, the PD/PI can only view the PRAM and may not edit it. To be able to allow the PD/PI to edit the PRAM, the SO needs to route the PRAM back to the PD/PI using steps similar to those above.
At the time of routing, an email is sent to the PD/PI and the selected SO (or other Next Reviewer) to notify them of the event.
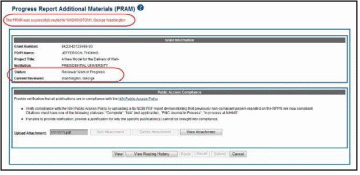
Public Access PRAM that has been routed to a reviewer can be recalled by the person who performed the routing action until the PRAM has been submitted to the Agency. This is useful in situations when the report was routed to the wrong person or the reviewer is unavailable. The last reviewer of the report is able to recall it; however, Signing Officials at the Institution and the Contact PD/PI who are not the last reviewer can also recall the report when it is in a status of Reviewer Work in Progress.

NOTE: A PD/PI delegate does not have the ability to recall the PRAM.

To recall Public Access PRAM, select the Recall button from the Progress Report Additional Materials (PRAM) screen.
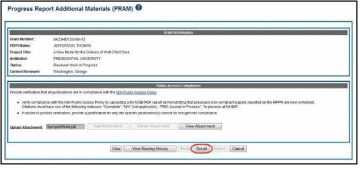
 A
message displays on the screen indicating: The
PRAM has been successfully recalled. You have been set as the Current
PRAM Reviewer.
A
message displays on the screen indicating: The
PRAM has been successfully recalled. You have been set as the Current
PRAM Reviewer.
The status of the PRAM is updated to PD/PI Work in Progress if recalled by the PI or Reviewer Work in Progress if recalled by the SO; the reviewer from whom the PRAM is recalled receives an email informing him of the action; and the PRAM routing audit history is updated to reflect the action.
Additionally, the ability to delete the attachment is restored and the Route button is enabled, providing the current reviewer the ability to update the PRAM and route it again.
When the Public Access PRAM is in Reviewer Work in Progress status, the Signing Official (SO) can submit it to the Agency. PD/PIs may also submit the information if they have been delegated Submit Progress Report authority by the SO.
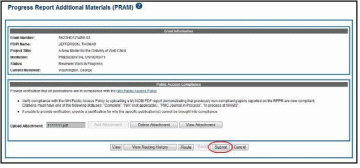
To submit the Public Access PRAM:
Access the Status screen on eRA Commons.
Enter the appropriate query parameters to locate the grant and select the Search button. The Status Result – General Search screen displays with the matching information.
From the Action column, select the link for PRAM.
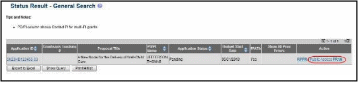
 The
Progress Report Additional Materials (PRAM) screen displays.
The screen displays Grant Information on top and the PD/PI
comments in the text box at the bottom of the screen. In addition to
submitting the PRAM, from this screen, the SO also may View the
PRAM as a PDF, Route it to another reviewer (or back to the
PD/PI), and view the Route History. Select any of the
appropriate buttons to perform these actions. Follow the steps below
to continue submitting the PRAM.
The
Progress Report Additional Materials (PRAM) screen displays.
The screen displays Grant Information on top and the PD/PI
comments in the text box at the bottom of the screen. In addition to
submitting the PRAM, from this screen, the SO also may View the
PRAM as a PDF, Route it to another reviewer (or back to the
PD/PI), and view the Route History. Select any of the
appropriate buttons to perform these actions. Follow the steps below
to continue submitting the PRAM.

 Select
the Submit
button.
Select
the Submit
button.
 The
Submit PRAM to Agency screen displays. By continuing from this
screen, the SO certifies that the submitting organization is in
compliance with the terms and conditions specified in the Notice of
Award and Grants Policy Statement. The SO also verifies that the
information provided in the PRAM is valid and accurate.
The
Submit PRAM to Agency screen displays. By continuing from this
screen, the SO certifies that the submitting organization is in
compliance with the terms and conditions specified in the Notice of
Award and Grants Policy Statement. The SO also verifies that the
information provided in the PRAM is valid and accurate.
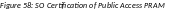 The
Progress Report Additional Materials (PRAM) screen displays
with a message indicating that the PRAM was successfully submitted.
The current reviewer is updated to the awarding agency, the PRAM
status is updated to Submitted to Agency, and the PRAM
submission date is recorded. The routing history is updated to
reflect the submission to Agency.
The
Progress Report Additional Materials (PRAM) screen displays
with a message indicating that the PRAM was successfully submitted.
The current reviewer is updated to the awarding agency, the PRAM
status is updated to Submitted to Agency, and the PRAM
submission date is recorded. The routing history is updated to
reflect the submission to Agency.
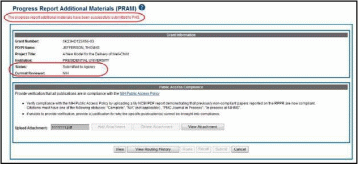
 When
PRAM is submitted to Agency, an email notification is sent to the
PD/PI (Contact PI) on the grant, the submitting SO, the SO assigned
to the RPPR, Interim-RPPR, or Final RPPR and AO assigned to the RPPR
and the Public Access PRAM link will no longer be available.
When
PRAM is submitted to Agency, an email notification is sent to the
PD/PI (Contact PI) on the grant, the submitting SO, the SO assigned
to the RPPR, Interim-RPPR, or Final RPPR and AO assigned to the RPPR
and the Public Access PRAM link will no longer be available.

NOTE: To view the submitted PRAM, select the View button on the Progress Report Additional Materials (PRAM) screen. This option opens the PRAM PDF in a separate window. The Public Access PRAM will appear as the final page of the PDF document. See below for display of IC Requested PRAM.

After submitting Public Access PRAM for multi-year funded awards, users with access to the grant information may view the PRAM via the Status Information screen. Status Information is accessed by selecting the Application ID hyperlink from the Status Result – General Search (SOs) and Status Result – List of Applications/Grants (PIs) screens.
Figure 60: Status Results Showing Hyperlink for Application ID
From the Status Information screen, select the hyperlink in the area marked Progress Report Additional Material (PRAM) in the Other Relevant Documents section. The PRAM links for multi-year funded awards display as PRAM Year <X> <date submitted>.
Figure 61: MYF Award PRAM Link in Status Information
The Agency Requested Progress Report Additional Materials (PRAM) feature provides a means for the grantee to enter, review, route, and submit information in response to specific request(s) by the Grants Management Specialist at the IC (or AHRQ, if applicable) for additional information following the submission of an RPPR.
As with the RPPR, a PD/PI (or Contact PI in the case of multiple PIs) can enter the PRAM, but can only submit it if the PD/PI is delegated with Submit Progress Report authority. Otherwise, only the SO can submit the PRAM to Agency.
The following sections cover the steps for initiating and submitting Agency Requested PRAM.

NOTE: Agency requested PRAM is not available for multi-year funded awards at this time.

The PD/PI (Contact PI) or PD/PI Delegate can initiate Agency Requested PRAM by following the steps below:
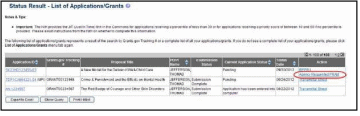
Access the eRA Commons Status Result – List of Applications/Grants screen.
Select the Agency Requested PRAM link from the Action column of the appropriate grant.
 The
Progress Report
Additional Materials (PRAM) screen
displays. Grant
Information,
including Grant Number, PD/PI Name, Project Title, Institution,
Status, and Current Reviewer, displays at the top of the screen. The
Additional Materials
Requested by Agency section
at the bottom provides a means for adding the requested materials. Up
to 100 attachments can be submitted, but all attachments must be in
the form of PDF files.
The
Progress Report
Additional Materials (PRAM) screen
displays. Grant
Information,
including Grant Number, PD/PI Name, Project Title, Institution,
Status, and Current Reviewer, displays at the top of the screen. The
Additional Materials
Requested by Agency section
at the bottom provides a means for adding the requested materials. Up
to 100 attachments can be submitted, but all attachments must be in
the form of PDF files.
Select the Add Attachment button in the Additional Materials Requested by Agency
section of the screen.
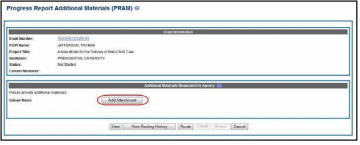
Figure 63: Add Attachment Button for Agency Requested PRAM
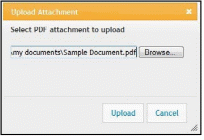
Use the Upload Attachment pop-up Browse and Upload buttons to search for and attach the appropriate file. Repeat for all necessary attachments.
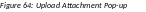 The
Additional Materials
Requested by Agency section
updates to show a table of all attachments. The table displays the
Document Name and
Action links
of View and
Delete for
each attachment.
The
Additional Materials
Requested by Agency section
updates to show a table of all attachments. The table displays the
Document Name and
Action links
of View and
Delete for
each attachment.
Optional: Select the document’s View link in the Action column to view the attachment.
Optional: Select the document’s Delete link in the Action column to remove the attachment.

NOTE: The options for View and Route History may be selected at this time. Selecting the option for Cancel closes the screen without saving or routing the PRAM information.

Select the Route button to send the PRAM for review.
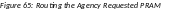 When
the Route button
is selected, the Route
PRAM to Next Reviewer screen
displays. A list of all available reviewers exists in the drop-down
for Next Reviewer.
When
the Route button
is selected, the Route
PRAM to Next Reviewer screen
displays. A list of all available reviewers exists in the drop-down
for Next Reviewer.
Select a name from the Next Reviewer drop-down list.
Enter text into the Comments field as necessary. This is not a mandatory field.
Select the Submit button to continue.
Figure 66: Route Agency Requested PRAM to Next Reviewer
The Route PRAM to Next Reviewer screen displays the PD/PI Assurance statement.
Read the assurance statement and select the Submit button to agree to the content and continue routing the PRAM to the next reviewer.

Figure 67: Agency Requested PRAM PD/PI Assurance Statement
The Progress Report Additional Materials (PRAM) screen displays with a message indicating that the PRAM was successfully routed to the selected reviewer. Additionally, the status is updated and shown as Reviewer Work in Progress. At this point, the PD/PI can only view the PRAM, the attachments, and the Route History; the PD/PI may not edit the PRAM. To be able to allow the PD/PI to edit the PRAM, the SO needs to route the PRAM back to the PD/PI using routing steps similar to those above.
At the time of routing, an email is sent to the PD/PI and the selected SO (or other Next Reviewer) to notify them of the event.
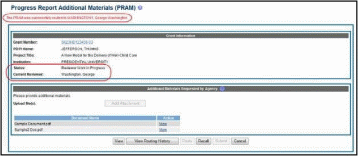
Figure 68: Successfully Routed Agency Requested PRAM
Agency Requested PRAM that has been routed to a reviewer can be recalled by the person who performed the routing action up until the submission of the current PRAM attachment(s) to the Agency. This is useful in situations when the report was routed to the wrong person or the reviewer is unavailable. The last reviewer of the report is able to recall it; however, Signing Officials at the Institution and the Contact PD/PI who are not the last reviewer can also recall the report when it is in a status of Reviewer Work in Progress.

NOTE: A PD/PI delegate does not have the ability to recall the PRAM.

To recall Agency Requested PRAM, select the Recall button from the Progress Report Additional Materials (PRAM) screen.
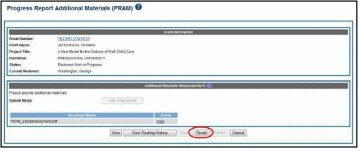
Figure 69: Recall Button for Agency Requested PRAM
A message displays on the screen indicating: The PRAM has been successfully recalled. You have been set as the Current PRAM Reviewer.

NOTE: Only items which have not already been submitted can be recalled. If prior Agency Requested PRAM submissions to the Agency exist, these attachments are not included in the recall.

The status of the PRAM is updated to PD/PI Work in Progress if recalled by the PI or Reviewer Work in Progress if recalled by the SO; the reviewer from whom the PRAM is recalled receives an email informing him of the action; and the PRAM routing audit history is updated to reflect the action.
Additionally, the ability to delete the attachment is restored and the Add Attachment and Route buttons are enabled, providing the current reviewer the ability to update the PRAM and route it again.
When the Agency Requested Progress Report Additional Materials (PRAM) is in Reviewer Work in Progress status, the Signing Official (SO) can submit it to the Agency. PD/PIs may also submit the information if they have been delegated Submit Progress Report authority by the SO.
To submit the PRAM:
Access the Status screen on eRA Commons.
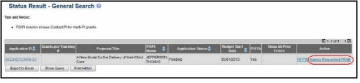
 The
Progress Report
Additional Materials (PRAM) screen
displays. The screen displays Grant
Information on top
and the files attached by the PD/PI in the Additional
Materials Requested by Agency portion
at the bottom. The attached files may be viewed or removed and
additional PDF files may be added if necessary.
The
Progress Report
Additional Materials (PRAM) screen
displays. The screen displays Grant
Information on top
and the files attached by the PD/PI in the Additional
Materials Requested by Agency portion
at the bottom. The attached files may be viewed or removed and
additional PDF files may be added if necessary.
Optional: Select the document’s View link in the Action column to view the attachment.
Optional: Select the document’s Delete link in the Action column to remove the attachment.
Optional: Select the Add Attachment button to attach additional files. Up to 100 PDF files may be attached.
Before submitting, the SO also may View the PRAM as a PDF, Route it to another reviewer (or back to the PD/PI), and view the Route History. Select any of the appropriate buttons to perform these actions. Follow the steps below to continue submitting the PRAM.
Select the Submit button.
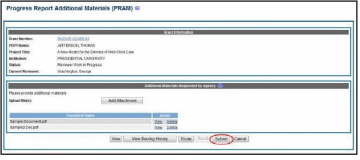
 The
Submit PRAM to Agency screen displays. By continuing from this
screen, the SO certifies that the submitting organization is in
compliance with the terms and conditions specified in the Notice of
Award and Grants Policy Statement. The SO also verifies that the
information provided in the PRAM is valid and accurate.
The
Submit PRAM to Agency screen displays. By continuing from this
screen, the SO certifies that the submitting organization is in
compliance with the terms and conditions specified in the Notice of
Award and Grants Policy Statement. The SO also verifies that the
information provided in the PRAM is valid and accurate.
Read certification agreement. Select the I Agree button to continue submitting the information. (Selecting the Cancel button closes the screen and returns the Progress Report Additional Materials screen without submitting the material.)
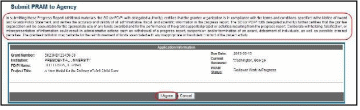
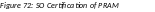 The
Progress Report Additional Materials (PRAM) screen displays
with a message indicating that the PRAM was successfully submitted.
The current reviewer is updated to NIH, the PRAM status is updated to
Submitted to Agency, and the PRAM submission date is recorded.
The routing history is updated to reflect the submission to Agency.
The
Progress Report Additional Materials (PRAM) screen displays
with a message indicating that the PRAM was successfully submitted.
The current reviewer is updated to NIH, the PRAM status is updated to
Submitted to Agency, and the PRAM submission date is recorded.
The routing history is updated to reflect the submission to Agency.
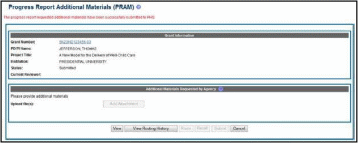
Figure 73: Agency Requested PRAM Submitted to Agency
When PRAM is submitted to Agency, an email notification is sent to the PD/PI (Contact PI) on the grant, the submitting SO, the SO assigned to the RPPR, and AO assigned to the RPPR.
Once the IC Requested PRAM is submitted, the View button remains on the PRAM screen to provide a preview of the latest PRAM submission; however, the ability to view or delete the individual attachments is removed. The ability to upload and submit additional attachments remains until the grant is awarded. Follow the steps provided in the Initiate Agency Requested PRAM section to add additional attachments (starting with Step 3).

