Form CCP- (W, 1, 2, 3, CCP- (W, 1, 2, 3, STUDY PROTOCOL FOR A COMPASSIONATE AQUACULTURE INVESTIGA
Administration of U.S. Fish and Wildlife Service Investigational New Animal Drug (INAD) Program
CCP protocol rev Dec 2021
Common Carp Pituitary (CCP) INAD #8391 - Private Sector
OMB:
OMB Control No. 1018-####
Expires ##/##/20##
STUDY PROTOCOL FOR A COMPASSIONATE AQUACULTURE INVESTIGATIONAL NEW ANIMAL DRUG (INAD) EXEMPTION FOR COMMON CARP PITUITARY (CCP) INAD 8391
Sponsor:
U.S. Fish and Wildlife Service, Fish and Aquatic Conservation
__________________________ ____________________
Sponsor Signature Date Approved
Manufacturer/Source of Supply:
Argent Aquaculture
8547 152nd Ave NE
Redmond, WA 98052
Office for Coordination of CCP INAD:
Aquatic Animal Drug Approval Partnership
4050 Bridger Canyon Road
Bozeman, Mt 59715
Proposed Starting Date: January 1, 2021
Proposed Ending Date: December, 2026
Study Director: Bonnie Johnson
Clinical Field Trial Location:
Facility:________________________________________________________
Investigator:____________________________________________________
Table of Contents
I. STUDY IDENTIFICATION AND TITLE 4
III. INVESTIGATORS/FACILITIES 4
IV. PROPOSED STARTING AND COMPLETION DATES 4
XII. TREATMENT RESPONSE PARAMETERS 13
XIII. FORMS FOR DATA COLLECTION 14
XIV. RECORD KEEPING PROCEDURES 15
XV. DISPOSITION OF INVESTIGATIONAL ANIMALS 15
XVI. DISPOSITION OF INVESTIGATIONAL DRUG 15
XVII. DATA HANDLING, QUALITY CONTROL, MONITORING, ADMINISTRATIVE RESPONSIBILITIES 15
XVIII. PLANS FOR DATA ANALYSIS 17
XIX. PROTOCOL AND PROTOCOL AMENDMENTS 17
All data must be entered through the online INAD database: 34
STUDY PROTOCOL FOR A COMPASSIONATE AQUACULTURE INVESTIGATIONAL NEW ANIMAL DRUG (INAD) EXEMPTION FOR COMMON CARP PITUITARY (CCP) UNDER INAD #8391
Clinical field trials to determine the efficacy of common carp pituitary (CCP) to induce gamete maturation (ovulation and spermiation) in a variety of fish species.
Dr. Marilyn Blair, U.S. Fish and Wildlife Service, Branch Chief, Aquatic Animal Drug Approval Partnership Program, 4050 Bridger Canyon Road, Bozeman, MT 59715; Phone: 406-994-9904; Fax: 406-582-0242; Email: [email protected]
Manufacturer: Argent Aquaculture
8547 152nd Ave NE
Redmond, WA 98052
Email: [email protected]
Study Director: Ms. Bonnie Johnson, U.S. Fish and Wildlife Service, Aquatic Animal Drug Approval Partnership (AADAP) Program, 4050 Bridger Canyon Road, Bozeman, MT 59715; Phone: 406-994-9905; Email: [email protected]
Principal Clinical Ms. Paige Maskill, USFWS – AADAP Program
Field Trial Coordinator: 4050 Bridger Canyon Road, Bozeman, MT 59715;
Phone: 406-994-9911; Email: [email protected]
INAD Study Monitors: See Appendix II for names and addresses.
See Appendix IIIa for names and addresses.
Proposed Starting Date: January 1, 2021
Proposed Completion Date: December, 2026
Background Information:
The use of hormones to induce spawning in fish is critical to the success of many fisheries programs in the United States. A wide variety of programs, including several that involve the restoration of threatened/endangered species are dependent upon hormone treatment to complete final gamete maturation and ensure successful spawning.
