Form AQSE- (W, 1, 2, 3) AQSE- (W, 1, 2, 3) STUDY PROTOCOL FOR A COMPASSIONATE AQUACULTURE INVESTIGA
Administration of U.S. Fish and Wildlife Service Investigational New Animal Drug (INAD) Program
AQS20E study protocol final Jan 2023
AQUI–S®20E INAD #11–741 - Private Sector
OMB:
OMB Control No. 1018-####
Expires ##/##/20##
STUDY PROTOCOL FOR A COMPASSIONATE AQUACULTURE
INVESTIGATIONAL NEW ANIMAL DRUG (INAD)
EXEMPTION FOR AQUI-S®20E (eugenol)
(INAD #11-741)
Sponsor:
U.S. Fish and Wildlife Service, Fish and Aquatic Conservation
______________________ ___________________
Sponsor Signature Date Approved
Manufacturer:
AQUI-S New Zealand, Ltd./Merck Animal Health
35500 W. 91st Street
Desoto, KS 66018
Facility for Coordination of AQUI-S®20E INAD:
Aquatic Animal Drug Approval Partnership Program
U.S. Fish and Wildlife Service
4050 Bridger Canyon Road
Bozeman, Mt 59715
Proposed Starting Date August 1, 2013
Proposed Ending Date December 31, 2027
Study Director Ms. Bonnie Johnson
Clinical Field Trial Location:
Facility: _________________________________________________________
Investigator: ______________________________________________________
Table of Contents
I. STUDY IDENTIFICATION AND TITLE 3
III. INVESTIGATORS/FACILITIES 4
IV. PROPOSED STARTING AND COMPLETION DATES 4
XII. TREATMENT RESPONSE PARAMETERS 12
XIII. FORMS FOR DATA COLLECTION 15
XIV. RECORD KEEPING PROCEDURES 15
XV. DISPOSITION OF INVESTIGATIONAL ANIMALS 15
XVI. DISPOSITION OF INVESTIGATIONAL DRUG 16
XVII. DATA HANDLING, QUALITY CONTROL, MONITORING, ADMINISTRATIVE RESPONSIBILITIES 17
XVIII. PLANS FOR DATA ANALYSIS 18
XIX. PROTOCOL AND PROTOCOL AMENDMENTS 18
All data must be entered through the online INAD database: 28
FORM AQSE-1.Report on Receipt of Drug 31
STUDY PROTOCOL FOR A COMPASSIONATE AQUACULTURE INVESTIGATIONAL NEW ANIMAL DRUG (INAD) EXEMPTION FOR
AQUI-S®20E (eugenol) UNDER INAD #11-741
I. STUDY IDENTIFICATION AND TITLE
Clinical field trials to determine the efficacy of AQUI-S®20E as an anesthetic for use in a variety of fish species. Clinical field trials will be conducted on various freshwater-reared and saltwater-reared finfish; sharks; and freshwater prawn at different facilities under a variety of environmental conditions under INAD #11-741.
Dr. Marilyn Blair, U.S. Fish and Wildlife Service, Branch Chief, Aquatic Animal Drug Approval Partnership Program, 4050 Bridger Canyon Road, Bozeman, MT 59715; Phone: 406-994-9904; Fax: 406-582-0242; Email: [email protected]
Manufacturer: AQUI-S New Zealand, Ltd./ Merck Animal Health
35500 W. 91st Street
Desoto, KS 66018
Contact Person at Merck Animal Health:
Jackie Zimmerman
Phone: (208) 603-0336
email: [email protected]
or
Merck
Animal Health Customer Service
Phone: 1-800-521-5767
email: [email protected]
Study Director: Ms. Bonnie Johnson, U.S. Fish and Wildlife Service, Aquatic Animal Drug Approval Partnership (AADAP) Program, 4050 Bridger Canyon Road, Bozeman, MT 59715; Phone: 406-994-9905; Email: [email protected]
Principal Clinical Ms. Paige Maskill, USFWS – AADAP Program
Field Trial Coordinator: 4050 Bridger Canyon Road, Bozeman, MT 59715;
Phone: 406-994-9911; Email: [email protected]
INAD Study Monitors: Appendix II will contain the names and addresses of the participating monitors that will be submitted directly to the Center of Veterinarian Medicine (CVM). This list is not available for the public.
Appendix IIIa will contain the names and addresses of the participating facilities that will be submitted directly to CVM and appropriate drug sponsor. This list is not available for the public.
IV. PROPOSED STARTING AND COMPLETION DATES:
Proposed Starting Date: August 1, 2013
Proposed Completion Date: December 31, 2027
Background Information:
The use of anesthetics is an important tool with broad application to fisheries management programs. Most often, anesthetics are used to reduce stress associated with the handling and/or transportation of fish. Anesthetics are widely used both in the culture of captive populations, and in field situations that involve the management of wildstock fish populations. Although a number of compounds have been used in the past, currently, the only approved anesthetic for use on fish is SYNCAINE (active ingredient methane tricainesulfonate, ANADA 200-226). SYNCAINE has a withdrawal period of 21 days. This restriction requires that potential food fish must be held for a minimum of 21 days following treatment before they can be released for legal harvest or slaughtered. While this product has been found to be effective anesthetics for use in fish, its required 21-day withdrawal period severely restricts approved use in many situations. In contrast, a zero-withdrawal (or immediate release) anesthetic would allow food fish to be released, stocked, or slaughtered “immediately” following treatment. In numerous fisheries management programs, and particularly those involving wildstock population assessment and evaluation, there is a critical need for such an anesthetic. AQUI-S®20E has been developed in New Zealand as an anesthetic for use on food-fish with no withdrawal period. The active ingredient in AQUI-S®20E, eugenol, is used in perfumeries, flavorings, essential oils, and in medicine as a local antiseptic and anesthetic.
Purpose of INAD:
The purpose of this compassionate INAD for AQUI-S®20E is to develop clinical efficacy field trial data that will be used to determine the most appropriate treatment regimen for AQUI-S®20E for use as an anesthetic in a variety of fish species. These data will be used to support a new animal drug application (NADA) for AQUI-S®20E.
The U. S. Fish and Wildlife Service (USFWS) anticipates requesting the U. S. Food and Drug Administration (FDA) to grant extensions of this INAD for additional years. The USFWS believes that data from at least 1-3 treatment seasons will be required in order to adequately assess the efficacy of AQUI-S-E as an anesthetic for use in fish, and to collect sufficient data to support a NADA.
The two major objectives of this study protocol are as follows:
1. Collect scientific data necessary to establish the effectiveness of AQUI-S®20E as an anesthetic in a variety of fish species under a variety of environmental conditions (e.g., temperature, water hardness, pH, turbidity, etc.).
2. Provide an opportunity for fish culturists and fisheries managers to legally use AQUI-S®20E as an anesthetic so that they can maintain and manage healthy stocks of fish during the period of time necessary for collection of efficacy, safety, and residue data required for an NADA for AQUI-S®20E in fish.