NOTE: If multiple PRAM submissions were completed, selecting the View button only provides a preview of the latest PRAM submission. To view all submissions as one document, access the Status Information screen for the grant and select the PRAM link. For more information, refer to the View Agency Requested PRAM from Status Information section of this document.

After submitting Agency Requested PRAM, Commons users with access to the grant information may view the PRAM via the Status Information screen. The Status Information is accessed by selecting the Grant Number hyperlink from the Progress Report Additional Materials (PRAM) screen or by selecting the Application ID hyperlink from Status Result – General Search (SOs) and Status Result – List of Applications/Grants (PIs) screens.
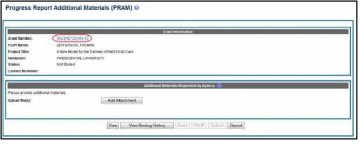
Figure 74: Grant Number Hyperlink on PRAM Screen
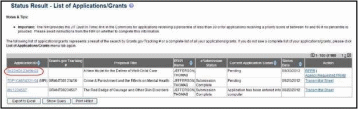
Figure 75: Application ID Hyperlink on Status Result for PIs
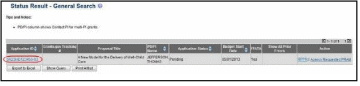
Figure 76: Application ID Hyperlink on Status Result for SOs
From the Status Information screen, select the hyperlink in the area marked Progress Report Additional Material (PRAM) in the Other Relevant Documents section.
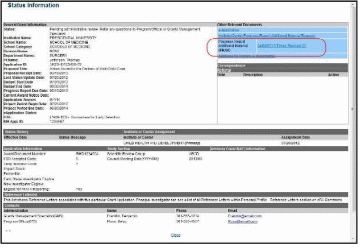
 The
Progress Report Additional Materials file opens as a PDF document.
The file is formatted to provide an information header section for
each PRAM submission followed by the attached documents provided
during that submission. If multiple submissions of Agency Requested
PRAM were completed, the additional materials are separated in the
document with the most recent submission displayed first followed by
earlier submissions in reverse chronological order. Information in
the document can be navigated using the provided bookmarks on the
left.
The
Progress Report Additional Materials file opens as a PDF document.
The file is formatted to provide an information header section for
each PRAM submission followed by the attached documents provided
during that submission. If multiple submissions of Agency Requested
PRAM were completed, the additional materials are separated in the
document with the most recent submission displayed first followed by
earlier submissions in reverse chronological order. Information in
the document can be navigated using the provided bookmarks on the
left.
Figure 78: PDF of Multiple Submitted PRAM

The instructions in chapter 6 apply to the following awards: D71, DP1, DP5, G08, G11, G13, P40, R00, R01, R03, R18, R21, R33, R34, R36, R37, R56, RC1, RC2, RL1, S21, S22, SC1,
SC2, SC3, U1B, UC2, UH1, UH2, and UH3; and also to the following NIH multi-year funded awards, including: C06, DP2, DP3, DP4, R15, R55, RC3, RC4, RF1, UA5, UC4, UC6, UF1.
Note that AHRQ has not yet implemented the RPPR for any AHRQ multi-year funded awards.
Many of these instructions apply to other awards, but there may be exceptions (items that are not applicable, replace, or are in addition) for awards not listed above. Refer to the table in chapter 7 Supplemental Instruction for Specific Grant Award Types, and follow the appropriate instruction for the applicable activity code of other awards. Activity codes listed in 7.6 Multi-Project Awards and Single-Project Awards with Complicated Structure that are issued under the Streamlined Non-competing Award Process (SNAP) will complete the RPPR as described in this section. The electronic RPPR display is dynamic and shows the appropriate questions and instructions based on the activity code and SNAP status of the award.
![]() Agency-specific
reporting requirements and instructions are denoted by the DHHS logo
displayed to the left of the requirement or instruction, as
illustrated here.
Agency-specific
reporting requirements and instructions are denoted by the DHHS logo
displayed to the left of the requirement or instruction, as
illustrated here.
Not Applicable next to a particular item indicates that item does not apply to the particular kind of award, and the item should be ignored.
References to competing application instructions means either the SF424 (R&R) Application Guides (http://grants.nih.gov/grants/funding/424/index.htm) or the PHS 398 Grant Application (http://grants.nih.gov/grants/funding/phs398/phs398.html).
The RPPR may not be used for prior approval requests, with the exception of requesting prior approval for a reduction in the level of effort of the PD/PI or other senior/key personnel named in the Notice of Award under D.2 of the RPPR. All other prior approval requests must be submitted directly to the Grants Management Officer of the awarding component in accord with the Grants Policy Statement, 8.1.2.
The RPPR section A. Cover Page includes information about the award, PD/PI, organization, and project/reporting/budget periods. Much of this information is pre-populated from data in eRA systems, but certain fields are editable.
The addresses, emails and phone numbers are pre-populated from the Commons Profile. To update contact information as displayed, go to the Commons Profile and save the changes there.
To select a Signing Official and Administrative Official, choose a name from the associated drop-down box. The SO and AO may be the same individual. The SO need not be the SO that submits the RPPR.
If there is a change to the Contact PD/PI (Multiple-PD/PI awards only), select the Yes radio button and enter the Commons ID of the new Contact PD/PI in the associated field. The change in Contact PD/PI does not take effect until the agency accepts the report and issues a NoA. The Contact PD/PI must have a PD/PI role in the eRA Commons and must be associated with the grantee institution. The RPPR is not an appropriate vehicle for a prior approval request to change, add, or delete PD/PIs.

The Recipient ID field allows the grantee to record an internal tracking number or identifier for its own use. It is not a mandatory field and the awarding agency will disregard the information.
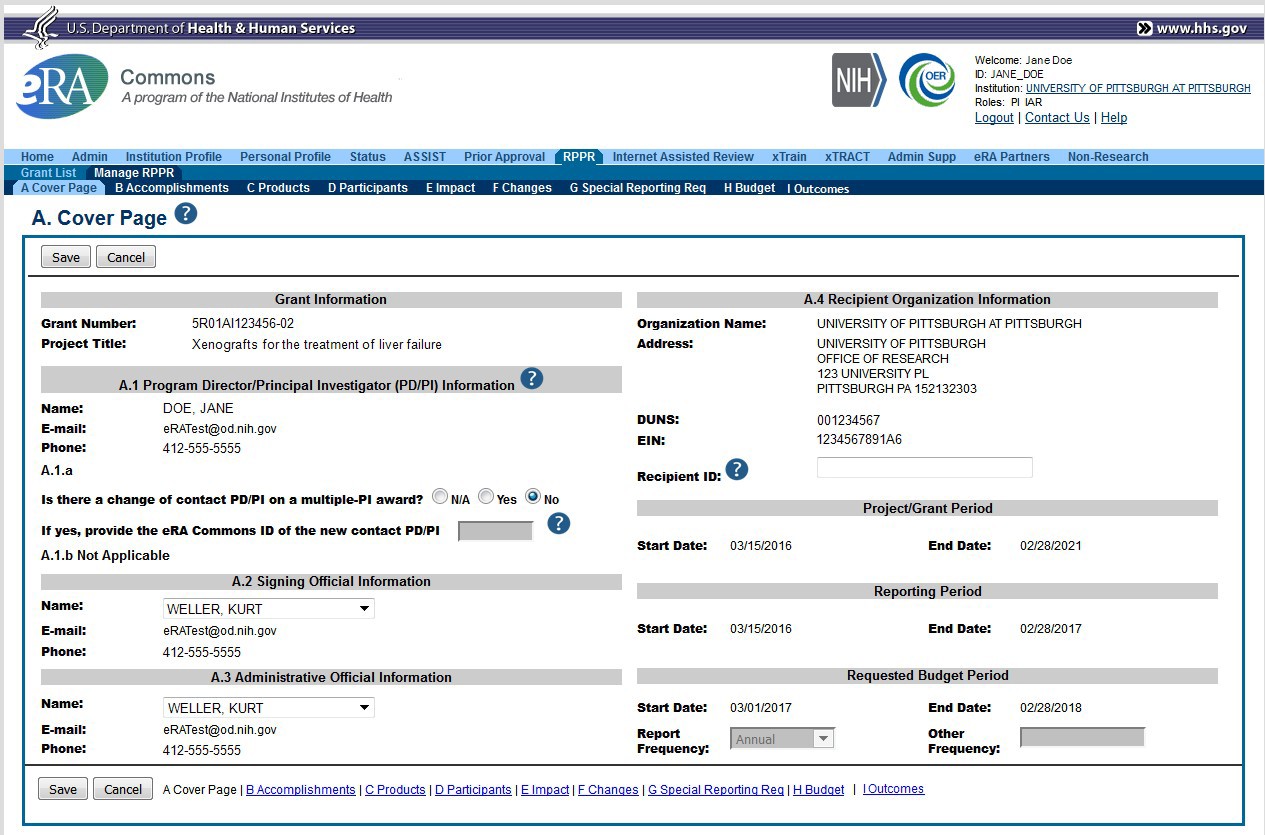
Figure 79: Section A, cover page
The RPPR section B. Accomplishments allows the agency to assess whether satisfactory progress has been made during the reporting period.
PD/PIs are reminded that the grantee is required to obtain prior written approval from the awarding agency grants official whenever there are significant changes in the project or its direction. See agency-specific instructions for submission of these requests.
What are the major goals of the project?
List the major goals of the project as stated in the approved application or as approved by the agency. If the application lists milestones/target dates for important activities or phases of the project, identify these dates and show actual completion dates or the percentage of completion.
Generally, the goals will not change from one reporting period to the next. However, if the awarding agency approved changes to the goals during the reporting period, list the revised goals and objectives. Also explain any significant changes in approach or methods from the agency approved application or plan.

![]() Goals
are equivalent to specific aims. Significant changes in
objectives and scope require prior approval of the agency (e.g., NIH
Grants Policy Statement, 8.1.2).
Goals
are equivalent to specific aims. Significant changes in
objectives and scope require prior approval of the agency (e.g., NIH
Grants Policy Statement, 8.1.2).
NOTE: The specific aims must be provided in the initial RPPR (i.e., first non-competing type 5 submission). In subsequent RPPRs this section will pre-populate with the aims/goals previously entered, and may be amended by answering Yes to question B.1.a.

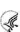

Select Yes if the major goals/specific aims have changed since the initial competing award or previous report, and provide a revised description of major goals/specific aims. Remember that written prior approval from the awarding agency grants official is required for significant changes in the project or its direction. The RPPR is not an appropriate vehicle to request such a change.
The first year that an RPPR is submitted any revised goals should be entered into the text box for
B.1. In subsequent years, if the user selects Yes the text box under B.1.a for entering revised major goals will be provided.
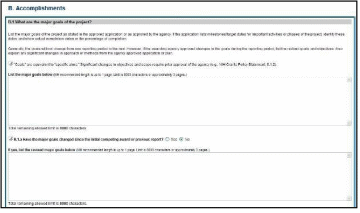
Figure 80: RPPR Section B. Accomplishments – Question B1
What was accomplished under these goals?
For this reporting period describe: 1) major activities; 2) specific objectives; 3) significant results, including major findings, developments, or conclusions (both positive and negative); and
4) key outcomes or other achievements. Include a discussion of stated goals not met. As the project progresses, the emphasis in reporting in this section should shift from reporting activities to reporting accomplishments. . (Note: If citing references in this section, please provide a full citation. List all products from this reporting period in section C.)
![]() Goals
are equivalent to specific aims. In the response,
emphasize the significance of the findings to the scientific field.
Include the approaches taken to ensure robust and unbiased results.
For most NIH awards, the response should not exceed 2 pages.
Goals
are equivalent to specific aims. In the response,
emphasize the significance of the findings to the scientific field.
Include the approaches taken to ensure robust and unbiased results.
For most NIH awards, the response should not exceed 2 pages.
For this reporting period, is there one or more Revision/Supplement associated with this award for which reporting is required?
If yes, identify the Revision(s)/Supplements(s) by grant number (e.g., 3R01CA098765-01S1) or title and describe the specific aims and accomplishments for each Revision/Supplement funded during this reporting period. Include any supplements to promote diversity or re-entry, or other similar supplements to support addition of an individual or a discrete project.
The NoA will indicate any reporting requirements. Be advised that the NoA incorporates requirements of the FOA that may also include reporting requirements.
Select the Add/New button to add the data to the table.
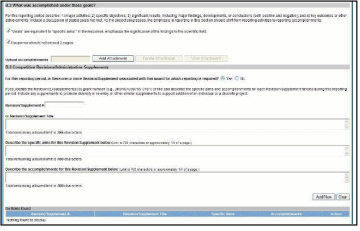
Figure 81: RPPR Section B. Accomplishments – Questions B2 & B3
What opportunities for training and professional development has the project provided?
If the research is not intended to provide training and professional development opportunities or there is nothing significant to report during the reporting period, select Nothing to Report.
Describe opportunities for training and professional development provided to anyone who worked on the project or anyone who was involved in the activities supported by the project. Training activities are those in which individuals with advanced professional skills and experience assist others in attaining greater proficiency. Training activities may include, for example, courses or one-on-one work with a mentor. Professional development activities result in increased knowledge or skill in one’s area of expertise and may include workshops, conferences, seminars, study groups, and individual study. Include participation in conferences, workshops, and seminars not listed under major activities.
![]() For
all projects reporting graduate students and/or postdoctoral
participants in Section D., describe whether your institution has
established Individual Development Plans
For
all projects reporting graduate students and/or postdoctoral
participants in Section D., describe whether your institution has
established Individual Development Plans
(IDPs) for those participants. Do not include the actual IDP, instead include information to describe how IDPs are used, if they are used, to help manage the training for those individuals. This information is not required for AHRQ grantees.
![]() For
T, F, K, R25, R13, D43 and other awards or award components designed
to provide training and professional development opportunities, a
response is required. Follow the
For
T, F, K, R25, R13, D43 and other awards or award components designed
to provide training and professional development opportunities, a
response is required. Follow the
Figure 82: RPPR Section B. Accomplishments – Question B4
Describe how the results have been disseminated to communities of interest. Include any outreach activities that have been undertaken to reach members of communities who are not usually aware of these research activities, for the purpose of enhancing public understanding and increasing interest in learning and careers in science, technology, and the humanities.
![]() Reporting
the routine dissemination of information (e.g., websites, press
releases) is not required. For awards not designed to disseminate
information to the public or conduct
Reporting
the routine dissemination of information (e.g., websites, press
releases) is not required. For awards not designed to disseminate
information to the public or conduct
similar outreach activities, a response is not required and the grantee should select Nothing to Report. A detailed response is only required for awards or award components that are designed to disseminate information to the public or conduct similar outreach activities. Note that scientific publications and the sharing of research resources will be reported under Products.
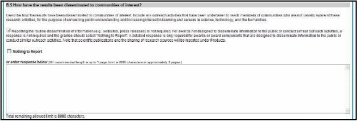
Figure 83: RPPR Section B. Accomplishments – Question B5
![]() Describe
briefly what you plan to do during the next reporting period to
accomplish the goals and objectives.
Describe
briefly what you plan to do during the next reporting period to
accomplish the goals and objectives.
Discuss efforts to ensure that the approach is scientifically rigorous and results are robust and unbiased.
![]() Remember
that significant changes in objectives and scope require prior
approval of the agency (e.g., NIH Grants Policy Statement, 8.1.2.).
Remember
that significant changes in objectives and scope require prior
approval of the agency (e.g., NIH Grants Policy Statement, 8.1.2.).
Include any important modifications to the original plans. Provide a scientific justification for any changes involving research with human subjects or vertebrate animals. A detailed description of such changes must be provided under Section F. Changes.
Not applicable for Interim and Final RPPRs.
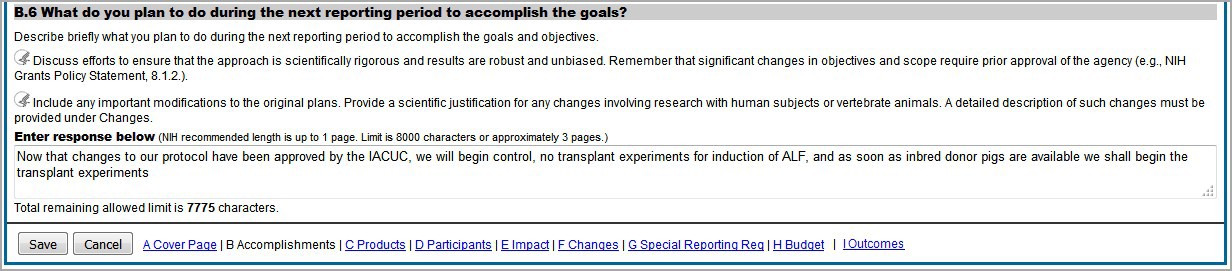
Figure 84: RPPR Section B. Accomplishments – Question B6
The RPPR section C. Products allows agencies to assess and report both publications and other products to Congress, communities of interest, and the public.
![]() The
product types
listed in
Sections C.1
– C.
5 below
are not
mutually exclusive.
Please select one
primary product
type under
which to
provide a
description for
the product,
and assign additional product types, if applicable.
Limit the response to this reporting period.
The
product types
listed in
Sections C.1
– C.
5 below
are not
mutually exclusive.
Please select one
primary product
type under
which to
provide a
description for
the product,
and assign additional product types, if applicable.
Limit the response to this reporting period.
 Publications.
Publications.
Are there publications or manuscripts accepted for publication in a journal or other publication (e.g., book, one-time publication, monograph, preprint) during the reporting period resulting directly from the award?
What to Report
Grantees must report publications in section C.1 if: (1) the publication was accepted for publication or made public during the the initial reporting period and the period since the last progress report was submitted”and (2) the publication directly arises from the award (e.g., the award supported trainees or personnel activity that contributed to the publication, such as authorship, consulting with authors, preparing manuscripts, running analyses reported in the publication).
Publications listed in other parts of the RPPR will not be tracked as award products.