The time of spawning is by its own nature a stressful period for all fish species. Both sexes are undergoing significant changes in physiology, morphology, and behavior (Hoar 1969). The handling required during the spawning of fish for artificial propagation complicates an already delicate situation. This is particularly true for wildstock species that must endure the added stresses of capture, handling, and confinement in an un-natural environment. The longer it is necessary to hold wild fish in captivity, the greater the likelihood of adversely affecting both the health of the fish and ultimate spawning success. In fact, with respect to some wildstock species, the stress of capture alone would be sufficient to cause complete reproductive failure unless spawning is induced by hormone treatment. Additionally, certain species have limited or depressed populations and in some cases may even be considered threatened/endangered. Hormone treatment of these fish is essential to ensure viable population numbers.
In order to maintain the health of both wildstock and domestic brood fish, it is beneficial to minimize overall fish handling. During the course of normal spawning operations at a hatchery, it may be necessary to handle and examine individual fish weekly over a 6-8 week period. Such procedures can be extremely stressful to valuable broodstocks, severely compromising general fish health. Successful hormone treatment can reduce handling requirements to a single hormone administration event followed by actual gamete collection, thereby greatly reducing overall fish handling.
Studies have shown that final gamete maturation in fish can be induced by the administration of a variety of hormones (Donaldson and Hunter 1983; Goetz 1983). The first reported studies investigating the hormonal control of reproduction in fish utilized intraperitoneal injection of freshly dissected pituitary glands (Houssay, 1931; von Ihering, 1937). The use of CCP was first reported in the United States by Hasler et al., (1939, 1940). These and many other early studies investigating the use of fish pituitaries to induce gamete maturation in a variety of fish species were thoroughly reviewed by Pickford and Atz (1957) in their comprehensive treatise on the fish pituitary gland.
The efficacy of CCP to induce ovulation and spermiation in fish is well documented (Chaudhuri, 1976). CCP has been shown to induce gamete maturation in a wide variety of species including; common carp, grass carp, silver carp, bighead carp, striped bass, white bass, goldfish, lake sturgeon, white sturgeon, channel catfish, flathead catfish, mullet, muskellunge, bigmouth buffalo, lake trout, brook trout, walleye, yellow perch, northern pike, and white crappie to name a few. Not only was carp pituitary injection one of the very first methods of inducing ovulation and spermiation in fish, it has stood the test of time and is still the preferred methodology of many fish culturists.
Currently, the use of CCP is an important management/production tool in the propagation of a number of important species. In some situations, it has been found to be the most efficient and reliable method of inducing final gamete maturation. The success many commercial aquaculture production programs, as well as a considerable number of supplementation/recovery/restoration programs, are dependent upon its continued availability for use as an aid in spawning fish.
B. Purpose of INAD:
The purpose of this compassionate INAD for CCP is to develop clinical field trial data that will be used to determine the efficacy and appropriate treatment regimens for gamete maturation (ovulation and spermiation) in a variety of cultured and wildstock fish species. These data will be used to support a new animal drug application (NADA) for CCP.
USFWS anticipates requesting that FDA grant an extension of the CCP INAD for additional years at the end of this treatment season. The USFWS is aware that opportunities for CCP therapy are unpredictable. There is no way of knowing in advance if, when, or where opportunities for pivotal studies will be encountered. USFWS feels that data from at least three treatment seasons will be required in order to adequately assess the efficacy of CCP treatment on induced gamete maturation in fish to support a NADA.
The two major objectives of this study protocol are as follows:
Collect scientific data necessary to establish the efficacy of CCP on gamete maturation in both cultured fish under typical hatchery situations and on critical wildstock species.
Provide the opportunity for fish culturists and fisheries managers to legally use CCP to maintain the genetic integrity and improve the reproductive potential of hatchery broodstocks during the period of time necessary for collection of efficacy, safety, and residue data required for a NADA on CCP in fish. Specifically, CCP will be used to induce ovulation and spermiation in both domestic and wildstock populations, including several species that are listed under the Endangered Species Act.
Test and Control Articles:
Drug Identity
Active ingredient
Common Name: Common Carp Pituitary
Appearance: Brownish/White powder
Odor: None
Strength and dosage form
CCP is obtained by dissection as a fresh material from adult common carp (Cyprinus carpio). Whole pituitaries are desiccated using an alcohol/acetone rinse, ground into a powder, and stored. CCP is prepared for injection by suspending the powder in sterile water or physiological saline.