A. Test and Control Articles:
1. Drug Identity
a. Active ingredient
Chemical Name: eugenol [2-methoxy-4-(propenyl) phenol]
C.A.S. Registry No.: 97-53-0
Molecular Formula: C10H12O2
Appearance: Yellow, viscous liquid
Odor: Spicy, pungent, clove-like
Specific Gravity: 1.124
b. Strength and dosage form
AQUI-S®20E is 10% eugenol (active ingredient). Fish are exposed by immersion bath.
c. Manufacturer, source of supply
AQUI-S New Zealand, Ltd./ Merck Animal Health
35500 W. 91st Street
Desoto, KS 66018
Contact Person at Merck Animal Health:
Jackie Zimmerman
Phone: (208) 603-0336
email: [email protected]
or
Merck
Animal Health Customer Service
Phone: 1-800-521-5767
email: [email protected]
2. Verification of Drug Integrity/Strength:
AQUI-S New Zealand, Ltd. will provide the analytical data necessary to establish the purity of each lot/batch of AQUI-S®20E supplied. The lot number and date of manufacture for each batch of AQUI-S®20E will be placed on the label of each container. The form Report on Receipt of Drug - Guide for Reporting Investigational New Animal Drug Shipments for Poikilothermic Food Animals (Form AQSE-1) will clearly identify the lot number and date of manufacture of AQUI-S®20E shipments. If the integrity of the AQUI-S®20E is compromised (i.e., by spilling or contamination of the stock container) the event will be carefully recorded, dated, and signed in the Drug Inventory Form (Form AQSE-2). All unusable AQUI-S®20E will be disposed of by following the Safety Data Sheet (SDS).
3. Storage Conditions
AQUI-S®20E will be stored in the original container supplied by the Manufacturer with the appropriate investigational label attached. AQUI-S®20E has high stability and should be stored at room temperature in a dry location away from direct sunlight. AQUI-S®20E should be stored in a secure location such as in a locked cabinet.
4. Handling Procedures
Each Study Monitor and Investigator will be required to have a current copy of the Safety Data Sheet (SDS) for AQUI-S®20E (Appendix IV). Each person involved with the study and each person who may be present during the use of AQUI-S®20E shall be required to read the SDS. Safety precautions as outlined in the SDS will be followed at all times when working with AQUI-S®20E. Standard laboratory equipment such as gloves, lab coats or aprons, eye protection, etc., will be worn at all times.
5. Investigational Labeling
A copy of the label to be attached to each container of AQUI-S®20E is provided in Appendix V. Although investigational labels will be affixed to AQUI-S®20E containers by the supplier, it is the responsibility of the Investigator to ensure proper labeling of all containers of AQUI-S®20E.
6. Accountability
Merck Animal Health will be the sole supplier of AQUI-S®20E to all Investigators
under this INAD.
1. All facilities using AQUI-S®20E:
Immediately upon receiving an order/shipment of AQUI-S®20E, the Investigator will complete “Report on Receipt of Drug - Guide for Reporting Investigational New Animal Drug Shipments for Poikilothermic Food Animals” (located in the “Manage/View Drug Inventory” section of the investigator account). The Study Director will forward a copy of this form to the FDA. Arrangements should be made between Investigators and Study Monitors to insure completed Form AQSE -1s are received by the Study Director within 10 days of drug receipt.
All Investigators are also responsible for maintaining an accurate inventory of AQUI-S®20E on-hand. A Drug Inventory Form (Form AQSE-2) must be completed and maintained by each Investigator. Each time AQUI-S®20E is used, it must be recorded by the Investigator in the Results Report form in the “Amount Of Drug Used” table.
At the conclusion of the study, all remaining AQUI-S®20E will be disposed of by following the SDS (note: unless AQUI-S®20E is planned for use in another approved field trial, and planned usage is within the storage guidelines established by the manufacturer). Disposition of all AQUI-S®20E must be properly recorded and accounted for on the Drug Inventory Form (Form AQSE-2). The Study Monitor will be responsible for verifying the quantity of AQUI-S®20E remaining on hand versus the amount indicated on Form AQSE-2. Note: AQUI-S®20E can be transferred to other facilities that are participating under INAD 11-741. Transfers must be shown on Form AQSE-2.
7. Preparation Procedures
AQUI-S®20E will be prepared according to label directions for normal use. This includes accurately measuring out (by volume or weight) the calculated amount of AQUI-S®20E needed to obtain the desired treatment dose. AQUI-S®20E is “ready-for-use” as supplied by the manufacturer and may be added directly to treatment water. Be sure to uniformly mix the AQUI-S®20E with treatment water before actual treatment of fish.
Visit the Aqui-S website to find the Aqui-S 20E calculator to help calculate the amount of AQUI-S®20E that will be needed. Select the Aqui-S 20E calculator and then fill in the fields. Please note that due to the specific gravity of AQUI-S®20E that there is a difference between the amount of AQUI-S®20E needed in grams versus milliliters. The calculator can be found at: Aqui-s 20E Calculator.
To calculate the volume (milliliters; mL) of Aqui-S 20E to add to the treatment bath use the following calculation:
The concentration of Aqui-S 20E is 112 mg/eugenol/mL
Aqui-S
20E (ml) = [(A x B)
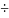 112]
112]
Where: A = target concentration eugenol (mg/L)
B = treatment water volume (liters)
To convert gallons to liters, multiply: (gallons x 3.79 L/gallon) = Liters
Example:
A = 30 mg/L target concentration eugenol
B = 37.9 L
(10 gallons x 3.79 L/gal)
[(30
x 37.9)
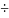 112] = 10.2 mL Aqui-S 20E to add
112] = 10.2 mL Aqui-S 20E to add
B. Items Needed for Treatment, Data Collection, Etc.:
Treatment and diagnostic equipment should include a balance, graduated cylinder or flask, treatment tank, recovery tank, thermometer, stopwatch, and a dissolved oxygen meter.
When the Study Protocol has been approved and treatments are scheduled, the Investigator at each facility covered by the AQUI-S®20E INAD will need to complete several forms located in the online INAD database. These forms are described in Section XIII. Copies of these forms are attached to this Study Protocol and will be used as a guide only for collecting the data that will be entered into the online INAD database.
The experimental unit in this clinical field trial will consist of a contained or isolated group of fish. This will generally be a group of fish contained in a tank, raceway, pond, or container used to hold fish treated in the field. In some cases, the experimental unit may be individual animals.
A. Facilities/Investigators
The proposed facility and the Investigator must be listed in Appendix IIIa (note: this appendix is only available for AADAP; CVM; and the appropriate drug sponsor) of the Study Protocol for the current calendar year before AQUI-S®20E can be ordered and dispensed under this INAD. Last minute deviations can be requested by the Sponsor, Investigator, or Study Monitor in case emergency use-pattern needs should arise (See Section XX). However, poor planning and/or a lack of preparation will not be considered an emergency situation.