Publications can include preprints, which are complete and public draft of a scientific document. Preprints are typically un-reviewed versions of peer-reviewed journal articles. Publications that are not peer-reviewed are not covered by the NIH Public Access Policy. See FAQ for more information.
To claim an interim research product as a product of an NIH award, the NIH expects that the awardee has:
Made the product publicly available. To maximize the impact of an interim research product, the NIH strongly encourages awardees to select a Creative Commons Attribution (CC-BY) license or dedicate their work to the public domain.
In the text of the document:
Acknowledged NIH funding in accordance with NIH Grants Policy Statement Chapter 8.2.1
Clearly stated that the work is not peer-reviewed
Declared any competing interests, as an author would do for any journal article
Publications that fall under the NIH Public Access Policy and are non-compliant must be reported. NIH awardees are responsible for public access compliance of all the publications listed in section C1. Generally, it takes weeks to bring non-compliant publications into compliance; PD/PIs are advised to do so as soon as possible to ensure their award is renewed in a timely manner. For more information, see Manage Compliance with the NIH Public Access Policy in My NCBI and the NIH Public Access website.
When an award’s only contribution to a publication is non-personnel resources (e.g., materials, equipment, data), applicants should not list the paper in section C. The awardee is neither responsible for providing a full listing of these publications, nor for ensuring compliance of these publications with the public access policy. Awardees submitting an RPPR may list these papers in Section B.2 which requests a description of accomplishments, including other achievements. These publications will not count against the section B.2's two-page limit and will not be tracked as a product of this award.
How to Report
If there are publications to report select Yes and ensure that the Associate with this RPPR box is checked as appropriate. If there are no publications to report select No. The tables draw information from the PD/PI’s My NCBI account.
Note that the publication data in these tables is dynamic until the progress report is submitted to the agency. Any change to the data occurring in PubMed, PubMed Central, the PD/PI’s My Bibliography account, or in the compliance status of a publication, will refresh upon saving the C.1 Products section, or opening the RPPR in another session. When the progress report is submitted to the agency, the publication data is frozen in the progress report.
Table 1: All Publications Associated with this Project in My NCBI
The first table, All Publications Associated with this Project in My NCBI, lists all publications that are in the PD/PI’s My Bibliography collection, are associated with this award, and have not been reported in previous electronic progress reports for this award.

The first column Associate with this RPPR is automatically checked. Leaving the box checked upon submission does the following: (1) associates the publication with this progress report; (2) results in the publication being displayed in RePORT; and (3) makes the award-publication association in My NCBI permanent and the association will be reported in PubMed. Unchecking the box does the following: (1) disassociates the publication with this progress report, and (2) upon submission of the RPPR to NIH, removes the award-publication association in My NCBI.
The second column, NIH Public Access Compliance, indicates the current compliance status with the NIH Public Access Policy. This information is from My NCBI. The compliance status for AHRQ grantees will be indicated as NA (not applicable) until such time as AHRQ implements a public access policy.
Table 2: Publications Not Associated with this Project in My NCBI
The second table, Publications not associated with this project in My NCBI, lists all other publications that are listed in the PD/PI’s My Bibliography collection with no association to this award. Checking Associate with this RPPR box will associate a publication with the award both in the progress report and in My NCBI.
Refreshing this screen (i.e., clicking the Save button) will also move the newly associated publications from this table to the first table. Similarly, publications disassociated in the first table will appear in this table when the screen is refreshed.
Table 3: Publications Previously Reported for this Project
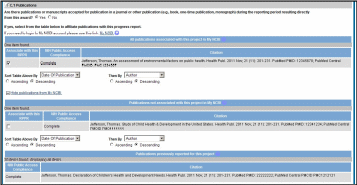
The
final table, Publications previously reported for this project,
only lists publications reported in a previous electronic progress
report for this award. Grantees are responsible for ensuring that
these publications comply with the Public Access policy even if they
were provisionally compliant (listed as in Process) when
previously reported.
Figure 85: RPPR Section C. Products – Question C1
Submitting an RPPR with Noncompliant Publications
Publications that fall under the NIH Public Access Policy and are non-compliant still must be reported. When non-compliant papers are reported, the system will generate an automated email to

the PD/PI (with cc to the AO and SO) requesting that the grantee provide evidence of compliance or an explanation by a specified due date two weeks prior to the next budget start date.
Responding to Non-compliance Notifications
To bring a publication into compliance, please follow the submission instruction wizard at http://publicaccess.nih.gov/determine-applicability.htm. NIH cannot accept anything other than compliance with the policy except in the rarest of circumstances, such as a death of the sole author.
The grantee must respond to this non-compliance notification either via an email to the GMS and PO, or may respond via the Progress Report Additional Materials (PRAM) link found on the eRA Commons Status page. The simplest way to provide evidence of compliance is to generate a My NCBI PDF report (https://www.ncbi.nlm.nih.gov/books/NBK53595/#mybibliography.Creating_an_Award_Complia) of the formerly non- compliant publications. The PRAM link provides a text box in which the grantee may respond through the eRA Commons and link to attach the My NCBI PDF report. The grantee will be able to view the PRAM in the grant folder. See Section 5.10 Public Access Progress Report Additional Materials (PRAM) for more information.
My NCBI Management
PD/PIs can log in to their My NCBI account. PD/PIs that do not have a My NCBI account can create one by simply logging in to My NCBI with their eRA Commons credentials, which will automatically create a My NCBI account. Any changes made to their My Bibliography collection will be reflected in the RPPR once the screen is refreshed (i.e., by clicking the Save button). For more information on My NCBI, see http://publicaccess.nih.gov/communications.htm, and My NCBI Help.
List the URL for any Internet site(s) that disseminates the results of the research activities. A short description of each site should be provided. It is not necessary to include the publications already specified above.
![]() A
description is only required for awards designed to create or
maintain one or more websites. Limit the response to this reporting
period. If the website disseminates a product that falls into one or
more of the other product categories, please select the appropriate
category(ies) from the pull-down menu (select multiple categories, if
appropriate, by holding down the Ctrl button while selecting
the categories). For awards not designed to create or maintain one or
more websites, select Nothing to Report.
A
description is only required for awards designed to create or
maintain one or more websites. Limit the response to this reporting
period. If the website disseminates a product that falls into one or
more of the other product categories, please select the appropriate
category(ies) from the pull-down menu (select multiple categories, if
appropriate, by holding down the Ctrl button while selecting
the categories). For awards not designed to create or maintain one or
more websites, select Nothing to Report.
Identify technologies or techniques that have resulted from the research activities. Describe the technologies or techniques and how they are being shared.
![]() PD/PIs
are required to report all technologies or techniques that arise from
their NIH award in this section. If there are technologies or
techniques to report, select Yes and
PD/PIs
are required to report all technologies or techniques that arise from
their NIH award in this section. If there are technologies or
techniques to report, select Yes and
enter a short description. If the technology or technique falls into one or more of the product categories, please select the appropriate category(ies) from the pull-down menu (select multiple categories, if appropriate, by holding down the Ctrl button while selecting the categories). If there are no technologies or techniques to report select Nothing to Report. Limit the response to this reporting period.
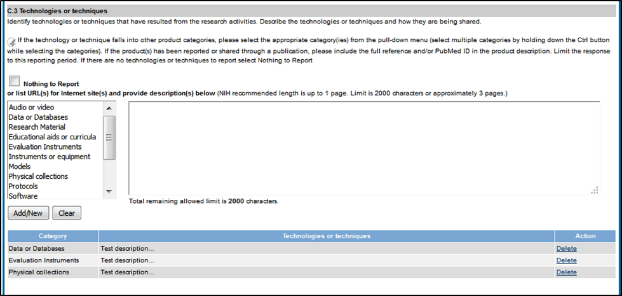
Figure 86: RPPR Section C. Products – Questions C2 & C3
Have inventions, patent applications and/or licenses resulted from the award during this reporting period?
If yes, has this information been previously provided to the PHS or to the official responsible for patent matters at the grantee organization?
Reporting of inventions through iEdison is mandatory.
Identify any other significant products that were developed under this project.
![]() PD/PIs
are required to report all products that arise from their NIH award
in section C. If there
are other products to report not
covered in Sections
C1 – C4, select Yes and
choose the appropriate
product category(ies) from the pull down menu (select
multiple categories, if
appropriate, by holding
down the Ctrl button while selecting the categories).
If there is more than one product to report,
select “add
product” to create a workspace for an additional
product. Limit the
response to this
reporting period.
PD/PIs
are required to report all products that arise from their NIH award
in section C. If there
are other products to report not
covered in Sections
C1 – C4, select Yes and
choose the appropriate
product category(ies) from the pull down menu (select
multiple categories, if
appropriate, by holding
down the Ctrl button while selecting the categories).
If there is more than one product to report,
select “add
product” to create a workspace for an additional
product. Limit the
response to this
reporting period.
Please assign products reported in Sections C.3 Technologies and Techniques and C.5a Other Products to the following categories. Please choose as many categories as applicable for each of the product(s) reported. If the product(s) has been reported or shared through a publication, please include the full reference and/or PubMed ID in the product description.
Product Categories (click on hyperlinks to review additional guidance for each category):
Audio or video
Data or Databases
Research Material
Educational aids or curricula
Evaluation Instruments
Instruments or equipment
Models
Physical collections
Protocols
Software
Survey Instruments
Interventions (e.g., clinical or educational)
New Business Creation
Other
The Other category can include interim research products, which are complete, public research products that are not final. Do not report interim versions of these products unless they are

For example, if an awardee made a draft of their survey public, it would be an interim research product/ listed under product category #. If the draft was not public, or was missing key questions, do not report it.
Citations for interim products must include the Digital Object Identifier and the Object type (e.g. preprint, protocol) in the citation. Also list any information about the document version (e.g. most recent date modified), and if relevant, the date the product was cited. For more information, see the FAQs.
Example: Bar DZ, Atkatsh K, Tavarez U, Erdos MR, Gruenbaum Y, Collins FS. Biotinylation by antibody recognition- A novel method for proximity labeling. BioRxiv 069187 [Preprint]. August 11, 2016 [cited 2017 Jan 12]. Available from: https://doi.org/10.1101/069187.
Specifically, to claim an interim research product as a product of an NIH award, the NIH expects that the awardee will:
Make the product publicly available. To maximize the impact of an interim research product, the NIH strongly encourages awardees to select a Creative Commons Attribution (CC-BY) license or dedicate their work to the public domain.
In the text of the document:
Acknowledge NIH funding in accordance with NIH Grants Policy Statement Chapter 8.2.1
Clearly state that the work is not peer-reviewed
Declare any competing interests, as an author would do for any journal article
 Resource
Sharing
Resource
Sharing
PD/PIs and grantee organizations are expected to make the results and accomplishments of their activities available to the research community and to the public at large. For additional information on NIH Sharing Policies and Related Guidance on NIH-Funded Research Resources see http://grants.nih.gov/grants/sharing.htm.
If the initial research plan addressed, or the terms of award require, a formal plan for sharing final research data, model organisms, Genome Wide Association Studies data, or other such project-specific data, describe the progress in implementing that plan. For sharing model organisms, include information on the number of requests received and number of requests fulfilled during this reporting period. If the sharing plan is fully implemented, provide a final statement on data sharing.
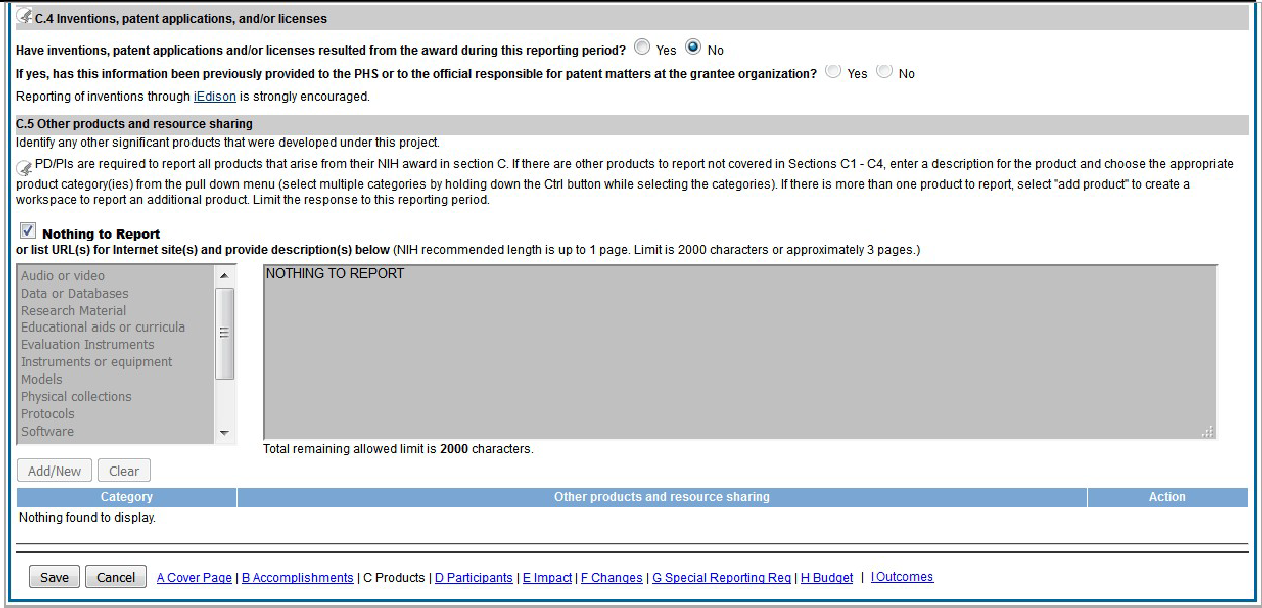
Figure 87: RPPR Section C. Products – Questions C4 & C5
6.4 Section D – Participants
The RPPR Section D. allows the agency to know who has worked on the project to gauge and report performance in promoting partnerships and collaborations.
What individuals have worked on the project?
Provide or update the information for: (1) program director(s)/principal investigator(s) (PDs/PIs); and (2) each person who has worked at least one person month per year on the project during the reporting period, regardless of the source of compensation (a person month equals approximately 160 hours or 8.3% of annualized effort).
Provide the name and identify the role the person played in the project. Indicate the person months, rounded to the nearest one-tenth (Calendar, Academic, Summer) that the individual worked on the project.
Show the most senior role in which the person has worked on the project for any significant length of time. For example, if an undergraduate student graduates, enters graduate school, and continues to work on the project, show that person as a graduate student.
![]() NIH Instructions:
NIH Instructions:
An individual's Commons user ID may be used to partially populate his or her information
A Commons ID is required for all individuals with a postdoctoral, graduate or undergraduate role (NOT-OD-13-097). The Commons ID is also required for individuals supported by a Reentry or Diversity Supplement. For all other project personnel, the Commons ID is strongly encouraged but currently optional. AHRQ only requires a Commons ID for individuals in a postdoctoral role.
Individuals with a postdoctoral-like role should be identified as Postdoctoral (scholar, fellow, or other postdoctoral position)
Do not include Other Significant Contributors who are not committing any specified measurable effort to this project