Manufacturer, source of supply
Argent Aquaculture
8547 152nd Ave NE
Redmond, WA 98052
Contact Person: Thomas Sawtell
Phone: 425-605-0933
email: [email protected]
Website: http://argentaquaculture.com/
Verification of Drug Integrity/Strength:
The Manufacturer will provide the analytical data necessary to establish the purity of each lot of CCP supplied. The lot number and date of processing for each batch of CCP will be placed on the label of each container. The form "Report on Receipt of Drug - Guide for Reporting Investigational New Animal Drug Shipments for Poikilothermic Food Animals" (Form CCP-1) will clearly identify the lot number for all CCP shipments. If the integrity of the CCP is compromised (i.e., by spilling or contamination of the stock container) the event will be carefully recorded, dated, and signed in the Chemical Use Log (Form CCP-2). All un-usable CCP must be destroyed by following the disposal methods described in the MSDS.
Storage Conditions
CCP will be stored in the original container supplied by the Manufacturer with the appropriate investigational label attached. The container will be stored in a cool, dry location. If CCP is stored in a refrigerator, the refrigerator must be labeled to indicate that it contains hazardous material and that "NO Food or Drink is to be Stored in this Refrigerator/Freezer". CCP should be stored in a secure location.
Handling Procedures
Each Study Monitor and Investigator will be required to have a current copy of the Safety Data Sheet (SDS) for CCP (see Appendix IV). Each person involved with the study and each person who may be present during the use of CCP shall be required to read the SDS. Safety precautions as outlined in the SDS will be followed at all times when working with CCP.
Investigational Labeling
A copy of the label to be attached to each container of CCP is provided in Appendix V. Although investigational labels will be affixed to containers by the manufacturer, it is the responsibility of the Investigator to ensure proper labeling of all containers of CCP.
Accountability
Argent Aquaculture will be the sole supplier of CCP to all Investigators under this INAD.
The Online INAD Database must be used by Investigators for ALL INAD reporting. The online INAD database has a built-in system of checks, balances, and email notifications to ensure that all information/data reporting and accountability follows established INAD Study Protocol guidelines. Unless data is entered directly into the online INAD database (i.e., not captured elsewhere at the time of observation or measurement and transcribed into the online INAD database) investigators must archive hard copies of all raw data.
Immediately upon receiving an order/shipment of CCP, the Investigator will complete Form CCP-1 “Report on Receipt of Drug - Guide for Reporting Investigational New Animal Drug Shipments for Poikilothermic Food Animals" (located in the “Manage/View Drug Inventory” section of the investigator account). The Study Director will in turn forward a copy to FDA. Arrangements should be made between Investigators and Study Monitors to ensure completed Form CCP-1s are received by the Study Director within 10 days of drug receipt.
All Investigators are also responsible for maintaining an accurate inventory of CCP on-hand. A Chemical Use Log (Form CCP-2) must be completed and maintained by each Investigator. Each time CCP is used, it must be recorded by the Investigator in the Results Report form in the “Amount of Drug Used” table.
At the conclusion of the study, all remaining CCP will be disposed of by following the disposal methods in the Safety Data Sheet (note: unless CCP is planned for use in another approved field trial, and planned usage is within the storage guidelines established by the manufacturer). Disposition of all CCP must be properly recorded and accounted for in the Drug Inventory Form of the database which is Form CCP-2. The Study Monitor will be responsible for verifying the quantity of CCP remaining on hand versus the amount indicated on Form CCP-2. Note: CCP can be transferred to other facilities that are participating under INAD 8391. Transfers must be shown in the Drug Inventory section of the database (formerly Form CCP -2).
7. Preparation Procedures
CCP for injection will be supplied in vials containing 1 - 25 g of desiccated powder. CCP is prepared for injection by suspending the powder in sterile water or physiological saline. The amount of CCP needed for each treatment will be weighed on an accurate laboratory scale, preferably to the nearest milligram. Dilution volume is dependent upon dosage, size and number of fish to be injected, and desired injection volume.