B. The characteristics of the study animals (species, number, etc.) in Appendix VIb will be sent directly to CVM. This list is not available for the public.
C. Environmental conditions
Environmental conditions will be variable and include a broad spectrum of temperatures and water quality parameters. Environmental conditions will be reported on Form AQSE-3.
D. Ability of investigator to fulfill all the requirements of the Study Protocol
See Appendix IIIb for example of knowledge required of hatchery managers (i.e., Investigators).
Prior to initiating each treatment event, the Investigator must first complete Form AQSE-W. “Worksheet for Designing Individual Field Trials” (located under the “New Study Request” tab in the investigator account) that pertains to each specific treatment event. The worksheet should be filled out and forwarded to the Study Monitor through the online INAD database. The Study Monitor will review the planned treatment (worksheet) and forward it to the Study Director at the AADAP Office. The Study Director will then review the worksheet, assign the approved treatment a Study Number, and then the online INAD database will notify both the Investigator and the Study Monitor of the assigned number and approval to proceed. In most cases, this entire process should be able to be accomplished within a single working day. After initiation of the field trial, the Investigator should also record the assigned study number on any paper forms that are being used as a guide to collect the data to enter in the online database (i.e., Form AQSE-2 and AQSE -3), as well as on any additional correspondence regarding that specific treatment event. If for some reason the Investigator is unable to reach the Study Monitor with regards to Worksheet approval and the need for treatment is immediate, the Investigator should contact the AADAP Office for permission to proceed.
Note: The Online INAD Database must be used by Investigators for ALL INAD reporting. The online INAD database has a built-in system of checks, balances, and email notifications to ensure that all information/data reporting and accountability follows established INAD Study Protocol guidelines. Unless data is entered directly into the online INAD database (i.e., not captured elsewhere at the time of observation or measurement and transcribed into the online INAD database) investigators must archive hard copies of all raw data.
A treatment group or experimental unit may be an entire tank, pond, raceway, or group of fish, or it may be individual animals.
Non-treated control groups will not be a requirement for clinical field trials evaluating the efficacy of AQUI-S®20E as an anesthetic. As the primary use of AQUI-S®20E will be to facilitate handling and reduce stress to fish when they are being handled, untreated controls would in most cases be extremely impractical, as well as detrimental to fish health. However, Investigators are encouraged to record observations with respect to the behavior and physiological state of fish prior to AQUI-S®20E treatment. This information will provide a “psuedo-control” as to fish condition without, or prior to, AQUI-S®20E treatment.
Although as stated above untreated control groups are not a required element of treatment under this INAD exemption, it is important for all Investigators to note that field trials conducted under a more stringent study protocol (i.e, including requirements for non-treated controls groups, replication, blinding, dose verification, etc.) will ultimately be required in order to support a NADA for AQUI-S®20E. It is also important to note that the INAD sponsor fully expects that a limited number of facilities/Investigators listed under this INAD exemption will agree to participate in such “pivotal” efficacy studies. These studies will be initiated only after direct consultation between facilities/Investigators and the sponsor. These studies will be conducted under a separate FDA-approved study protocol (i.e., not the INAD study protocol), and will also be conducted with assistance from, and under the direct supervision of, the sponsor. If for any reason it becomes apparent to the sponsor that facilities/Investigators listed under this INAD are not willing to participate in such “pivotal” studies, the sponsor will request that FDA terminate the INAD.
A. Route of administration
AQUI-S®20E will be administered as a static immersion bath treatment. AQUI-S®20E will be prepared according to label directions for normal use. This includes accurately measuring out (by volume or weight) the calculated amount of AQUI-S®20E needed to obtain the desired treatment dose. AQUI-S®20E is “ready-for-use” as supplied by the manufacturer and may be added directly to treatment water. Be sure to uniformly mix the AQUI-S®20E with treatment water before actual treatment of fish.
Note: An Aqui-S 20E calculator to help calculate the amount of AQUI-S® can be found at: Aqui-s 20E Calculator. See Section VII.A.7. Preparation Procedures for calculation formulas to use.
B. Dose to be administered and duration of treatment
AQUI-S®20E should be applied as a static immersion bath at eugenol concentrations ranging from:
Light Sedation: 1 – 15 mg/L (note: AQUI-S®20E is 10% eugenol as the active ingredient) for up to 8 hours exposure. After completion of treatment and handling, fish should immediately be placed in fresh water.
Handleable: The Efficacy Technical Section has been completed for fish that will be sedated to handleable levels. Please follow the below potential label claim dosing for salmonids and non-salmonids. If there are any deviations to the below doses, then a justification is needed in Form AQSE-3.
Treatment doses and duration for freshwater or saltwater finfish for the handleable level of sedation:
Option A: Handleable sedation for freshwater/saltwater salmonids is between 25 – 40 mg/L; sedation time no longer than 5 minutes; and recovery time less than 20 minutes. If treatment dose, sedation, or recovery times are not in this dosing range then a justification must be provided in Form AQSE-3.
Option B: Handleable sedation for freshwater non-salmonids is between 40 – 100 mg/L; sedation time no longer than 5 minutes; and recovery time less than 20 minutes. If treatment dose, sedation, or recovery times are not in this dosing range then a justification must be provided in Form AQSE-3.
Option C: Handleable sedation for saltwater non-salmonids is between 30 – 40 mg/L; sedation time no longer than 5 minutes; and recovery time less than 20 minutes. If treatment dose, sedation, or recovery times are not in this dosing range then a justification must be provided in Form AQSE-3.
Surgery Level; or Euthanasia: 10 - 100 mg/L (note: AQUI-S®20E is 10% eugenol as the active ingredient) for up to 15-minute exposure. Treatment durations will not exceed 15 minutes. If fish do not become sedated to the desired level of anesthesia within 15 minutes, then treatment must be stopped. Investigators will need to decide if a higher dose is needed to achieve the correct level of anesthesia within the 15-minute time frame before continuing treatments. After completion of treatment and handling, fish should immediately be placed in fresh water.
Within this range, the actual concentration applied will be at the discretion of the Investigator for the different levels of sedation as long as it is within the dosing range listed above. Dosage will likely vary with respect to species, water temperature, and level of anesthesia desired.
Note: the term Light Sedation is used to describe fish that are lightly sedated. Handleable (or a handleable level of sedation) is used to describe sedation that is typically used when handling fish (i.e., lengths and weights; spawning; Floy tags; PIT tags; etc). Anesthesia for surgery (or surgery level of sedation) is used to describe sedation that is required to perform surgery on fish. See section XII for a description of the four levels of sedation that can be used under this protocol. It is possible that the same activity may be reported as different levels of anesthesia depending on how sedated the fish need to be. If this happens then AADAP will review the study and classify the level of sedation that best meets the purpose of the activity.