Do not report personnel for whom a PHS 2271 Appointment form has been submitted through xTrain
Required fields are marked with an * eRA Commons User ID: Entering the User ID allows selection of “Populate from Profile” which will partially populate the individual’s information. Those with an Administrator role in the eRA Commons may search for user IDs by following the instructions at:
Search for Commons User Accounts
Senior/key personnel are defined as the PD/PI and other individuals who contribute to the scientific development or execution of a project in a substantive, measurable way, whether or not they receive salaries or compensation under the grant. Typically these individuals have doctoral or other professional degrees, although individuals at the masters or baccalaureate level may be considered senior/key personnel if their involvement meets this definition. Consultants and those with a postdoctoral role also may be considered senior/key personnel if they meet this definition.
Project Role: PD/PI names and information from their Commons Profile(s) will be prepopulated. To update the PD/PI information as displayed, go to the Commons Profile and save the changes there. For all other personnel, select from a dropdown menu of the following options:
Supplement Support: If personnel are supported by a Reentry or Diversity Supplement indicate type of supplement in this field.
Person Months: The metric for expressing the effort (amount of time) devoted to a specific project. The effort is based on the type of appointment of the individual with the organization; e.g., calendar year, academic year, and/or summer term; and the organization's definition of such. For instance, some institutions define the academic year as a 9-month appointment while others define it as a 10-month appointment.
Include (1) the PD/PI regardless of effort devoted to the project and (2) each person who has

worked at least one person month per year on the project during the reporting period, regardless of the source of compensation.
Round to the nearest one-tenth (i.e., one decimal place) of a person month that the individual worked on the project. For example, if the individual worked 2.25 person months, indicate 2.3 person months. If the individual worked 2.24 person months, indicate 2.2 person months. If the PD/PI worked 0.74 person months, round down to 0.7 person months. If the PD/PI worked 0.76 person months, round up to 0.8 person months. For any amount less than 0.05 for a PD/PI (who by definition must contribute measurable effort), enter 0.1 person months. For all other participants, round down to zero (0.0) when the amount is less than 0.05 person months.
To calculate person months, multiply the percentage of effort associated with the project by the number of months of the appointment. For example:
25% of a 9 month academic year appointment equals 2.25 (academic year) person months (.25 x 9 = 2.25). Round down to 2.3
90% of a 12 month calendar appointment equals 10.8 (calendar year) person months (.90 x 12 = 10.8).
35% of a 3 month summer term appointment equals 1.05 (summer) person months (.35 x 3= 1.05). Round down to 1.1
If the regular pay schedule of an institution is a 9 month academic year and the PD/PI will devote 9 academic months at 30% time/effort and 3 months summer term at 30% time/effort , then 3 academic months (.30% x 9 = 2.7, round up to 3), and 1 summer month (.30 x 3 = .9, round up to 1) should be reported
Note that a PD/PI must have measurable effort. Change in Level of Effort for the PD/PI(s) and other senior key/personnel designated in the NoA is reported under D.2.a below.
 For Final and
Interim RPPR under D.1 recipients should only report on the
individuals/staff that worked on the project during the last budget
period minus any approved no-cost extensions.
For Final and
Interim RPPR under D.1 recipients should only report on the
individuals/staff that worked on the project during the last budget
period minus any approved no-cost extensions.
 Is the individual’s primary affiliation with a foreign
organization?
Is the individual’s primary affiliation with a foreign
organization?
Check No if the individual’s primary affiliation is with a foreign organization but the individual is working on this award solely while in the U.S.
If Yes, provide the name of the organization and country. Select the Add/New button to add the data to the table.
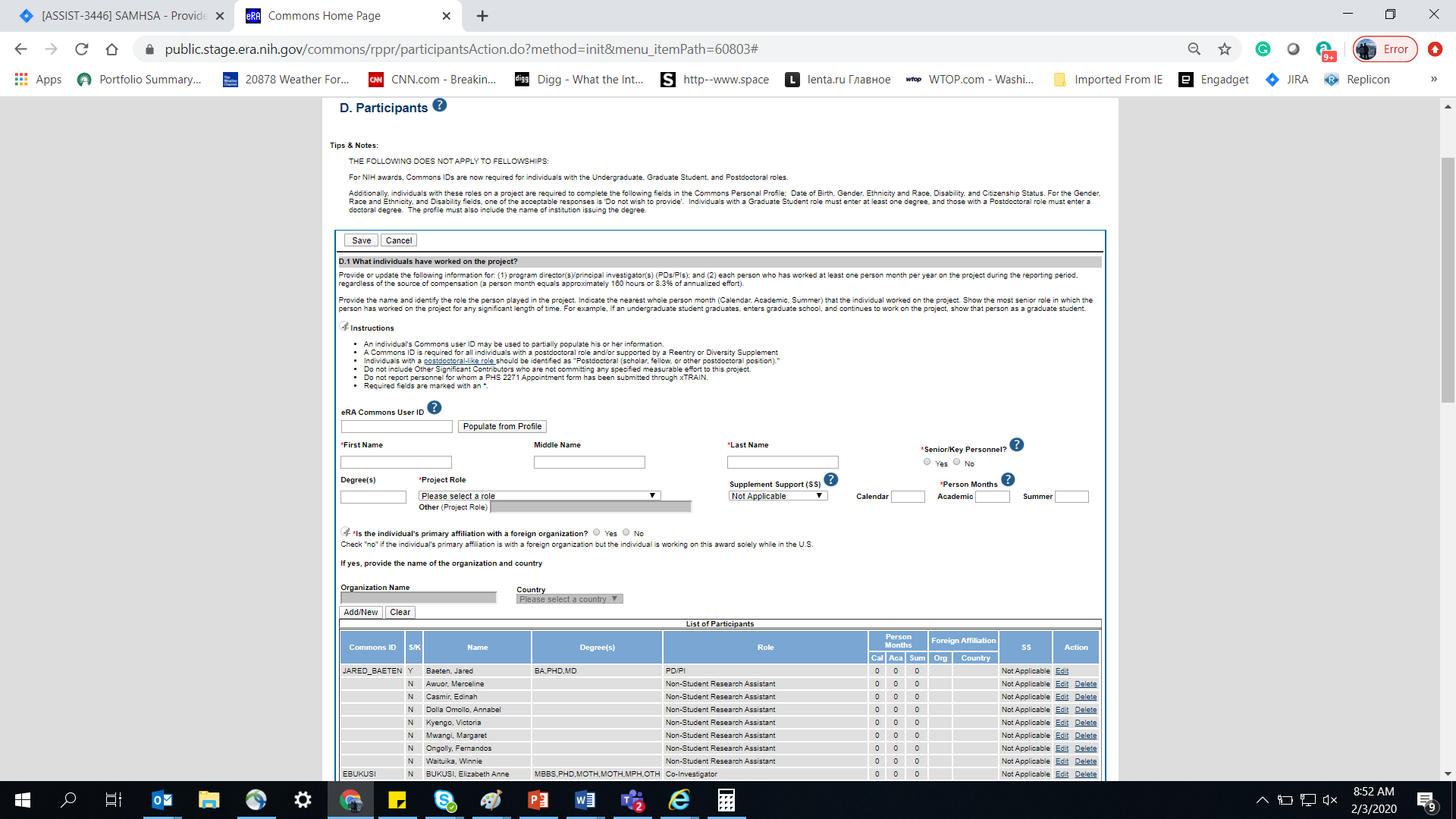
Figure 88: RPPR Section D. Participants – Question D1
D.2.a Level of effort.
Will there be, in the next budget period, either (1) a reduction of 25% or more in the level of effort from what was approved by the agency for the PD/PI(s) or other senior/key personnel designated in the Notice of Award, or (2) a reduction in level of effort below the minimum amount of effort required by the Notice of Award?
Reductions are cumulative, i.e., the 25% threshold may be reached by two or more successive reductions that total 25% or more. Once agency approval has been given for a significant change in the level of effort, then all subsequent reductions are measured against the approved adjusted level. Selecting Yes constitutes a prior approval request to the agency and the issuance of a subsequent year of funding constitutes agency approval of the request. D.2.b New senior/key personnel.
Are there, or will there be, new senior/key personnel?
Senior/key personnel are those identified by the grantee institution as individuals who contribute in a substantive measurable way to the scientific development or execution of the project, whether or not salaries are requested. Typically these individuals have doctoral or other professional degrees, although individuals at the masters or baccalaureate level may be considered senior/key personnel if the involvement meets this definition. Consultants may be considered senior/key personnel if they meet this definition.
If yes, upload biosketches and other support for all new senior/key personnel.
Follow the biosketch instructions in the competing application guide and provide active other support for all new senior/key personnel. Combine all biosketches and other support into a single PDF.