B. Items Needed for Treatment, Data Collection, Etc.:
Treatment equipment should include clean glassware, sterile physiological saline, and sterile syringes and needles. A compound microscope should be available for evaluation of sperm motility.
When the Study Protocol has been approved and treatments are scheduled, the Investigator at each facility covered by the CCP INAD will need to complete several forms in the online INAD database. These forms are described in Section XIII. Copies of these forms are attached to this Study Protocol and will be used as a guide only for collecting the data that will be entered into the online INAD database.
The experimental unit in this clinical field trial may consist of a contained or isolated group of fish. This will be a group of fish contained in a tank, raceway, or pond. It could also be a group of fish held in confinement in a lake or stream. However, it is strongly encouraged that whenever possible, the experimental unit in clinical field trials is individual animals. Whenever individual animals are considered to be the experimental unit, treatment response parameters for each animal must be evaluated separately.
Facilities/Investigators
The proposed facility and the Investigator must be listed in Appendix IIIa of this Study Protocol for the current calendar year before CCP can be ordered and dispensed under this INAD. Last minute deviations can be requested by the Sponsor or by an Investigator to address emergency-use situations (See Section XX). However, poor planning and/or a lack of preparation will not be considered an emergency situation.
The characteristics of the study animals (species, size, number, etc.) is presented in Appendix VIb.
Period of use
CCP treatment has been shown to be most effective when administered during the final stages of gamete maturation. In most cases, CCP will be used within 4 weeks of the time fish are normally expected to spawn.
Environmental conditions
Since CCP activity is rapidly lost in dilute aqueous solution, there will be no drug discharge from participating facilities. Therefore, CCP qualifies for a categorical exclusion from the requirement to prepare an environmental assessment under 21 CFR 25.33(e).
Environmental conditions will be variable and include a spectrum of water temperatures and water quality parameters. Environmental conditions will be reported on Form CCP-3.
Ability of investigator to fulfill all the requirements of the Study Protocol
See Appendix IIIb for example of knowledge required of hatchery managers (i.e., Investigators).
Prior to initiating each treatment event, the Investigator must first complete Form CCP-W: “Worksheet for Designing Individual Field Trials” (located under the “New Study Request” tab in the investigator account) that pertains to each specific treatment event. The worksheet should be filled out and forwarded to the Study Monitor through the online INAD database. The Study Monitor will review the planned treatment (worksheet) and forward it to the Study Director at the AADAP Office. The Study Director will then review the worksheet, assign the approved treatment a Study Number, and then the online INAD database will notify both the Investigator and the Study Monitor of the assigned number and approval to proceed. In most cases, this entire process should be able to be accomplished within a single working day. After initiation of the field trial, the Investigator should also record the assigned study number on any paper forms that are being used as a guide to collect the data to enter in the online database (i.e., Form CCP-2 and CCP-3), as well as on any additional correspondence regarding that specific treatment event. If for some reason the Investigator is unable to reach the Study Monitor with regards to Worksheet approval and the need for treatment is immediate, the Investigator should contact the AADAP Office for permission to proceed.
Note: The online INAD database, which must be used by Investigators for all INAD reporting, has a built-in system of checks, balances, and email notifications to ensure that all information/data reporting follows established INAD Study Protocol guidelines.
A treatment group or experimental unit may be an entire tank, pond, raceway, or group of fish. However, the experimental unit should be considered individual fish whenever possible.