C. Dosing interval and repetition
AQUI-S®20E will be applied as a single treatment event and will not require repeated treatments. Treatment baths will not be re-dosed, so new treatment baths will need to be made once it is determined that additional baths of fish are no longer becoming sedated in the expected timeframe. Fish will only be treated a single time that day.
D. Disposition of anesthetic solution
If at all possible, discharge of anesthetic solution remaining in the treatment containers following completion of treatment should be to the ground. If ground discharge is not possible, anesthetic solution may be released/mixed with facility effluent or released directly into public surface water. In situations where minimal dilution of anesthetic solution occurs prior to release to public surface waters, a pulsed release of anesthetic solution should be employed to minimize discharge levels.
E. Detailed procedures for drug administration
Standard laboratory equipment such as gloves, lab coats or aprons, eye protection, etc. should be worn at all times when working with AQUI-S®20E. The amount of AQUI-S®20E necessary for each treatment should be accurately measured out (by volume or weight) immediately prior to treatment.
Note: An Aqui-S 20E calculator to help calculate the amount of AQUI-S® can be found at: Aqui-s 20E Calculator. See Section VII.A.7. Preparation Procedures for calculation formulas to use.
F. Permissible concomitant therapy
Since efficacy data are being collected during the INAD process, there should be little or no concomitant therapy. There should be no other therapy during a period extending from 2 weeks prior to treatment lasting until 2 weeks after treatment. In addition to no concomitant therapy, Investigators need to keep fish cultural procedures and environmental conditions consistent following treatment with AQUI-S®20E.
An exception to this is that the food use authorization allows for the use of certain drug treatments with AQUI-S®20E treatments, provided the withdrawal time and use pattern identified in the food use authorization for that concomitant treatment is observed (see section XV for specific concomitant treatment information). The drugs must be used under the conditions of the respective INAD protocol or approved labeling. If another drug is used, please note its use on Form AQSE-3 under the description of results section. Please consult the Study Director to find out if AQUI-S®20E can be used with another drug prior to treatments.
XII. TREATMENT RESPONSE PARAMETERS
The collection and reporting of source data begins with the decision to treat valuable fish based on hatchery records or field management practices that indicate treatment is warranted. Daily morbidity and mortality records, case history records, as well as any extenuating or mitigating circumstances that may affect treatment response needs to be documented. All pertinent treatment response parameters should be reported on Form AQSE-3. Treatment response parameters that should be addressed include the following:
1. Primary Response Parameters
The primary treatment response parameters in this study will be a reflection of the physiological condition of fish following treatment with AQUI-S®20E. The primary physiological conditions evaluated will include when a fish is considered to be: 1) “lightly sedated”; 2) “handleable”; 3) “anesthetized”; 4) euthanized; or 5) “recovered”. In most cases, dependent upon level of anesthesia desired and treatment duration, a study will involve either handleable or anesthetized (surgery level of sedation) and recovered. Data should be reported in time (minutes) to reach a specific level of sedation, how long fish were held in the treatment bath, and time to recovery from sedation.
It is possible that some activities may overlap different sedation levels due to the level of sedation needed or how quickly a fish may need to be sedated. The reason for the sedation will need to be entered in Form AQS-W in the “Study Design” section and in Form AQS-3 in the “Description of Results”. This will help identify what activities are most likely to be conducted under the different levels of sedation.
Light Sedation
A fish will be considered lightly sedated when it has partial to total loss of reactivity to external visual and tactile stimuli, slight decrease in opercula rate, and equilibrium remains normal. Pivotal research work conducted by AADAP shows that fish will be lightly sedated if they can be caught by hand; not lose equilibrium; able to swim; and are spread throughout the water column. This is similar to Stages 1 - 2 of anesthesia as described by Summerfelt and Smith (1990).
The light sedation level will be used when working with fish that need to be lightly sedated over an extended amount of time (typically 15 minutes to an hour but no longer than 8 hours) for fish handling procedures that may include tagging; fish health; or moving fish to different tanks within the facility. Typically, these fish will become lightly sedated in over 5 minutes and recover within 5 - 10 minutes. Treatment baths may require supplemental oxygen during the extended exposure period. Note: light sedation will not be used for transporting fish.
Handleable
A fish will be considered handleable when it loses partial or total equilibrium, has slow but regular opercular rate, can be caught easily by hand, placed on a measuring board with minimal fish movement, and easily measured for length. As a general rule, a fish will be considered handleable when it can be captured and held for several seconds without difficulty. This is similar to Stages 3 - 4 of anesthesia as described by Summerfelt and Smith (1990).
The handleable level is the most common sedation level that will be used. This level will be used for insertion of PIT, coded, or floy tags; length and weight measurements; spawning; and other fish handling procedures that don’t require surgery.
Anesthetized (surgery level anesthesia)
A fish will be considered anesthetized when it loses all reflex activity. This condition generally occurs after a fish has completely lost equilibrium. As a general rule, a fish will be considered anesthetized when it can be easily held out of water, and when lifting the operculum and touching the gill lamellae does not elicit a reflexive “cough” within 5 seconds. This is similar to Stage 5 of anesthesia as described by Summerfelt and Smith, (1990).
The anesthetized level will only be used for surgery level sedation for fish. For example, anesthetizing the fish for insertion of a radio tag would require this level of sedation. A reason for the surgery level of anesthesia should be recorded on Form AQSE-3.
Euthanized
A fish will be considered euthanized when all opercular movements have ceased for a period of 10 minutes. The current timeframe has been established from the American Veterinary Medical Association (AVMA) Guidelines for the Euthanasia of Animals: 2020 edition. Aqui-S 20E concentrations and length of time required for euthanasia may vary depending on fish species and water temperature. As part of the INAD investigation for euthanizing fish, the AADAP Research Team will conduct pivotal studies in the future to better define parameters that can be used to help define this claim.
Note: Euthanized fish must not be sent to slaughter, made available for food, or returned to a water source as streambank enhancement.
Recovered
An anesthetized fish will be considered recovered when it exhibits normal swimming behavior, including avoidance of obstacles. For AQUI-S®20E treatments, the fish must recover in less than 30 minutes of exposure to fresh water to be considered “recovered”.
As a result of the potential diversity of treatment conditions and unique treatment response parameters (e.g., specific level of anesthesia desired) that may be involved in these studies, Investigators are encouraged to provide detailed descriptions of all study variables, including specific definitions/descriptions of level of anesthesia and criteria used to establish anesthesia levels. Investigators may also choose to create their own forms for purposes of recording source data under this INAD. Supplementary data forms should be attached to Form AQSE-3.
2. Secondary Response Parameters
Secondary parameters include general observations on fish behavior and response to routine culture/management activities. Secondary parameters would include such responses as feeding activity, apparent level of stress, or other negative fish behavior (including, but not limited to, gill coughing, agitation, and/or jumping when exposed to treatment). All post-treatment mortality should be documented.