Figure 89: RPPR Section D. Participants – Questions D2a & D2b
D.2.c Changes in other support.
Has there been a change in the active other support of senior/key personnel since the last reporting period?
If yes, upload active other support for senior/key personnel whose support has changed and indicate what the change has been. List the award for which the progress report is being submitted and include the effort that will be devoted in the next reporting period.
Select Yes only if active support has changed for the PD/PI(s) or senior/key personnel.
If a previously active grant has terminated and/or if a previously pending grant is now active, submit complete Other Support information using the suggested format and instructions found at http://grants.nih.gov/grants/funding/2590/Non-competing_othersupport.docx. Annotate this information so it is clear what has changed from the previous submission.
Submission of other support information is not necessary if support is pending or for changes in the level of effort for active support reported previously.
Other support information should be submitted only for the PD/PI and for those individuals considered by the grantee to be key to the project for whom there has been a change in other support. Senior/key personnel are defined as individuals who contribute in a substantive measurable way to the scientific development or execution of the project, whether or not a salary is requested. Do not include other support information for Other Significant Contributors; e.g., those that may contribute to the scientific development or execution of the project, but are not committing any specified measurable effort to the project. D.2.d New other significant contributors.
Are there, or will there be, new other significant contributors?
Other significant contributors are individuals who have committed to contribute to the scientific development or execution of the project, but are not committing any specified measurable effort (i.e., person months) to the project.
If yes, upload biosketches for all new other significant contributors.
D.2.e Will there a change in the MPI Leadership Plan for the next budget period?
Change in status of PD/PI requires prior approval of the agency (e.g., NIH Grants Policy Statement, 8.1.2.6). In accord with the NIH GPS, 9.5, revision of the Leadership Plan during the project period may be accomplished through a joint decision of the PD/PIs and reported in the RPPR. Prior approval of a change in the MPI Leadership Plan is not required.
If yes, upload a revised MPI Leadership Plan that includes a description of the change(s).
All multiple PD/PI awards have a Leadership Plan that describes the roles and areas of responsibility of the named PD/PIs, the process for making decisions concerning scientific directions, allocation of resources, disputes that may arise, and other information related to the management of the proposed team science project. If there has been any change in the governance and/or organizational structure of the Leadership Plan, provide a description, including communication plans and procedures for resolving conflicts, and any changes to the administrative, technical, and scientific responsibilities of the PD/PIs. If the progress report includes a change in the Contact PD/PI (Cover Page, A.1) address this change and the impact, if any, the change has on the administrative, technical, and scientific responsibilities of the PD/PIs. A request to change from a multiple PD/PI model to a single PD/PI model, or a change in the number or makeup of the PD/PIs on a multiple PD/PI award, requires the prior approval of the GMO. The progress report is not the appropriate vehicle to request such a change.
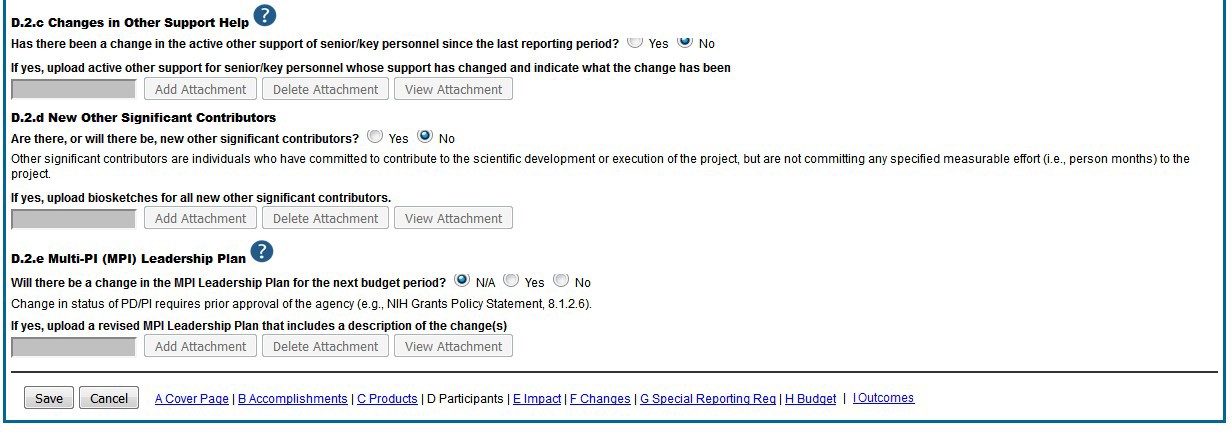
Figure 90: RPPR Section D. Participants – Questions D2c – D2e
6.5 Section E – Impact
The RPPR Section E Impact will be used to describe ways in which the work, findings, and specific products of the project have had an impact during this reporting period. E.1 Not Applicable for most awards. See chapter 7 Supplemental Instructions.
Describe ways, if any, in which the project made an impact, or is likely to make an impact, on physical, institutional, and information resources that form infrastructure, including:
physical resources (such as facilities, laboratories, or instruments);
institutional resources (such as establishment or sustenance of societies or organizations); or
information resources, electronic means for accessing such resources or for scientific communication, or the like.
![]() If
the award or award component(s) is not intended to support physical,
institutional, or information resources that form infrastructure,
select Nothing to
Report. E.3
Not Applicable for most awards. See chapter 7 Supplemental
Instructions.
If
the award or award component(s) is not intended to support physical,
institutional, or information resources that form infrastructure,
select Nothing to
Report. E.3
Not Applicable for most awards. See chapter 7 Supplemental
Instructions.
E. 4 What dollar amount of the award’s budget is being spent in foreign country(ies)?
![]() For
domestic awardees provide the dollar amount obligated to first-tier
subawards to foreign entities for this reporting period. For foreign
awardees provide the dollar amount of the award, excluding all
first-tier subawards to U.S. entities, for this reporting period.
For
domestic awardees provide the dollar amount obligated to first-tier
subawards to foreign entities for this reporting period. For foreign
awardees provide the dollar amount of the award, excluding all
first-tier subawards to U.S. entities, for this reporting period.
Dollars provided should reflect total costs.
If more than one foreign country identify the distribution between the foreign countries.
Report only cumulative first-tier subawards dollars by country. Do not report foreign travel, purchases, etc., unless part of a first-tier subaward to a foreign country.
Select the Add/New button to add the data to the table.
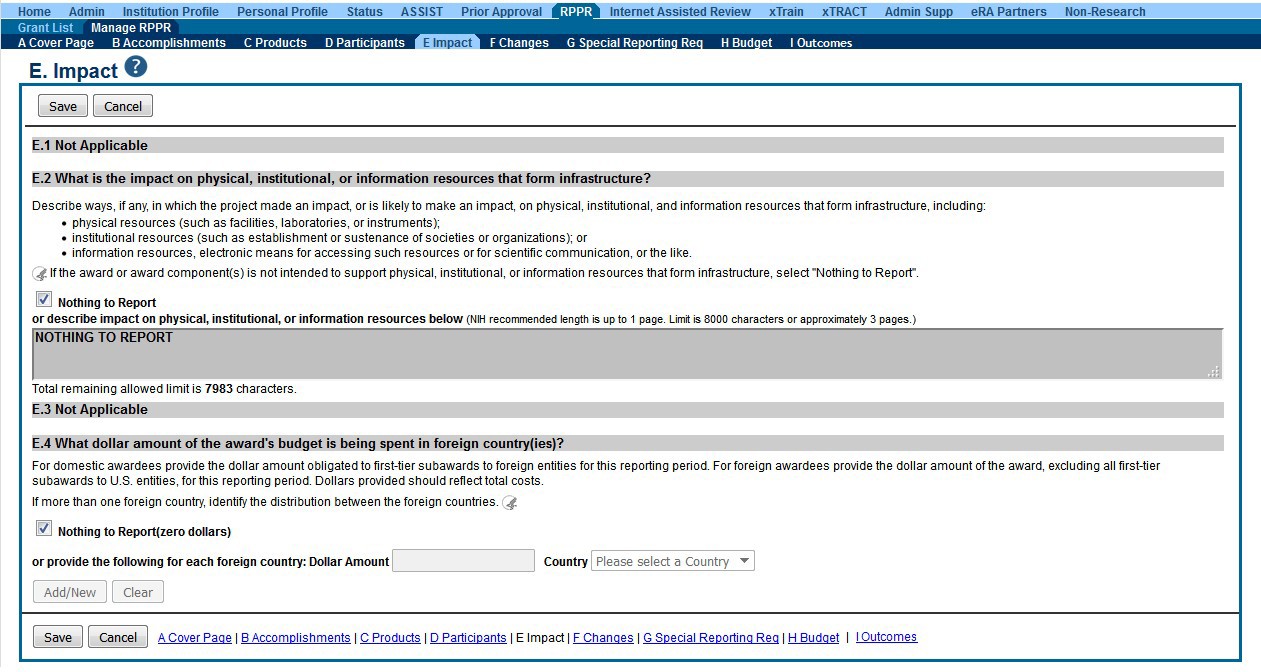
Figure 91: RPPR Section E. Impact – Questions E1 through E4
6.6 Section F – Changes
The RPPR Section F addresses Changes. Grantees are reminded that significant changes in objectives and scope require prior approval of the agency.
Describe challenges or delays encountered during the reporting period and actions or plans to resolve them.
![]() Describe
only significant challenges that may impede the research (e.g.,
accrual of patients, hiring of personnel, need for resources or
research tools) and emphasize their resolution.
Describe
only significant challenges that may impede the research (e.g.,
accrual of patients, hiring of personnel, need for resources or
research tools) and emphasize their resolution.
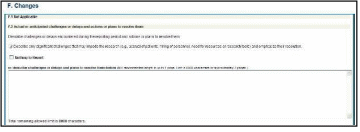
Figure 92: RPPR Section F. Changes – Questions F1 & F2
 Significant
changes to
human subjects,
vertebrate animals,
biohazards, and/or
select agents.
Significant
changes to
human subjects,
vertebrate animals,
biohazards, and/or
select agents.
Describe significant deviations, unexpected outcomes, or changes in approved protocols for human subjects, vertebrate animals, biohazards and/or select agents during this reporting period.
Remember that significant changes in objectives and scope require prior approval of the agency (e.g., NIH Grants Policy Statement, 8.1.2.). If there are changes in any of the following areas, check the appropriate box and provide a description of the changes.
F.3.a Human Subjects
If human subject studies are or will be different from the previous submission, include a description and explanation of how the studies differ and provide new or revised Protection of Human Subjects Section and Inclusion of Women, Minorities, and Children sections as described in the competing application instructions. Additional or modified inclusion enrollment reports may also be necessary and should be provided by clicking the Human Subjects link in Section G.4 of the RPPR to make necessary updates in the Human Subjects System (HSS).
If there are or will be significant changes to the uses of vertebrate animals from the previous submission, provide a description of the changes. Examples of changes considered to be significant include, but are not limited to, changing animal species, changing from noninvasive to invasive procedures, new project/performance site(s) where animals will be used, etc. If studies involving live vertebrate animals are planned and were not part of the originally proposed research design, provide a new or revised Vertebrate Animal Section as described in the competing application instructions.
If the use of biohazards is or will be different from that in the previous submission, provide a description and explanation of the difference(s).
F.3 d Select Agents
If the possession, use, or transfer of Select Agents is or will be different from that proposed in the previous submission, including any change in the select agent research location and/or the required level of biocontainment, provide a description and explanation of the differences. If the use of Select Agents was proposed in the previous submission but has not been approved by regulatory authorities, provide an explanation. If studies involving Select Agents are planned and were not part of the originally proposed research design, provide a description of the proposed use, possession, transfer, and research location as described in the competing application instructions.
U.S. Select Agent Registry information: https://www.selectagents.gov
Figure
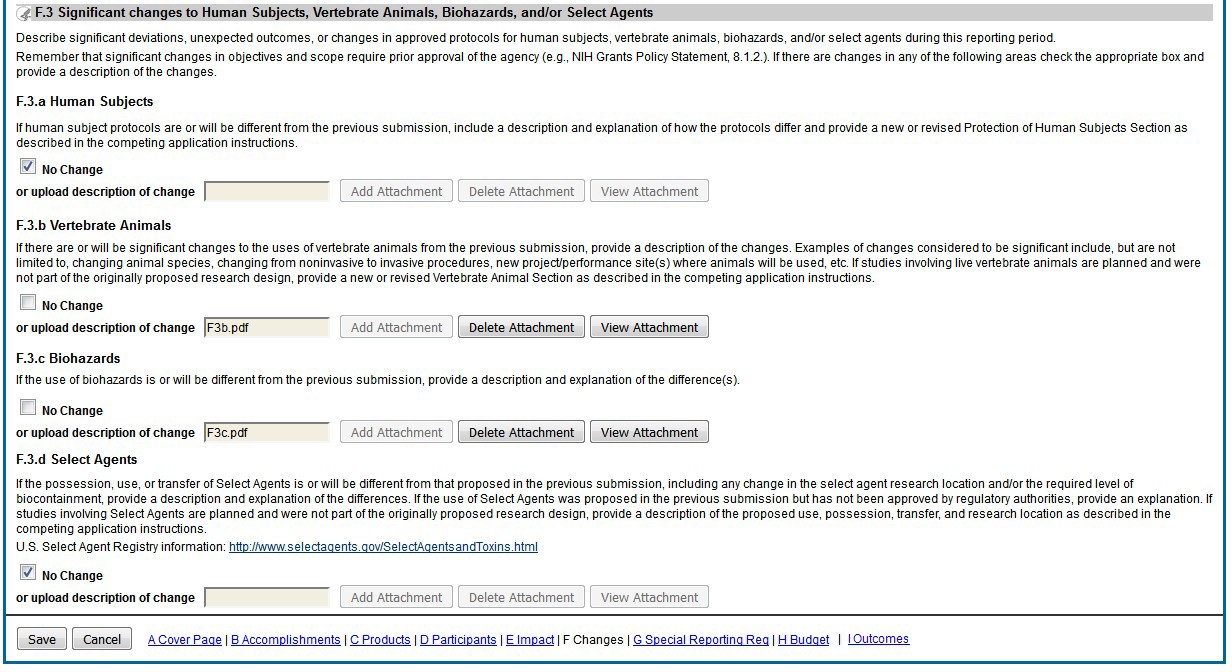
Figure 93: RPR Section F Changes- Question F3
6.7 Section G – Special Reporting Requirements
![]() The
RPPR Section G Special Reporting Requirements address agency-specific
award terms and conditions, as well as any award specific reporting
requirements.
The
RPPR Section G Special Reporting Requirements address agency-specific
award terms and conditions, as well as any award specific reporting
requirements.
Address any special reporting requirements specified in the award terms and conditions in the Notice of Award (NoA) or Funding Opportunity Announcement (FOA).
Not Applicable to most awards. See chapter 7 Supplemental Instructions.
Figure 94: RPPR Section G. Special Reporting Requirements – Questions G1 through G3
G.4. Human Subjects Inclusion enrollment data.
If conducting NIH-defined clinical research, reporting the cumulative enrollment of subjects and the distribution by sex/gender, race, and ethnicity is required, as defined in the competing application instructions. If there are details or concerns related to inclusion enrollment progress, or if the cumulative enrollment data does not reflect the planned enrollment by sex/gender, race, and/or ethnicity, the reasons for this should be addressed in Section F.3.a of the RPPR.
Update the inclusion enrollment with the total cumulative enrollment data collected to-date. Grantees can access the inclusion enrollment record(s) in Section G.4. by clicking the “Human Subjects” link. The link will be available when inclusion monitoring is required.
Grantees may have more than one inclusion enrollment report. If new clinical studies have started and planned enrollment was not previously provided, create a new Planned Enrollment record in the Human Subjects System.
Refer to the section of this guide titled Editing Inclusion Enrollment Data for more information.
AHRQ grantees only: If inclusion enrollment reporting is required, download and complete the Cumulative Inclusion Enrollment Report, and upload it in Section G.4. If inclusion enrollment reporting is not required, select “Nothing to Report.”
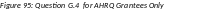 Guidance
for Collecting and Reporting Inclusion Data: Below are
instructions for how to collect and report data on the basis of
sex/gender, race, and ethnicity with additional guidance for handling
subpopulations, non-U.S. populations, changes to planned enrollment
data, and NIH-defined Phase III clinical trials.
Guidance
for Collecting and Reporting Inclusion Data: Below are
instructions for how to collect and report data on the basis of
sex/gender, race, and ethnicity with additional guidance for handling
subpopulations, non-U.S. populations, changes to planned enrollment
data, and NIH-defined Phase III clinical trials.
For questions about the NIH policies for inclusion, please refer to: http://grants.nih.gov/grants/funding/women_min/women_min.htm or contact the program officer.