Control groups will not be a requirement for clinical field trials evaluating the efficacy of CCP treatment. In some cases, particularly with respect to wildstock populations, the number of broodfish available at a given time for CCP treatment may be extremely limited. It is likely that some facilities may need to initiate treatment on groups of ten or fewer brood fish. To establish meaningful control groups with such a limited number of animals would be difficult. Therefore, it is proposed that treatment groups of 10 or fewer fish be exempted from the requirement to establish control groups. It is also proposed that species listed under the authority of the Endangered Species Act (ESA) be exempted from the requirement to establish control groups. With respect to species listed under the ESA, every fish may be critical to the restoration effort. In all other situations, investigators should make a serious effort to include a control group in the trial. Fish should be assigned to control or treatment groups randomly. Study fish should be crowded into a confined space where segregation and escape is impossible, and captured using dip nets. Fish in alternating nets should be assigned to control or treatment groups until desired fish numbers are obtained. Suggested control groups will be based on treatment population size according to the following schedule:
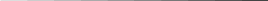
Treatment Group Size Control Group Size1
0 - 10 fish 0 (too few fish for data analysis)
11 - 30 fish 5 fish
31 - 50 fish 10 fish
51 - 75 fish 15 fish
76 -100 fish 20 fish
101-300 fish 25 fish
>300 fish 30 fish
1Minimum number of fish per control group
Although untreated control groups are not a required element of treatment under this INAD exemption and are at the discretion of the Investigator, they are strongly encouraged whenever circumstances permit. Control groups are extremely important to not only document response to treatment, but also to validate potential adverse reactions in treated animals. Assignment to control and treatment groups should be random and designed to avoid bias. It is important that all fish are treated in a similar fashion. If fish are physically moved into separate test groups or different rearing units, caution should be used so that handling and rearing conditions are as similar as possible. Control fish should be kept under conditions as similar as possible to treated fish for valid comparison. Use of control groups will help to ensure that results of efficacy studies provide useful information that will support a NADA.
Although as stated above untreated control groups are not a required element of treatment under this INAD exemption, it is important for all investigators to note that field trials conducted under a more stringent study protocol (i.e including requirements for non-treated controls groups, replication, blinding, dose verification, etc) will ultimately be required in order to support a NADA for CCP. It is also important to note that the INAD sponsor fully expects that a limited number of facilities/investigators listed under this INAD exemption will agree to participate in such “pivotal” efficacy studies. These studies will be initiated only after direct consultation between facilities/investigators and the sponsor. These studies will be conducted under a separate FDA-approved study protocol (i.e. not the INAD study protocol), and will also be conducted with assistance from, and under the direct supervision of, the sponsor. If for any reason it becomes apparent to the sponsor that facilities/investigators listed under this INAD are not willing to participate in such “pivotal” studies, the sponsor will request that FDA terminate the INAD.
Route of administration
CCP should be dissolved in sterile physiological saline or sterile water and administered as either an intraperitoneal (IP) or intramuscular (IM) injection.
Dose to be administered
Standard dosage rates will be 4-10 mg CCP/kg body weight. Although certain situations may require a higher dosage rate, dosage will never exceed 25 mg CCP/kg body weight.
Dosing interval and repetition
Dependent upon the species/strain involved, CCP may be administered as a single treatment, or as a multiple treatment. Determination of whether single or multiple treatment regimen is used will be largely a matter of past experience of the investigator and literature citations reporting successful protocol with respect to specific species/strains. Multiple treatment regimen will generally consist of a single "priming" dose followed by a single "resolving" dose.
Drug preparation procedures
CCP will be supplied by Argent Aquaculture in vials containing 1- 25 g of a desiccated brownish/white powder. CCP is prepared for injection by suspending the powder in sterile water or physiological saline. The amount of CCP needed for each treatment will be weighed on an accurate laboratory scale, preferably to the nearest milligram. Dilution volume is dependent upon dosage, size and number of fish to be injected, and desired injection volume.
Permissible concomitant therapy
Since efficacy data are being collected during the INAD process, there should be little or no concomitant therapy. Preferably, there should be no other therapy during a period extending from 2 weeks prior to treatment to 2 weeks after treatment. Investigators must be prepared to make no changes in fish cultural procedures or environmental conditions, and apply no other hormone therapy once a decision has been made to conduct CCP treatment. However, if concomitant therapy is required in order to protect/propagate valuable fish stocks, AADAP should be contacted right away.
XII. TREATMENT RESPONSE PARAMETERS
The collection and reporting of source data begin with the decision to treat valuable fish based on hatchery records or other pertinent species information indicating treatment is warranted. Daily morbidity and mortality records, case history records, as well as any extenuating or mitigating circumstances that may affect treatment response need to be documented. All pertinent treatment response parameters should be reported on Form CCP-3. Treatment response parameters that should be addressed include the following:
Primary Parameters
The primary response parameter for evaluating the effect of CCP on fish will be whether a fish is “ripe” or “non-ripe” following treatment. In the case of females, ripe fish are those that have released their eggs in response to normal artificial spawning procedures. In the case of males, ripe fish are those undergoing active spermiation. Non-ripe fish are the obvious converse. With respect to data reporting under this INAD, eggs will only be collected one time from individual fish.