3. Adverse Drug Events
Any adverse event related to treatment should be reported immediately to the Study Monitor, who will in turn notify the Study Director. Such responses might include changes in water quality, negative responses/behavior by the fish (e.g., gill coughing, agitation, and/or jumping when exposed to treatment) or hazards to personnel. All adverse drug events must also be documented on Form AQSE-3. It is possible adverse drug events may occur under certain environmental conditions or with respect to specific species/strains of fish. Careful observation of all treated fish for signs of any adverse reaction to treatment is extremely important, and all observations of adverse drug events should be documented. If any signs of drug toxicity are detected, they should also be documented and immediately reported to the Study Monitor, who will in turn notify the Study Director.
Note: Investigators are strongly encouraged to record observations/comments with respect to all phases of treatment. This may include a description of events before, during, and post-treatment. All extenuating or mitigating treatment circumstances need to be described in detail. Such information is imperative so that accurate study/data analysis can be performed.
XIII. FORMS FOR DATA COLLECTION
When the Study Protocol has been approved and treatments are scheduled, the Investigator at each facility covered by the AQUI-S®20E INAD will need to complete the following forms:
Form AQSE-W. Worksheet for Designing Individual Field Trials - located in the New Study Request tab
Form AQSE-1. Report on Receipt of Drug – located in the Manage/View Drug Inventory tab
Form AQSE-2. Chemical Use Log for Field Trials under AQUI-S®20E under INAD #11-741 – located in the Manage/View Drug Inventory tab and filled out in Form AQSE-3 to show use
Form AQSE-3. Results Report Form for use of AQUI-S®20E under INAD #11-741 – located in the Active Studies table on the home page
Copies of these forms are attached to this Study Protocol. Actual reporting is accomplished on forms located in the online INAD database.
XIV. RECORD KEEPING PROCEDURES
As stated immediately above, all data reporting is accomplished via forms located in the online INAD database. All current and completed studies conducted under the Investigator account will be stored and available in the online INAD database to the current study Monitor, study Investigator, and Study Director. Note: Unless data is entered directly into the online INAD database (i.e., not captured elsewhere at the time of observation or measurement and transcribed into the online INAD database) investigators must archive hard copies of all raw data.
XV. DISPOSITION OF INVESTIGATIONAL ANIMALS
Animals that die during treatment should be disposed of by burial or incineration.
All fish treated at hatchery facilities, or of immediate hatchery origin, or fish treated at light sedation at 1 to 15 mg/L from 15 minutes to 8 hours exposure must be held for at least 72 hours following treatment with AQUI-S®20E before they are stocked or allowed to enter the food chain.
Fish that are treated as part of field-based fisheries management activities may be released immediately following treatment as long as treatment duration does not exceed 15 minutes.
Fish that are illegal for harvest during that 72-hour period may be released immediately after treatment.
Note: Euthanized fish must not be sent to slaughter, made available for food, or returned to a water source as streambank enhancement.
For treatments where other drugs may be used at the same time as AQUI-S®20E then the longest withdrawal period must be followed. See below for the drugs listed on the current food use authorization and the withdrawal period that must be followed. Contacting AADAP before use of a concomitant drug is advised.
The use of Chorulon® needs to follow the approved label dosing instructions for this drug. A 72-hour withdrawal period is required for use of Aqui-S®20E and Chorulon®.
The use of CCP is used under the conditions of INAD 8391. A 72-hour withdrawal period is required for use of Aqui-S®20E and CCP.
The use of GnRH IIa is used under the conditions of INAD 13-345. A 72-hour withdrawal period is required for use of Aqui-S®20E and GnRH IIa.
The use of LHRHa is used under the conditions of INAD 8061. A 72-hour withdrawal period is required for use of Aqui-S®20E and LHRHa.
The use of sGnRHa (Ovaplant-L) is used under the conditions of INAD 13-298. No fish can be released if Ovaplant-L is used since there is no food use authorization in place for this INAD.
The use of oxytetracycline injectable is used at 20 mg/kg body weight. A 45-day withdrawal period is required for use of Aqui-S®20E and oxytetracycline injection.
The use of erythromycin is used under the conditions of INAD 12-781 and is only for salmonid species. A 60-day withdrawal period is required for use of Aqui-S®20E and erythromycin.
The Investigator must record the disposition of all treated fish on Form AQSE-3.
XVI. DISPOSITION OF INVESTIGATIONAL DRUG
AQUI-S®20E will be used only in the manner and by the individuals specified in the Study Protocol. If any unused or outdated AQUI-S®20E remains at the end of the study period, Investigators should contact Study Monitors for instructions regarding drug disposal. Drug disposal information is available in the Safety Data Sheet (SDS) located in Appendix IV of this protocol. Disposition of all AQUI-S®20E must be properly recorded and accounted for on the Chemical Use Log (Form AQSE-2). The Study Monitor will be responsible for verifying the quantity of AQUI-S®20E remaining on hand versus the amount indicated on Form AQSE-2. The investigational drug may not be redistributed to others not specified by the protocol and should not be retained by the Investigator after completion of the study (note: unless AQUI-S®20E is planned for use in another approved field trial, and planned usage is within the storage guidelines established by the manufacturer).
XVII. DATA HANDLING, QUALITY CONTROL, MONITORING, ADMINISTRATIVE RESPONSIBILITIES
A. Drug distribution
See Section VII.A.6. Accountability for information and details.
B. Study Monitors
The Study Monitors are generally fish health professionals with experience in diagnosing and treating fish diseases. A Study Monitor will be selected by each facility that is authorized to treat fish with AQUI-S®20E under this INAD. The Study Monitors are responsible for supervision of the trials, adherence of the Investigator to the Study Protocol, and inspection of the site.
C. Special equipment and materials
Most of the equipment and materials required for this study (with the exception of the AQUI-S®20E itself) are already available at each participating facility. The use of anesthetics to aid in the handling of fish is a common occurrence at most fish hatcheries and in many fisheries management programs. Fish hatchery managers and fisheries managers (i.e., Investigators) are well trained and well equipped to supervise these procedures (see Appendix IIIb). If any additional equipment or materials are required, they will be provided by the Study Monitors (See Section VII.B. Items needed for sample collection, observations, etc..,).
D. Administrator of the drug
AQUI-S®20E will be administered directly by the assigned Investigator (fish hatchery manager or fisheries manager) or under the Investigator's direct supervision. AQUI-S®20E will be maintained in a secure location, and only the Investigator or a person under his/her direct supervision will have access.
E. Drug accountability records
See protocol Section VII.A.6. Accountability for details and the following forms will be used as guides for data collection: Form AQSE-W, Form AQSE-1, Form AQSE-2, and Form AQSE-3.
F. Recording observations
The Investigator or a person under his/her direct supervision will be responsible for implementing the Study Protocol, making observations, collecting samples, and recording data during the clinical field trials. After the data have been collected and recorded on the forms, the Investigator will send the data to the Study Monitor who will ensure that all required information is provided. The Study Monitors will in turn send the data to the Study Director. The Study Director will analyze and summarize the data and prepare summary reports that will be submitted to the FDA. Note: If the Study Monitor does not think all required information has been provided, or forms have not been satisfactorily completed, he/she should contact the Investigator and rectify the situation before forwarding the package to the Study Director.