Standards for Collecting Data from Study Participants: The Office of Management and Budget (OMB) Directive No. 15 defines minimum standards for maintaining, collecting and presenting data on ethnicity and race for all Federal (including NIH) reporting purposes. The categories in this classification are social-political constructs and should not be interpreted as being anthropological in nature. The standards were revised in 1997 and now include two ethnic categories: Hispanic or Latino, and Not Hispanic or Latino. There are five racial categories: American Indian or Alaska Native, Asian, Black or African American, Native Hawaiian or Other Pacific Islander, and White. Reports of data on ethnicity and race should use these categories. The definitions below apply for the ethnic and racial categories.
Ethnic Categories:
Hispanic or Latino: A person of Cuban, Mexican, Puerto Rican, South or Central American, or other Spanish culture or origin, regardless of race. The term, “Spanish origin,” can be used in addition to “Hispanic or Latino”. Not Hispanic or Latino
Racial Categories:
American Indian or Alaska Native: A person having origins in any of the original peoples of North, Central, or South America and maintains tribal affiliation or community.
Asian: A person having origins in any if the original peoples of the Far East, Southeast Asia, or the Indian subcontinent including, for example, Cambodia, China, India, Japan, Korea, Malaysia, Pakistan, the Philippine Islands, Thailand, and Vietnam.
Black or African American: A person having origins in any of the black racial groups of Africa. Terms such as “Haitian” or “Negro” can be used in addition to “Black or African American.”
Native Hawaiian or Other Pacific Islander: A person having origins in any of the original peoples of Hawaii, Guam, Samoa, or other Pacific Islands.
White: A person having origins in any of the original peoples of Europe, North Africa, or the Middle East.
Reporting Data on Race and Ethnicity: NIH is required to use the above standards and definitions for race and ethnicity to allow comparisons to other federal databases, especially the census and national health databases. Federal agencies shall not present data on detailed categories if doing so would compromise data quality or confidentiality standards.
When collecting data on ethnicity and race, as well as sex/gender, use the categories listed to obtain the data from individuals on the basis of self-identification. Participants should be asked to identify their ethnicity and their race. The OMB recommends collecting this information using two separate questions, with ethnicity information collected first followed by race, with the option to select more than one racial designation (http://www.whitehouse.gov/omb/fedreg_1997standards). The NIH inclusion enrollment format is not designed for use as a data collection instrument. Collect the data using instruments prepared for the study, and use that information to complete the NIH inclusion enrollment form(s). Study participants who self-identify with more than one of the racial categories should be reported in the aggregate in the "More Than One Race" category.
Collecting and Reporting Data on Subpopulations: Each ethnic/racial group contains subpopulations that are delimited by geographic origins, national origins, and/or cultural differences. It is recognized that there are different ways of defining and reporting racial and ethnic subpopulation data. The subpopulation to which an individual is assigned depends on self-reporting of specific origins and/or cultural heritage. Attention to subpopulations also applies to individuals who self-identify with more than one ethnicity or race. These ethnic/racial
combinations may have biomedical, behavioral, and/or social-cultural implications related to the scientific question under study. The collection of greater detail is encouraged, e.g., on ethnic/racial subpopulations; however, any collection that uses more detail needs to be organized in such a way that the additional categories can be aggregated into the OMB categories for reporting data on ethnicity, race, and more than one race. Investigators who have data on subpopulations are encouraged to provide that information in the Comments field of the inclusion enrollment forms and/or in the text of their progress report.
Collecting and Reporting Data on Non-U.S. Populations: If conducting NIH-defined clinical research outside of the United States, design culturally appropriate data collection instruments that allow participants to self-identify their ethnic and/or racial affiliation in a way that is meaningful in the cultural and scientific contexts of the study. However, investigators will need to use the OMB-defined categories for reporting sex/gender, race and ethnicity to NIH (see definitions for each ethnic and racial category above), which will allow for completion of the inclusion enrollment form(s). Since OMB categories reference world-based geographic origin, this should facilitate completion of the form(s). Enrollment of participants at non-U.S. sites should be reported to NIH on a separate inclusion enrollment form from that for reporting participants at U.S. sites, even if they are part of the same study. For additional guidance and FAQs related to this topic, please refer to: http://grants.nih.gov/grants/funding/women_min/women_min.htm or contact the program officer.
Changes to Planned Enrollment: If there are changes from the planned enrollment originally approved for funding, contact the program officer to discuss updating/revising the planned enrollment, address the change in Section F.3.a of the RPPR, and revise the existing Planned Enrollment for that study by clicking the Human Subjects link to update the record in the Human Subjects System.
Reporting Data on NIH-defined Phase III Clinical Trials: If conducting an NIH-defined Phase III Clinical Trial, report on the cumulative enrollment (as described above) and indicate in Section F.3.a if any data analysis has begun for the trial. If analysis has begun or data have been published, report any progress made in evaluating potential differences on the basis on sex/gender, racial, and/or ethnicity.
Figure 96: RPPR Section G. Special Reporting Requirements – Question G.4
Are there personnel on this project who are or will be newly involved in the design or conduct of human subjects research?
If yes, provide the following:
Does this project involve human embryonic stem cells?
Only hESC lines listed as approved in the NIH Registry may be used in NIH funded research.
If yes, identify the hESC Registration number(s) from the NIH Registry.
Select the Add/New button to add the data to the table.
If there is a change in the use of hESCs provide an explanation.
Does this project involve vertebrate animals?
Figure 97: RPPR Section G. Special Reporting Requirements – Questions G5 through G7
If there are changes to the project/performance site(s) displayed, edit as appropriate.
One of the sites indicated must be the identified as the Primary Performance Site. If including a new Project/Performance Site where either human subjects or vertebrate animals will be involved, address the change under F.3.a or F.3.b. If a Project/Performance Site is engaged in research involving human subjects, the grantee organization is responsible for ensuring that the Project/Performance Site operates under an appropriate Federal Wide Assurance for the protection of human subjects and complies with 45 CFR Part 46 and other NIH human subject related policies described in Part II of the competing application instructions and the NIH Grants Policy Statement.
For research involving live vertebrate animals, the grantee organization must ensure that all Project/Performance Sites hold OLAW-approved Assurances. If the grantee organization does not have an animal program or facilities and the animal work will be conducted at an institution with an Assurance, the grantee must obtain an Assurance from OLAW prior to the involvement of vertebrate animals.
Select the Add/New button to add the data to the table.
Figure 98: RPPR Section G. Special Reporting Requirements – Question G8
Provide the organization name, country, and description of each foreign component.
Foreign component is defined as significant scientific activity that was performed outside of the United States, either by the grantee or by a researcher employed by a foreign organization, whether or not grant funds were expended. The following grant-related activities are significant and must be reported:
involvement of human subjects or research with live vertebrate animals;
extensive foreign travel by grantee project staff to collect data, or conduct surveys or sampling activities; or
any grantee activity that may have an impact on U.S. foreign policy. Examples of other grant-related activities that may be significant are:
collaborations with investigators at a foreign site anticipated to result in co-authorship;
use of facilities or instrumentation at a foreign site; or
receipt of financial support or resources from a foreign entity.
Foreign travel for consultation does not meet the definition of foreign component. Select the Add/New button to add the data to the table.
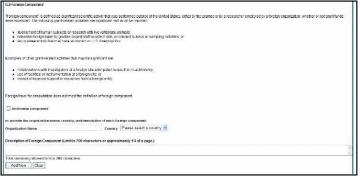
Figure 99: RPPR Section G. Special Reporting Requirements – Question G9
The total approved budget equals the current fiscal year award authorization plus any approved carryover of funds from a prior year(s). The numerator equals the total amount available for carryover and the denominator equals the current year’s total approved budget.
AHRQ ONLY: The RPPR is automatically set to “yes” for an AHRQ award because AHRQ grantees must provide the estimated cumulative total cost dollar amount of any unobligated balance that is anticipated, regardless of whether the percentage of unobligated funds is over or under 25%. If the estimated cumulative total cost dollar amount of the unobligated balance is
$0, enter “0” in G.10.a.
AHRQ ONLY: If the estimated unobligated balance exceeds 25% of the current year’s total approved budget, provide an explanation of why this significant balance remains. If the estimated cumulative total cost dollar amount of the unobligated balance is less than 25%, enter “N/A” in G.10.b.
Grantees not authorized to carryover unobligated balances automatically must submit a prior approval request to the awarding IC. See instructions in NIH Grants Policy Statement Section
8.1.2.4 Carryover of Unobligated Balances, or HHS GPS, Part II, Prior Approval Requirements, as applicable.
Is program income anticipated during the next budget period? If yes, provide the amount and source(s).
Program Income is defined as gross income earned by the grantee organization, a consortium participant, or a contractor under the grant that is directly generated by the grant-supported project or activity or earned as a result of the award. Program income includes, but is not limited to, income from fees for services performed; charges for the use or rental of real property, equipment or supplies acquired under the grant; the sale of commodities or items fabricated under an award; charges for research resources; registration fees for grant-supported conferences, and license fees and royalties on patents and copyrights. Program income from license fees and royalties from copyrighted material, patents, and inventions is exempt from reporting requirements unless otherwise specified in the terms and conditions of award.
Select the Add/New button to add the data to the table.
Is there a change in performance sites that will affect F&A costs? If yes, provide an explanation.
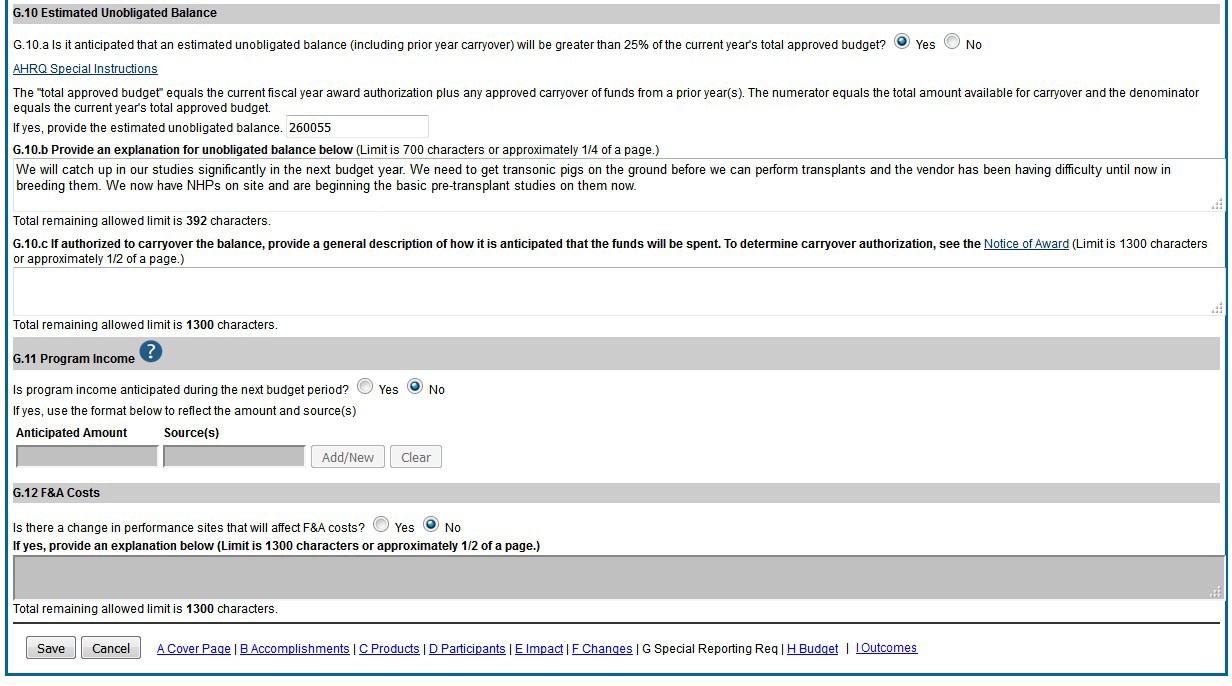
Figure 100: RPPR Section G. Special Reporting Requirements – Questions G10 through G12
6.8 Section H – Budget [Applicable to non-SNAP awards only]
H.1 Budget Form
Select the SF424 Research and Related Budget from the drop down menu and follow the instructions in the SF424 (R&R) Application Guide for NIH and Other PHS Agencies, Section I, 4.7 Budget Form, Section G.300 R&R Budget Form, to complete the R&R budget, sections A-K, and the R&R Cumulative Budget, for the remainder of the project period. The budget justification should be uploaded as item K and must include detailed justification for those line items and amounts that represent a significant change from previously recommended levels (e.g., total re-budgeting greater than 25 percent of the total award amount for this budget period).
AHRQ Grantees only: Total costs (direct and indirect) should not exceed the committed level listed on the current NoA for the upcoming budget period. A detailed budget is required because AHRQ does not utilize the NIH SNAP process. If consortia are involved, include a detailed budget for each in H.2.
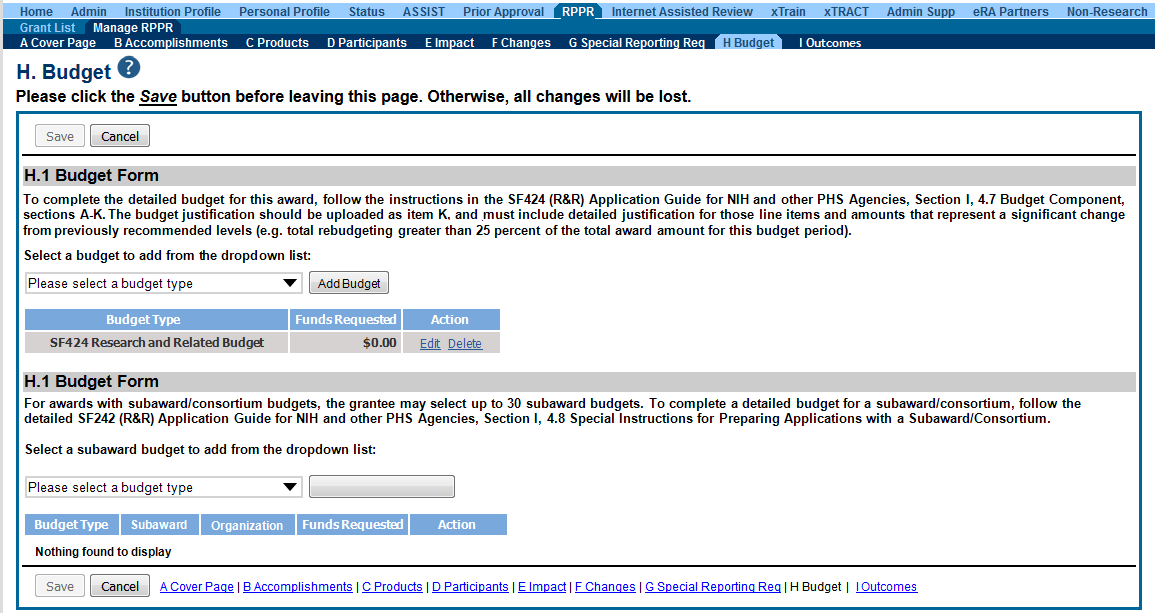
Figure 101: Section H.Budget of RPPR for a Non-SNAP Award
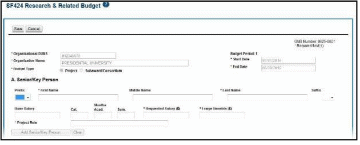
Figure 102: SF 424 Research & Related Budget Form Opened for Editing

NOTE: If subaward budgets are completed, the system will not calculate the budget line item
F.5 for the main budget (see figure below). Total consortium costs for the main budget MUST be computed and entered manually into budget line item F.5.

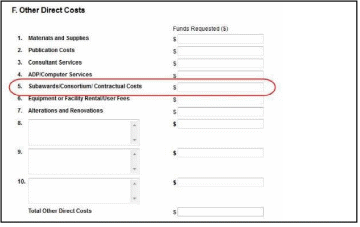
Figure 103: SF 424 R&R Budget Form - Question F.5
H.2 Subaward Budget Form
For awards with subaward/consortium budgets, select the SF424 Research and Related Budget Subaward Budget from the drop down menu and follow the SF424 (R&R) Application Guide for NIH and Other PHS Agencies, Section I, 4.8 Special Instructions for Preparing Applications with a Subaward/Consortium.
6.9 Section I – Outcomes
This component is used to provide information regarding the cumulative outcomes or findings of the project.
![]() For NIH
Section I.
Outcomes will be made publicly
available, thus allowing recipients to
provide the
general public
with a
concise summary
of the
cumulative outcomes
or findings
of the project at the end of a competitive segment. For NIH
awards the length should not exceed
half a page. In addition,
for the interim or final RPPR the summary of outcomes or findings of
the award must be written in the following
format:
For NIH
Section I.
Outcomes will be made publicly
available, thus allowing recipients to
provide the
general public
with a
concise summary
of the
cumulative outcomes
or findings
of the project at the end of a competitive segment. For NIH
awards the length should not exceed
half a page. In addition,
for the interim or final RPPR the summary of outcomes or findings of
the award must be written in the following
format:
Is written for the general public in clear, concise, and comprehensible language;
Is suitable for dissemination to the general public, as the information may be available electronically;
Does not include proprietary, confidential information or trade secrets Please refer to the following link for samples of acceptable project outcomes.
NOTE: This section is only applicable to the Interim and Final RPPR. See NOT-OD-17-022 and NOT-OD-17-037
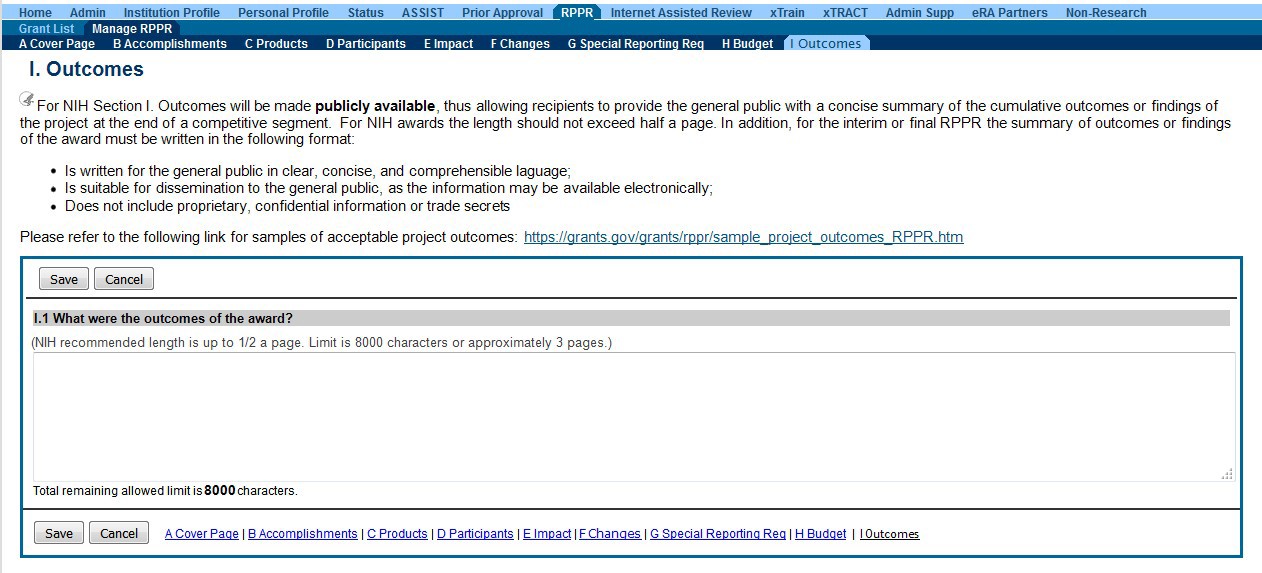
Figure 104: Section I. Outcomes
* This section was added based on the updated version of the Federal-wide performance progress reporting format published in the Federal Register on November 16, 2016.
7 Supplemental Instructions for Specific Grant RPPR Types
The RPPR Instructions in chapter 6, Sections A–H, apply to the following awards: D71, DP1, DP5, G08, G11, G13, P40, R00, R01, R03, R18, R21, R33, R34, R36, R37, R56, RC1, RC2,
RL1, S21, S22, SC1, SC2, SC3, U1B, UC2, UH1, UH2, UH3, and awards listed in Section 7.6 if they issued under SNAP. For all other awards, see Table 1 below and applicable supplemental instructions for specific grant award types that either replace or are in addition to the Instructions for RPPR Sections A–I.
Table 1: Applicable Supplemental Instructions
-
Applicable Supplemental Instructions
Award Activity Codes
7.1 Individual Career Development (K) Awards
K01, K02, K05, K06, K07, K08, K18, K22, K23, K24, K25, K26, K99, KL1
7.2 Fellowship Awards
F05, F30, F31, F32, F33
7.3 SBIR/STTR Awards
R41, R42, R43, R44, U43, U44, UT1, UT2
7.4 Training Awards
K12, KL2, R90, RL9, T15, T32, T34, T35, T37, T90, TL1
7.5 Educational Awards
D43, DP7, K30, R13, R25, RL5, T14, T36, U13, U2R
-
7.6 Multi-Project Awards and SingleProject Awards with Complicated Structure
G12, R34, M01, P01, P20, P2C, P30,
P41, P42, P50, P51, P60, PL1, PM1, PN1, PN2, R24, R28, RM1, S06, S11,
U01, U10, U19, U24, U2C, U34, U41,
U42, U45, U54, U56, UC7, UL1, UM1,
UM2
7.1 Individual Career Development (K) RPPRs
For Career Development Awards (i.e., K01, K02, K05, K06, K07, K08, K18, K22, K23, K24, K25, K26, K99, and KL1) follow the Instructions for RPPR Sections A–I in chapter 6, with the exceptions noted below:
B.4 What opportunities for training and professional development has the project provided?
Describe activities such as teaching, clinical care, professional consultation, service on advisory groups, and administrative activities. Indicate percent of time spent in each of these activities and the relationship to the awardee's research career development.
For mentored career development awards to postdoctoral researchers (e.g., K01, K22, K99), indicate whether the institution uses Individual Development Plans (IDPs), and if so, describe how they were used in this reporting period to help manage the career development of the awardee (do not include the actual IDP). This information is not required for AHRQ grantees.
For awards that include a requirement to mentor others (e.g., K05 and K24), indicate the percent of time devoted to mentoring activities, individuals mentored during the reporting period, the frequency and kinds of mentoring, financial and other support provided to mentees, and the productivity of the mentoring relationship.
B. 6. What do you plan to do for the next reporting period to accomplish the goals?
Provide a timeline for the activities planned for the next year, including plans to apply for subsequent grant support. Recipients of transition awards (e.g., K22, K99) should report on progress in identifying an independent research position. Additionally, awardees charged with mentoring others (e.g., K05, K24) should provide information describing planned mentoring activities and proposed mentees (e.g., backgrounds, interests, professional levels, etc.) sufficient to evaluate the quality of the mentoring.
C.2. Not Applicable.
C.3. Not Applicable.
D.2.e Not Applicable.
Not Applicable.
Not Applicable.
Not Applicable.
F.1 Not Applicable.
Responsible Conduct of Research
Describe the responsible conduct of research instruction received (or instruction given as a course director, discussion leader, etc., in the case of mid-career or senior career awardees) by formal and/or informal means, during this reporting period. If instruction or participation as a course director/discussion leader occurred in a prior budget period, note the dates of occurrence. Any activities undertaken to individualize instruction appropriate to career stage should be discussed. Address the five components: Format, Subject Matter, Faculty Participation, Duration, and Frequency. Additional detailed guidance on this requirement is found in the competing application instructions.
For mentored K awards, provide a letter signed by the mentor, in PDF format, assessing the awardee's progress and performance during this reporting period, both in research and in terms of development into an independent investigator in the area of the award. Include information on the continued commitment of the required minimum professional effort by the awardee (for most mentored K awardees this will be nine person months, or 75% effort) to the career development award and the availability of support for the candidate’s research project during the next budget segment. For applicable career transition awards (e.g., K22, K99), the mentor should describe the awardee’s efforts to transition into a permanent research position and the mentor’s contributions to that process. If required to submit letters from more than one mentor, letters should be assembled in one PDF file. For non-mentored K awards, select “Not Applicable.”
G.11 Not Applicable.
G.12 Not Applicable.
H. Budget. [Applicable to non-SNAP awards only.]
H.1 Budget Form
Follow the instructions for SF 424 (R&R) for K awards in SF424 Part I, Section 7.4.6. Base the awardee's salary and fringe benefits request on a full-time, 12-month appointment following the guidelines in the appropriate career award instructions. Support for other personnel and amounts in other budget categories may be requested in accordance with applicable CDA guidelines.

NOTE: If subaward budgets are completed, the system will not calculate the budget line item
F.5 for the main budget (see figure below). Total consortium costs for the main budget MUST be computed and entered manually into budget line item F.5.

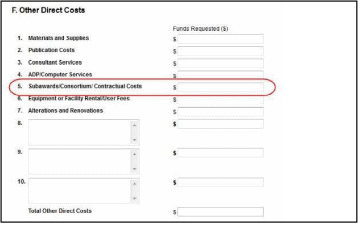
Figure 104: SF 424 R&R Budget Form - Question F.5
H.2 Subaward Budget Form
For awards with subaward/consortium budgets, the grantee may select up to 30 subaward budgets. To complete a detailed budget for a subaward/consortium, follow the SF424 (R&R) Application Guide for NIH and Other PHS Agencies, Section I, 4.8 Special Instructions for Preparing Applications with a Subaward/Consortium.
7.2 Fellowship RPPRs
For Fellowship Awards (i.e., F05, F30, F31, F32, and F33), follow the Instructions for RPPR Sections A–H in chapter 6, with the exceptions noted below:
B.4 What opportunities for training and professional development has the project provided? Indicate whether the institution uses Individual Development Plans (IDPs), and if so, describe how they were used in this reporting period to help manage the fellow’s training (do not include the actual IDP). This information is not required for AHRQ recipients.
B.6 What do you plan to do during the next reporting period to accomplish the goals?
Include any course work and any important modifications to the original plans. Provide a scientific justification for any changes involving research with human subjects or vertebrate animals. A detailed description of such changes must be provided under Changes.
C.2 Not Applicable.
D.1 Not Applicable.
D.2.e Not Applicable.
Not Applicable.
Not Applicable.
Not Applicable.
F.1 Not Applicable.
Responsible Conduct of Research
Describe the responsible conduct of research instruction received (or instruction given as a course director, discussion leader, etc., in the case of senior fellows or senior career awardees) by formal and/or informal means, during this reporting period. If instruction or participation as a course director/discussion leader occurred in a prior budget period, note the dates of occurrence. Any activities undertaken to individualize instruction appropriate to career stage should be discussed. Address the five components: Format, Subject Matter, Faculty Participation, Duration, and Frequency. Additional detailed guidance on this requirement is found in the competing application instructions.
Provide a letter signed by the sponsor, in PDF format, assessing the progress of the research training (including academic work) and the fellow’s accomplishments during this reporting period. For fellowships supporting dual-degree training (e.g. F30) the sponsor should report on the fellow’s progress with respect to the proposed dual-degree timeline and whether the fellow is expected to complete the requirements for the research doctoral degree in the upcoming budget period.
Not Applicable.
Not Applicable.
7.3 SBIR/STTR RPPRs
For SBIR/STTR Awards (i.e., R41, R42, R43, R44, U43, U44, UT1, and UT2), follow
the Instructions for RPPR Sections A–I in chapter 6, with the exceptions noted below:
Goals is equivalent to specific aims and/or milestones.
For this reporting period, is there one or more Revision/Supplement associated with this award for which reporting is required?
If yes, identify the Revision(s) by grant number (e.g., 3R01CA098765-01S1) or title and describe the specific aims and/or milestones for each Revision. Include any supplements to promote or enhance diversity and re-entry, or other similar supplements to support addition of an individual or a discrete project.
B.6 What do you plan to do during the next reporting period to accomplish the goals?
For FastTrack and Phase II progress reports include a one-page abstract describing the research plan for Phase II and, as necessary, an updated commercialization plan.
C.5.a Other products
For SBIR/STTR awards commercial technologies will be addressed under Impact.
E.1 Not Applicable.
What is the impact on technology transfer?
Describe ways in which the project made an impact, or is likely to make an impact, on commercial technology or public use, including:
transfer of results to entities in government or industry;
instances where the research has led to the initiation of a start-up company; or
adoption of new practices.
E.3.a Commercialization Activities.
Report on the status of commercialization activities resulting from the award:
Nothing to report or select one or more of the following:
Sales = $
Licensing revenue = $
3rd Party investment since award start (Non-federal) = $
Sale of company
Sale of technology rights
Company merger related to product
Joint venture agreement
Marketing/Distribution agreement(s)
Manufacturing agreement(s)
R&D agreements
Customer alliance(s)
Other [60 character limit] E.3.b FDA Interactions.
Report on interactions with the Food and Drug Administration during the reporting period related to the technology that is the subject of the award:
Not applicable to this technology or select one or more of the following:
Discussion with FDA not initiated
Discussion with the FDA initiated o
Approval in Progress
Applied for approval
Review ongoing
In human clinical trials
Other
Approval Granted: Type
Not approved
F.1 Not Applicable.
G.1: Special Notice of Award and Funding Opportunity Announcement Reporting Requirements. NIH requires SBIR/STTR grant receipients to submit the Life Cycle Certifications within the Interim and Final RPPR in this section. Forms must be uploaded directly and SBIR/STTR recipients should not select the “Nothing to Report” box in this section. The I-RPPR or F-RPPR will not be accepted unless all completed Life Cycle Certification(s) are received. Life Cycle Certification forms are available for SBIR and STTR applicants and awardees in fillable format at: https://grants.nih.gov/grants/forms/manage_a_small_business_award.htm.
G.12 F & A Costs. [Applicable to SNAP awards only.]
H. Budget [Applicable to non-SNAP awards only.]
H.1 Budget Form
To complete the detailed budget for this award, follow the instructions in the SF424 (R&R) SBIR/STTR Application Guide for NIH and Other PHS Agencies, Section I, 4.6 Budget Component, sections A-K. The budget justification should be uploaded as item K, and must include detailed justification for those line items and amounts that represent a significant change from previously recommended levels (e.g., total re-budgeting greater than 25 percent of the total award amount for this budget period).

NOTE: If subaward budgets are completed, the system will not calculate the budget line item
F.5 for the main budget (see figure below). Total consortium costs for the main budget MUST be computed and entered manually into budget line item F.5.


Figure 105: SF 424 R&R Budget Form - Question F.5
H.2 Subaward Budget Form
For awards with subaward/consortium budgets, the grantee may select up to 30 subaward budgets. To complete a detailed budget for a subaward/consortium, follow the SF424 (R&R) SBIR/STTR Application Guide for NIH and Other PHS Agencies, Section I, 4.7 Budget Component.
7.4 Training RPPRs
For Training Awards (i.e., K12, KL2, R90, RL9, T15, T32, T34, T35, T37, T90, and TL1) and multi-year funded training awards (i.e., KM1) follow the Instructions for RPPR Sections A–H in chapter 6, with the exceptions noted below.
AHRQ Grantees only: T32 grantees should consult their AHRQ Program Official for additional requirements.
B.1. What are the major goals of the project?
Provide a description of the training objectives and goals. List the major goals of the project as stated in the approved application or as approved by the agency. If the application lists milestones/target dates for important activities or phases of the project, identify these dates and show actual completion dates or the percentage of completion.