G. Data storage
The Investigator is responsible for complete and accurate data collection and must complete all required data forms (see protocol Section XIII). The Investigator should forward all completed forms to the Study Monitor for review. Study Monitors should carefully check each set of data for accuracy and completeness. If a form is incomplete or inaccurate, it should be returned to the Investigator. If a form is complete and accurate, it should be forwarded to the Study Director at the AADAP Office. Note: data that is entered through the online INAD database will be archived in the database. These archived forms will be available as long as the study participant accounts remain open. Unless data is entered directly into the online INAD database (i.e., not captured elsewhere at the time of observation or measurement and transcribed into the online INAD database) investigators must archive hard copies of all raw data.
XVIII. PLANS FOR DATA ANALYSIS
Data analysis will be completed by the Study Director located at the AADAP Office. Data from the treatment year will be summarized through tabulation and appropriate statistical analysis. INAD reports will be prepared and submitted to the FDA as required. This submission may include a request for an extension of the INAD based on the data collected during that year. When sufficient data are collected, the entire INAD data set will be summarized in a final report for submission to support a full NADA.
XIX. PROTOCOL AND PROTOCOL AMENDMENTS
A signed copy of the Study Protocol must be retained by each Investigator. At any time before the study begins, desired changes in the Study Protocol should be brought to the attention of the Study Director. The desired changes will be fully described in the form of an amendment along with the reason for the change. The amendment will be signed by the Sponsor (or its representative) and forwarded to FDA for review. The requested changes cannot occur until FDA concurrence has been received. Copies of the signed amendment will be attached to each copy of the Study Protocol. Investigators will be liable for non-compliance violation if drugs are used without a Study Protocol or differently than specified in the Study Protocol, if forms are not filed on time, or if the study data are not properly collected, maintained, and reported. The Study Monitor is responsible for ensuring that all INAD procedures are being followed as defined by the Study Protocol.
Deviations from the established Study Protocol occasionally cannot be avoided. If deviations occur, the Study Monitor should be contacted immediately for advice. Protocol deviations should be fully documented and should be accompanied by a written explanation of what happened, why, and what steps were taken to mitigate the deviation. Deviation statements should be signed and dated. These statements should be forwarded to the Study Monitor along with Form AQSE-3, and ultimately be submitted to the Study Director.
Please note, if there are any deviations to the below doses then a justification is needed in Form AQSE-3. Treatment doses and duration for freshwater or saltwater finfish for the handleable stage:
Option A: Handleable sedation for freshwater/saltwater salmonids is between 25 – 40 mg/L; sedation time no longer than 5 minutes; and recovery time less than 20 minutes. If treatment dose, sedation, or recovery times are not in this dosing range then a justification must be provided in Form AQSE-3.
Option B: Handleable sedation for freshwater non-salmonids is between 40 – 100 mg/L; sedation time no longer than 5 minutes; and recovery time less than 20 minutes. If treatment dose, sedation, or recovery times are not in this dosing range then a justification must be provided in Form AQSE-3.
Option C: Handleable sedation for saltwater non-salmonids is between 30 – 40 mg/L; sedation time no longer than 5 minutes; and recovery time less than 20 minutes. If treatment dose, sedation, or recovery times are not in this dosing range then a justification must be provided in Form AQSE-3.
The contents of this document do not have the force and effect of law and are not meant to bind the public in any way. This document is intended only to provide clarity to the public regarding existing requirements under the law or agency policies.
American Veterinary Medical Association (AVMA). 2020. AVMA Guidelines for the Euthanasia of Animals: 2020 Edition. Schaumburg, IL. pp 84.
Summerfelt, R.C. and L.S. Smith. 1990. Anesthesia, surgery, and related techniques. In: Methods for Fish Biology. C.B. Schreck and P.B. Boyle editors. American Fisheries Society. Bethesda, Maryland. pp. 213-272
Appendix I. Sponsor Contact Information for Aqui-S® 20E INAD #11-741
Sponsor: Dr. Marilyn Blair, U.S. Fish and Wildlife Service, Aquatic Animal Drug Approval Partnership (AADAP) Program
Phone: (406) 994-9904
Fax: (406) 582-0242
Email: [email protected]
Sponsor Address: 4050 Bridger Canyon Road, Bozeman, MT 59715
Study Director: Ms. Bonnie Johnson
Aquatic Animal Drug Approval Partnership
(AADAP) Program
Phone: (406) 994-9905
Fax: (406) 582-0242
Email: [email protected]
Principal Clinical Field
Trial Coordinator: Ms. Paige Maskill
Aquatic Animal Drug Approval Partnership
(AADAP) Program
Phone: (406) 994-9911
Fax: (406) 582-0242
Email: [email protected]
Appendix II. Study Monitors for Aqui-S® 20E INAD #11-741
Note: This information will be provided directly to CVM
Appendix IIIa. Facilities and Names of Investigators Participating under Aqui-S® 20E INAD #11-741
Note: This information will be provided directly to CVM and Merck Animal Health
Appendix IIIb. Sample of Knowledge Required for Position of Hatchery Manager (i.e., Investigators)
Professional knowledge of all facets of fishery biology as well as the ability to apply new scientific findings, developments, and advances toward the resolution of critical propagation problems involving the rearing a variety of fish species under a variety of water quality conditions, water temperatures, water chemistry, etc.
Knowledge of general bacteriology, parasitology, and water chemistry sufficient to treat fish for various diseases.
Skill in interpreting biological observations and ability to draw sound conclusions from available data.
Skill in developing and coordinating available resources to ensure effective management and utilization of manpower, equipment, and funds relative to established priorities and needs.
Skill in coordination of sometimes divergent resource issues to obtain common objectives, including interaction with other Federal, State, Tribal, and private agencies/facilities.
Knowledge of and skill in the use of effective management and supervisory techniques to provide support, guidance, and motivation to hatchery staff.
Appendix IV. Safety Data Sheet (SDS) for Aqui-S® 20E INAD #11-741
The SDS for Aqui-S 20E(eugenol) can be found at the drug sponsors website https://www.aqui-s.com/products/aqui-s-20e
Appendix V. Investigational Label for Aqui-S® 20E INAD #11-741
1. Investigational label for tests in vitro and in laboratory research animals [511.1(a)]:
"Caution. Contains a new animal drug for investigational use only in laboratory animals or for tests in vitro. Not for use in humans."
2. Investigational label for use in clinical field trials [511.1(b)]:
"Caution. Contains a new animal drug for use only in investigational animals in clinical field trials. Not for use in humans. Edible products of investigational animals are not to be used for food unless authorization has been granted by the U.S. Food and Drug Administration or by the U.S. Department of Agriculture."
Appendix VIa. Fish Species Treated under Aqui-S® 20E INAD #11-741
All freshwater-reared finfish
Freshwater prawn
All saltwater-reared finfish
Sharks
Appendix VIb. Table of Facilities and Fish Stocks Treated under Aqui-S® 20E INAD #11-741
Note: This information will be provided directly to CVM
All data must be entered through the online INAD database:
The following forms are to be used as a guide for collecting data that will be entered
into the online INAD database. Any paper forms that are submitted to AADAP will be
sent back to the study participants.
Form AQSE-W: Worksheet for Designing Individual Field Trials under
AQUI-S® 20E INAD 11-741
INSTRUCTIONS
Investigator must fill out Form AQSE-W for each trial conducted under this INAD before actual use of AQUI-S® 20E. The Investigator is responsible that Form AQSE-W is completed accurately.
Investigator should forward a copy of AQSE-W to the Study Monitor for review.
After review, the Study Monitor should forward a copy to the AADAP Office for review and assignment of the Study Number.
SITE INFORMATION
Facility |
|
||
Address |
|
||
|
|
||
Investigator |
|
||
Reporting Individual (if not Investigator) |
|
||
Phone |
|
Fax |
|
FISH CULTURE AND DRUG TREATMENT INFORMATION
Fish species |
|
||
Number of treated fish |
|
||
Average fish weight (gm) |
|
Average fish length (in) |
|
Estimated total weight of fish treated (lbs) |
|
||
Intended level of anesthesia (Light Sedation – LT; Handleable - H; Anesthetized - AN; Euthanized - E) |
|
||
Intended dosage (mg/L eugenol) – LT: 1 – 15 mg/L H: 25 – 40 mg/L salmonids fresh or saltwater; 40 – 100 mg/L freshwater non salmonids; 30 – 40 mg/L saltwater non salmonids AN or E: <100 mg/L |
|
Planned duration of treatment - LT: 15 min – 8 hrs H: 5 minutes AN or E: up to15 minutes |
|
Estimated total amount of AQUI-S® 20E needed for proposed treatment (ml) |
|
||
Anticipated date treatment will be initiated |
|
||
AQUI-S® 20E manufacturer |
AQUI-S New Zealand, Ltd. |
AQUI-S® 20E lot number |
|
STUDY DESIGN: Provide a brief description of your planned study. The description should include the reason you feel fish should be treated, the treatment dates, the number of fish that will be treated, and if the fish are a threatened or endangered species.
Study designed by |
|
DISPOSITION OF TREATED FISH (Human Food Safety Considerations):
|
Estimated time (days, months) from last treatment day to first possible harvest for human consumption |
Note: Euthanized fish must not be sent to slaughter or be otherwise available for food. If another drug is used during the Aqui-S® 20E treatment, then the longest withdrawal period must be followed – see section XV of the protocol.
|
|
Investigator should initial here to indicate awareness that fish disposition must be in compliance with FDA-mandated withdrawal times as described in the Study Protocol. |
|
|
|
|
|
WORKER SAFETY CONSIDERATIONS:
|
|
Investigator should initial here to indicate that all personnel handling the drug have read the Safety Data Sheet for AQUI-S® 20E and have been provided protective equipment, in good working condition, as described in the SDS. |
|
|
Date Prepared: |
|
Investigator: |
|
|
|
|
|
Date Reviewed: |
|
Study Monitor: |
|
FORM AQSE-1. Report on Receipt of Drug - Guide for Reporting Investigational New Animal Drug Shipments for Poikilothermic Food Animals
INSTRUCTIONS
1. Investigator must fill out Form AQSE-1 immediately upon receipt of AQUI-S®20E.
2. Investigator should forward a copy of Form AQUI-S®20E to the Study Director at the AADAP Office.
The sponsor, U.S. Fish and Wildlife Service, submits a notice of claimed investigational exemption for the shipment or delivery of a new animal drug under the provisions of Section 512 of the Federal Food, Drug, and Cosmetics Act.
Name of Drug |
AQUI-S® 20E |
INAD Number |
11-741 |
Proposed Use of Drug |
Sedation/anesthesia in a variety of fish species |
||
Date of CVM Authorization Letter |
March 29, 2018 |
||
Date of Drug Receipt |
|
Amount of Drug Received |
|
Drug Lot Number |
|
Trial Number |
|
Name of Investigator |
|
||
Address of Investigator |
|
||
Location of Trial |
|
||
Pivotal Study |
No |
Non-pivotal Study |
Yes |
Approximate Number of Treated Animals |
|
Approximate Number of Control Animals |
|
Study Protocol Number |
11-741 |
||
Approximate dates of trial (start/end) |
|
||
Species, Size, and Type of Animals |
|
||
Maximum daily dose and duration |
100 mg/L eugenol for 15 minutes 15 mg/L eugenol for 8 hours |
||
Methods(s) of Administration |
Immersion |
||
Withdrawal Period |
Hatchery use and light sedation up to 8 hours = 72 hours; Field-based use = no withdrawal (immediate release) Note: Euthanized fish must not be sent to slaughter or be otherwise available for food If another drug is used with Aqui-S® 20E then the longest withdrawal time is needed |
||
Date Prepared: |
|
Investigator: |
|
Date Reviewed: |
|
Study Monitor: |
|
Date Reviewed: |
|
Study Director: |
|
Form AQSE-2: Chemical Use Log for Use of AQUI-S®E under INAD 11-741
INSTRUCTIONS
Investigator should initiate a new form AQSE-2 immediately upon receipt of each shipment of AQUI-S® 20E.
Each lot of AQUI-S® 20E may be used for multiple treatment regimens.
Form AQSE-2 should be updated whenever drug is used, transferred, or discarded.
Qty of AQUI-S® 20E from
previous page (ml) Facility Reporting individual
Date |
Amount of AQUI-S® 20E received (ml) |
Lot number of AQUI-S® 20E received |
Study Number |
Amount AQUI-S® 20E used in treatment (ml) |
AQUI-S® 20E transferred (ml)1 |
AQUI-S® 20E discarded (ml) |
AQUI-S®20E remaining on hand (ml) |
Inventory by (initials) |
|
|
|
|
|
|
|
|
|
|
xxxx |
xxxx |
|
|
|
|
|
|
|
xxxx |
xxxx |
|
|
|
|
|
|
|
xxxx |
xxxx |
|
|
|
|
|
|
|
xxxx |
xxxx |
|
|
|
|
|
|
|
xxxx |
xxxx |
|
|
|
|
|
|
|
xxxx |
xxxx |
|
|
|
|
|
|
|
xxxx |
xxxx |
|
|
|
|
|
|
|
xxxx |
xxxx |
|
|
|
|
|
|
|
xxxx |
xxxx |
|
|
|
|
|
|
|
xxxx |
xxxx |
|
|
|
|
|
|
|
xxxx |
xxxx |
|
|
|
|
|
|
|
xxxx |
xxxx |
|
|
|
|
|
|
|
xxxx |
xxxx |
|
|
|
|
|
|
|
xxxx |
xxxx |
|
|
|
|
|
|
1 Unused AQUI-S® 20E that is shipped to another facility participating in AQUI-S® 20E INAD 11-741 (Note: AQUI-S® 20E can only be shipped to another facility with prior authorization by the AADAP Office).