B.2 What was accomplished under these goals?
Since the last report or application, describe implementation of training and other specific programmatic objectives, and the recruitment and retention of trainees, including those from diverse groups.
B.4 What opportunities for training and professional development has the project provided?
For all awards, provide a PDF that includes the following items: (1) a completed Trainee Diversity Report, covering the individuals supported by the award during the the initial reporting period and the period since the last progress report was submitted. (generally not applicable for FIC awards); and (2) a paragraph for each trainee/scholar supported by the award describing activities and progress during the reporting period or period since the last progress report. Include the following information for each trainee/scholar, as applicable:
Degrees working toward or received
Mentor(s)
Description of the trainee/scholar’s research project and progress
Career development related activities (e.g., individualized coursework or workshops attended)
Conference presentations
A description of the trainee/scholar’s role in any planned or published papers resulting from research conducted while supported by this award (e.g., designed or conducted experiment, analyzed data, drafted paper)
Note that full citations of all publications arising from work conducted while the trainee/scholar was supported by the award should not be reported here, as they will be collected in Section C.1.
Honors, awards, fellowships, and any other support (note that support before and after the appointment is reported on Table 8 and should not be reported here)
This description should be sufficient to allow evaluation of the appointees’ progress towards the goals of the training grant.
Indicate whether the institution uses Individual Development Plans (IDPs), and if so, describe how they were used to help manage the training and career development of the trainees/scholars (do not include actual IDPs or blank IDP forms). This information is not required for AHRQ grantees.
For T awards, provide updated information in Table 8A, 8B and/or 8C, reflecting new appointments and other changes over the initial reporting period or period since the last progress report .
For D43, TU2, T15, T32, T37, T90, U2R, U90, and TL1 awards, include program statistics for doctoral training in Table 8A.
The Trainee Diversity Report format page is available at: http://grants.nih.gov/grants/funding/2590/2590.htm
B.6 What do you plan to do during the next reporting period to accomplish the goals?
Include plans for any modification based on the findings of your internal evaluations.
Recipients must report trainee publications associated with the training award in section C.1 of the RPPR if: (1) the publication was accepted for publication or made public during the “the initial reporting period and the period since the last progress report was submitted.; and (2) the publication resulted from work conducted while a trainee was supported by the award (i.e., receiving a stipend or salary from the award). Publications resulting from work the trainee conducted while not actively supported by the training grant should not be reported in section C.1.
Trainee publications must be entered into My Bibliography to display in section C.1 of the RPPR electronic interface. PD/PIs have the option in My NCBI to hide trainee papers for which they were not involved as an author from public display. (See the My Bibliography guide for instructions on how to hide citations from display for a public collection.)
C.5.b Not Applicable.
D.1 What individuals have worked on the project?
Provide or update the following information only for K12 and KL2 progress reports: (1) program director(s)/principal investigators(s) (PDs/PIs); and (2) each person who has worked at least one person month per year on the project during the reporting period, regardless of the source of compensation (a person month equals approximately 160 hours or 8.3% of annualized effort).
Do not report personnel for whom a PHS 2271 Appointment form has been submitted through xTRAIN. If not reporting on a K12 or KL2 award, disregard this section.
D.2.b New senior/key personnel.
Are there new training faculty?
If yes, provide biosketches and other support for all new training faculty.
Not Applicable.
Not Applicable.
Not Applicable.
F. 1 Changes in approach and reasons for change
Describe changes in the program for the next budget period, including changes in training faculty. Include, as appropriate, the role of external advisory committees, significant new training content, procedures or experiences, and indicate how these aid in strengthening and realizing the objectives and goals of the program.
F.2 Not Applicable.
F.3 Significant changes to Human Subjects, Vertebrate Animals, Biohazards, and/or Select Agents
Complete this section only if the use or care of human subjects, vertebrate animals, biohazards and/or select agents is not reported under another NIH award.
Responsible Conduct of Research
Describe the nature of the responsible conduct of research instruction and the extent of trainee (or scholar, in the case of the Institutional Career Development Programs) and faculty participation. Include a description of any enhancements and/or modifications to the five instructional components (Format, Subject Matter, Faculty Participation, Duration, and Frequency) from the plan described in the competing application. Faculty members who were contributors to formal instruction in responsible conduct of research during the last budget period must be named. Additional detailed guidance on this requirement is found in the competing application instructions.
G.6 Human Embryonic Stem Cells (hESCs)
Complete this section only if the use of hESCs is not reported under another NIH award.
Not Applicable.
Not Applicable.
H. Budget
For training awards, grantees should select the applicable RPPR budget type (e.g., SF424 (R&R) or PHS 398 Training Budget) from the drop down menu. For a small number of NIH training programs the grantee is required to submit both the SF424 (R&R) and PHS 398 Training Budget; the RPPR will accommodate this.
H.1 Budget Form
If completing the SF424 (R&R), follow the instructions in the SF424 (R&R) Application Guide for NIH and Other PHS Agencies, Section I, 4.7 R&R Budget Component, sections A-K. The budget justification should be uploaded as item K, and must include detailed justification for those line items and amounts that represent a significant change from previously recommended levels (e.g., total rebudgeting greater than 25 percent of the total award amount for this budget period).
If completing the PHS 398 Training Budget, follow the instructions in the SF424 (R&R) Application Guide for NIH and Other PHS Agencies, Section I, 8.5 PHS 398 Training Budget Component, items A-F. The budget justification should be uploaded as item F, and must include detailed justification for those line items and amounts that represent a significant change from previously recommended levels (e.g., total re-budgeting greater than 25 percent of the total award amount for this budget period).

NOTE: If subaward budgets are completed, the system will not calculate the budget line item
F.5 for the main budget (see figure below). Total consortium costs for the main budget MUST be computed and entered manually into budget line item F.5.


Figure 106: SF 424 R&R Budget Form - Question F.5
H.2 Subaward Budget Form
For awards with subaward/consortium budgets, the grantee may select up to 30 subaward budgets. To complete a detailed budget for a subaward/consortium, follow the SF424 (R&R) Application Guide for NIH and Other PHS Agencies, Section I, 4.8 Special Instructions for Preparing Applications with a Subaward/Consortium or 8.6 PHS 398 Training Subaward Budget Attachment(s) Form.
7.5 Education RPPRs
For Education Awards (i.e., D43, DP7, K30, R13, R25, RL5, T14, T36, U13, and U2R), follow the Instructions for RPPR Sections A–H in chapter 6, with the exceptions noted below:
B.4 What opportunities for training and professional development has the project provided?
Describe opportunities for training and professional development provided to anyone who worked on the project or anyone who was involved in the activities supported by the project. Training activities are those in which individuals with advanced professional skills and experience assist others in attaining greater proficiency. Training activities may include, for example, courses or one-on-one work with a mentor. Professional development activities result in increased knowledge or skill in one’s area of expertise and may include workshops, conferences, seminars, study groups, and individual study. Include participation in conferences, workshops, and seminars not listed under major activities
Programs that involve participants should also provide the following information, as applicable:
A completed Training Diversity Report covering the individuals supported by the award during the initial reporting period or period since the last progress report, for subsequent reports (generally not applicable for FIC awards).
If relevant to the goals of the program, indicate whether the institution uses Individual Development Plans (IDPs) for graduate students and postdoctoral researchers, and if so, describe how they were used to help manage the training and career development of participants (do not include actual IDPs). This information is not required for AHRQ grantees.
Grantees with NIH grant awards with the following activity codes are required to provide program statistics for doctoral training in Table 8A: D43 and U2R.
C.3. Not Applicable.
C.4 Not Applicable.
C.5.b Not Applicable.
E.1 What is the impact on the development of human resources?
Describe how the project made an impact or is likely to make an impact on human resource development in science, engineering, and technology. For example, how has the project: 1) provided opportunities for research and teaching in the relevant fields; 2) improved the performance, skills, or attitudes of members of underrepresented groups that will improve their access to or retention in research, teaching, or other related professions; 3) developed and disseminated new educational materials or provided scholarships; or 4) provided exposure to science and technology for practitioners, teachers, young people, or other members of the public?
E.2 Not Applicable.
E.3 Not Applicable
F.1 Changes in approach and reasons for change
Describe changes for the next budget period. Include, as appropriate, the role of external advisory committees, significant new content, procedures or experiences, and indicate how these aid in strengthening and realizing the objectives and goals of the award.
G.2 Responsible Conduct of Research
If required in the FOA for this award, describe the nature of the responsible conduct of research instruction and the extent of participant and faculty involvement. Include a description of any enhancements and/or modifications to the five instructional components (Format, Subject Matter, Faculty Participation, Duration, and Frequency) from the plan described in the competing application. Faculty members who were contributors to formal instruction in responsible conduct of research during the last budget period must be named. Additional detailed guidance on this requirement is found in the competing application instructions.
G.3 Not Applicable.
G.12 F&A Costs [Applicable to SNAP awards only.]
7.6 Multi-Project RPPRs and Single-Project RPPRs with Complicated Structure
For the purposes of the RPPR, the following activity codes are always categorized as multiproject awards or single-project awards with complicated structures: G12, M01, P01, P20, P2C. P30, P41, P42, P50, P51, P60, PL1, PM1, PN1, PN2, R24, R28, RM1, S06, S11, U01, U10, U19, U24, U2C, U34, U41, U42, U45, U54, U56, UC7, UL1, UM1, and UM2. These
activity codes awards may or may not include multiple components (projects, cores), but they all could potentially include multiple components. For multi-project awards, the grantee will follow the instructions for the overall portion of the RPPR below and for each component of the RPPR the grantee will folloiw the instructions under component instructions in section 7.6.2.
The Instructions for RPPR Sections A–H in chapter 6, are applicable to these activity codes (even if the award does not include multiple components) with the following exceptions.
7.6.1 Overall
Emphasize the synergy, collaboration and integration of major activities of the project. Report the major goals specific to an individual component under that component.
What was accomplished under these goals?
For this reporting period describe for the overall award: 1) major activities; 2) significant results, including major findings, developments, or conclusions (both positive and negative), and 3) key outcomes or other achievements. Include a discussion of stated goals not met. Report the accomplishments of individual projects and cores under that component.
If the Revision is associated with a specific project or core, identify the component.
How have the results been disseminated to communities of interest?
If there are individual projects/cores designed to disseminate information or conduct outreach activities, report those activities under that component.
Report goals and objectives of individual projects or cores under that component.
Other products
Identify any other significant products that were developed under the overall project. Report other products and resources resulting from an individual project or core under that component.
Report resource sharing for an individual project or core under that component.
Participants
In addition to the instructions in Section 6.4, specify the component(s) on which the individual worked in the appropriate text box. This personnel information is for the entire project. Use the Ctrl key to select multiple components.

NOTE: If an individual is associated with multiple components, some components may be hidden to reduce the size table. Where applicable, use the show more link to display all of an individual’s components in the row. Use the show less link to collapse the information.

Figure 107: D.1 Specifying the Components for an Added Individual
Personnel questions (D.2.a.-e.) are applicable to entire project. For D.2.b, new senior/key personnel, identify the component(s) on which the individuals worked or will work. For D.2.e,
new other significant contributors, identify the component(s) on which the individual worked or will work.
E.1 Not Applicable.
E.3 Not Applicable.
F.1 Not Applicable.
F.3 Significant changes to Human Subjects, Vertebrate Animals, Biohazards, and/or Select Agents.
If there are changes in any of the following areas check the appropriate box and provide a d3escription of the changes. If applicable, report the change under the relevant component.
Figure 108: Associating a Component with the NCT Number
G.12 F&A Costs [Applicable to SNAP awards only.]
H. Budget
For multi-project RPPRs complete the budget for each component and for each subaward; see Section 7.6.1. A summary budget will be system-generated based on the budgets completed for the components and will be included in the final .pdf submitted to the Agency. The composite budget summaries will reflect the direct costs for the grantee. Although the direct and indirect costs for subawards are direct costs to the grantee institutions, these costs will be listed as a separate line item, called “consortium” and will include all consortium costs. The total consortium costs for the summary budget are automatically calculated by the system and reflect the sum of the consortium costs (budget line item F.5 of the project budget) for the project budgets with the grantee institution DUNS and the total direct and indirect costs (budget line item I.) for project budgets with a DUNS different from that of the grantee institution.

H.1 Budget Form [Single-Project RPPRs with Complicated Structure only]
For Single-Project RPPRs with Complicated Structure , follow the instructions in the SF424 (R&R) Application Guide for NIH and Other PHS Agencies, Section I, 4.7 Budget Component, sections A-K. The budget justification should be uploaded as item K, and must include detailed justification for those line items and amounts that represent a significant change from previously recommended levels (e.g., total re-budgeting greater than 25 percent of the total award amount for this budget period).

NOTE: If subaward budgets are completed, the system will not calculate the budget line item
F.5 for the main budget (see figure below). Total consortium costs for the main budget MUST be computed and entered manually into budget line item F.5.

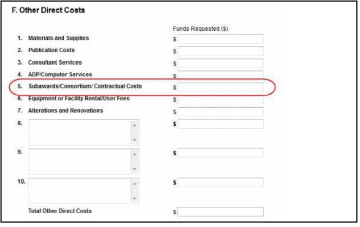
Figure 109: SF 424 R&R Budget Form - Question F.5
H.2 Subaward Budget Form[Single-Project RPPRs with Complicated Structure only]

For awards with subaward/consortium budgets, the grantee may select up to 30 subaward budgets. To complete a detailed budget for a subaward/consortium, follow the SF424 (R&R) Application Guide for NIH and Other PHS Agencies, Section I, 4.8 Special Instructions for Preparing Applications with a Subaward/Consortium.
7.6.2 Component Instructions
For each component, click the Add Component button, and complete the instructions. The Instructions for RPPR Sections A–H in chapter 6 are applicable to each individual component with the following exceptions:
A.1 Provide the name, email, phone number and address of the PI of the component.
Figure 110: Sample of Section A. Cover Page for a Component
3 Not Applicable.
B.5 How have the results been disseminated to communities of interest?
Reporting the routine dissemination of information (e.g., websites, press releases) is not required. If the Component is not designed to disseminate information to the public or conduct similar outreach activities, select Nothing to Report. If the Component is designed to disseminate information or conduct outreach activities, report those activities here. Note that scientific publications and the sharing of research resources will be reported under Products.

C.4 Not Applicable.
D. Not Applicable.
Describe ways in which the component made an impact, or is likely to make an impact, on commercial technology or public use, including:
F.1 Not Applicable.
.
G.5 Not Applicable.
H. Budget
H.1 Budget Form [Multi-Project RPPRs only]
When a grantee institution is the lead on the Component, follow the instructions in the SF424 (R&R) Application Guide for NIH and Other PHS Agencies, Section I, 4.7 Budget Component, sections A-K. The budget justification should be uploaded as item K, and must include detailed justification for those line items and amounts that represent a significant change from previously recommended levels (e.g., total re-budgeting greater than 25 percent of the total award amount for this budget period).
When a collaborating institution is the lead on the Component, the information from the collaborating institution should be used to complete the project budget, following the
instructions in the SF424 (R&R) Application Guide for NIH and Other PHS Agencies, Section I, 4.7 Budget Component, sections A-K.
For multi-projects RPPRs the grantee must complete the DUNS and Organization Name fields, as the DUNS number will not automatically populate to the DUNS number.

NOTE: If subaward budgets are completed, the system will not calculate the budget line item
F.5 for the main budget (see figure below). Total consortium costs for the main budget MUST be computed and entered manually into budget line item F.5.


Figure 111: SF 424 R&R Budget Form - Question F.5
H.2 Subaward Budget Form [Multi-Project RPPRs only]
If the component has subaward/consortium budgets, follow the SF424 (R&R) Application Guide for NIH and Other PHS Agencies, Section I, 4.8 Special Instructions for Preparing Applications with a Subaward/Consortium.

The list of Assurances, and Certifications, and other Policies that apply to progress reports submitted to NIH and other PHS agencies are explained in Part III: Policies, Assurances, Definitions, and Other Information. Applicants and grantees must comply with a number of additional public policy requirements. Refer to the NIH Grants Policy Statement (http://grants.nih.gov/grants/policy/policy.htm), or the HHS Grants Policy Statement (http://www.hhs.gov/asfr/ogapa/grantinformation/hhsgps107.pdf), as applicable, for additional information.
The policies, assurances and certifications listed in Part III may or may not be applicable to the project, program, or type of applicant organization. If unable to certify compliance, provide an explanation and upload it in G.1 Special Notice of Award and Funding Opportunity Announcement Reporting Requirements.
Submission of the RPPR to the agency includes the following certification:
In submitting this RPPR, the SO (or PD/PI with delegated authority), certifies that the grantee organization is in compliance with the terms and conditions specified in the Notice of Award and Grants Policy Statement, and verifies the accuracy and validity of all administrative, fiscal, and scientific information in the progress report. The SO (or PD/PI with delegated authority) further certifies that the grantee organization will be accountable for the appropriate use of any funds awarded and for the performance of the grant-supported project or activities resulting from the progress report. Deliberate withholding, falsification, or misrepresentation of information could result in administrative actions such as withdrawal of a progress report, suspension and/or termination of an award, debarment of individuals, as well as possible criminal penalties. The grantee institution may be liable for the reimbursement of funds associated with any inappropriate or fraudulent conduct of the project activity.
Assurances/Certifications

Privacy Act Statement. The NIH maintains application and grant records as part of a system of records as defined by the Privacy Act: NIH 09-25-0036, Extramural Awards and Chartered Advisory Committees (IMPAC 2), Contract Information (DCIS), and Cooperative Agreement Information, HHS/NIH: http://oma.od.nih.gov/ms/privacy/pa-files/0036.htm.
Gov’t Use of Info Under the Privacy Act
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| File Title | NIH and Other PHS Agency Research Performance Progress Report (RPPR) Instruction Guide |
| Subject | Guide for completing the RPPR |
| Author | eRA Communications |
| File Modified | 0000-00-00 |
| File Created | 2022-09-26 |
© 2026 OMB.report | Privacy Policy
 –OR–
–OR–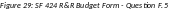
 5.5
5.5
 5.6
Submit the
5.6
Submit the  View
Routing
View
Routing From
From Recall
Public Access
Recall
Public Access