Date Prepared: |
|
Investigator: |
|
|
|
|
|
|
|
Date Reviewed: |
|
Study Monitor: |
|
|
STUDY NUMBER |
|
Page 1 of 3 |
Form AQSE-3: Results Report Form for AQUI-S® 20E Use under INAD 11-741
INSTRUCTIONS
1. Investigator must fill out Form AQSE-3 no later than 10 days after completion of the trial. Attach lab reports and other information.
2. If AQUI-S® 20E was not used under the assigned Study Number, contact the Study Director at the AADAP Office on how to close-out the study.
3. Investigator should forward a copy of Form AQSE-3 to the Study Monitor. Within 10 days of receipt, the Study Monitor should forward a copy to the Study Director at the AADAP Office
SITE INFORMATION
Facility |
|
Reporting Individual |
|
FISH CULTURE AND DRUG TREATMENT INFORMATION
AQUI-S® 20E lot number |
|
Total amount of AQUI-S® 20E used in treatments (ml) |
|
Fish species treated
|
|
Dosage used (mg/L eugenol) |
|
Average fish weight (gm)
|
|
Average fish length (in) |
|
Total number of treated fish |
|
Approximate fish age (fingerling/juvenile/adult) |
|
Treatment bath vol. (gal)
|
|
Number of fish/bath |
|
Treatment duration (minutes)
|
|
Treatment date(s) |
|
WATER QUALITY PARAMETERS
Ave treatment temp (oF)
|
|
Dissolved Oxygen (mg/L) |
|
pH
|
|
Hardness - CaCO3 (mg/L) |
|
Anesthesia Record - Version 1
INSTRUCTIONS
Investigator should fill out the Anesthesia Record as completely as possible.
2. Enter the number of fish in the treatment tank at one time. Dependent upon the size of the tank, fish size, etc., this number could vary from 1 to 20 or possibly even higher.
3. Enter the level of anesthesia desired. Use “LT” for light sedation, “H” for handleable, “AN” for anesthetized, and “E” for euthanized as described in the Study Protocol. If other measurements or parameters are used to determine level of desired anesthesia, describe anesthesia level in detail on a separate sheet of paper and attach to Form AQSE-3.
4. Use additional copies of this form if more than 20 individual treatments are involved in the trial.
5. If a different dose; level of anesthesia; or purpose (i.e., spawning, tagging, fish health) is needed then a different study number is needed.
Date |
Treatment Number |
Species |
Fish per Treatment |
Level of Anesthesia (LT, H; AN; or E) |
Aqui-S® 20E Dose (mg/L) |
Time to Anesthesia (min) |
Time to Recovery (min) |
Observer Initials |
|
1 |
|
|
|
|
|
|
|
|
2 |
|
|
|
|
|
|
|
|
3 |
|
|
|
|
|
|
|
|
4 |
|
|
|
|
|
|
|
|
5 |
|
|
|
|
|
|
|
|
6 |
|
|
|
|
|
|
|
|
7 |
|
|
|
|
|
|
|
|
8 |
|
|
|
|
|
|
|
|
9 |
|
|
|
|
|
|
|
|
10 |
|
|
|
|
|
|
|
|
11 |
|
|
|
|
|
|
|
|
12 |
|
|
|
|
|
|
|
|
13 |
|
|
|
|
|
|
|
|
14 |
|
|
|
|
|
|
|
|
15 |
|
|
|
|
|
|
|
|
16 |
|
|
|
|
|
|
|
|
17 |
|
|
|
|
|
|
|
|
18 |
|
|
|
|
|
|
|
|
19 |
|
|
|
|
|
|
|
|
20 |
|
|
|
|
|
|
|
RESULTS: Describe in detail treatment results. Was treatment successful? If treatment did not appear to be successful, explain why not? Were there any mitigating environmental conditions that may have impacted treatment results? Were there any deviations from the Study Protocol? Attach any supplemental reports.
TOXICITY OBSERVATIONS: Report any apparent drug toxicity including a description of unusual fish behavior.
OBSERVED WITHDRAWAL PERIOD OF TREATED FISH:
-
Observed withdrawal period:
72 hours
_______ No withdrawal (immediate release; field use only up to 15 minutes)
_______ If another drug was used during the Aqui-S 20E study then the longest withdrawal period needs to be followed – see section XV of the protocol.
Estimated number of days between last treatment and first availability of fish for human consumption (ensure this time period meets the withdrawal period). |
|
|
Note: Euthanized fish must not be sent to slaughter or be otherwise available for food.
NEGATIVE REPORT AQUI-S® 20E was not used at this facility under this Study Number during the ______reporting period. The study will be closed out in the online INAD database.
Date Prepared: |
|
Investigator: |
|
|
|
|
|
Date Reviewed: |
|
Study Monitor: |
|
NOTICES
Paperwork Reduction Act
In accordance with the Paperwork Reduction Act (44 U.S.C. 3501 et seq.), the U.S. Fish and Wildlife Service collects information necessary to permit the use of an investigational new animal drug to generate data to support a new animal drug approval (NADA) as part of the Fish and Aquatic Conservation fish health network. Your response is voluntary, but is required to obtain or retain a benefit. According to the Paperwork Reduction Act of 1995, an agency may not conduct or sponsor and a person is not required to respond to a collection of information unless it displays a currently valid OMB control number. OMB has approved this collection of information and assigned Control No. 1018-####.
ESTIMATED BURDEN STATEMENT
We estimate public reporting for this collection of information to average 4 hours, including time for reviewing instructions, gathering and maintaining data, and completing and reviewing the form. Direct comments regarding the burden estimate or any other aspect of the form to the Service Information Clearance Officer, Fish and Wildlife Service, U.S. Department of the Interior, 5275 Leesburg Pike, MS: PRB (JAO/3W), Falls Church, VA 22041-3803, or via email at [email protected]. Please do not send your completed form to this address.
FREEDOM OF INFORMATION ACT STATEMENT
Information provided to the Service is generally subject to release to the public under the Freedom of Information Act (FOIA). Certain information, however, may be subject to withholding if the Service determines that the information is a trade secret and/or commercial or financial information that is privileged or confidential. To the extent you are submitting business information that falls into one of these categories, you must clearly mark this information as "Business Confidential" in order for the Service to assess the applicability of FOIA Exemption 4. Any information provided by you that is not marked as “Business Confidential” will be considered releasable to the public under the FOIA [43 CFR 2.26 – 2.33].
Revised: 01/2023
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | Johnson, Bonnie |
| File Modified | 0000-00-00 |
| File Created | 2023-12-03 |
© 2026 OMB.report | Privacy Policy