Secondary Parameters
Secondary response parameters for females will include percent hatch. Secondary response parameters for males will include the volume of milt (ml) available from individual fish and an evaluation of milt motility (percent motile spermatozoa). Motility evaluations will be reported using a scoring system that assigns each milt sample a motility score of either 0, 1, 2, 3 or 4. Motility scores will be based on the following schedule:

 Percent
Motility Motility Score
Percent
Motility Motility Score
0 0
1-25 1
26-50 2
51-75 3
76-100 4

Secondary parameters may also include general observations on fish behavior and response to routine culture/handling activities. This would include such responses as apparent level of stress, negative fish behavior, etc.
Adverse Reactions
Any adverse reaction that occurs during the study period (whether considered/suspected to be treatment-related or not) should be reported immediately to the Study Monitor, who will in turn notify the Study Director. Such responses might include extremely negative responses/behavior by the fish or hazards to the applicator. Although CCP has been used extensively with beneficial effect in fish culture, it is possible adverse reactions may occur under certain environmental conditions or with respect to specific species/strains of fish. Carefully observe all treated fish for any signs of any adverse reaction to treatment. The Investigator should carefully document all observations of adverse reactions. If any signs of drug toxicity are detected, they should also be documented and immediately reported to the Study Monitor, who will in turn notify the Study Director.
Note: Investigators are strongly encouraged to record observations/comments with respect to all phases of treatment. This may include a description of events before, during, and post-treatment. All extenuating or mitigating treatment circumstances need to be described in detail. Such information is imperative so that accurate study/data analysis can be performed.
Mortalities and Moribund Fish
Any fish that die or are euthanized during the study period should undergo a complete necropsy. Necropsy should include examination of the implant site. Necropsy results should be recorded in the Results Report –Form 3 in the Necropsy Report Data Entry table. If it appears that fish died due to handling stress that needs to be reported in the Results Report - Form 3.
XIII. FORMS FOR DATA COLLECTION
When the Study Protocol has been approved and treatments are scheduled, the Investigator at each facility covered by the CCP INAD will need to complete the following forms:
Form CCP-W. Worksheet for Designing Clinical Field Trials under INAD
8391
Form CCP-1. Report on Receipt of Drug - Guide for Reporting
Investigational New Animal Drug Shipments for Poikilothermic Food Animals
Form CCP-2. Drug Inventory Form for use of CCP under
INAD 8391
Form CCP-3. Results Report Form for use of CCP under
INAD 8391
Form CCP-4N. Necropsy Report
Copies of these forms are attached to this Study Protocol and are to be used as a guide for collecting the data that will be entered into the online INAD database. Actual reporting is accomplished on forms located on the online INAD database.
XIV. RECORD KEEPING PROCEDURES
As stated immediately above, all data reporting are accomplished via forms located in the online INAD database. All current and completed studies conducted under the investigator account will be stored and available in the online INAD database to the current study monitor, study investigator, and AADAP.
Fish may be released immediately following treatments. Edible tissues derived from experimental animals treated under this protocol may be marketed for human consumption or fish may be released into public waters for possible human consumption.
CCP will be used only in the manner and by the individuals specified in the Study Protocol. If any unused or out-dated CCP remains at the end of the study period, Investigators should contact Study Monitors for instructions regarding drug disposal. Drug disposal information is available in the Safety Data Sheet (SDS) located in Appendix IV of this protocol. Disposition of all CCP must be properly recorded and accounted for on the Chemical Use Log (Form CCP -2). The Study Monitor will be responsible for verifying the quantity of CCP remaining on hand versus the amount indicated on Form CCP -2. The investigational drug may not be redistributed to others not specified by the protocol and should not be retained by the Investigator after completion of the study (note: unless CCP is planned for use in another approved field trial, and planned usage is within the storage guidelines established by the manufacturer).
Drug distribution
See Section VII.A.6. Accountability for information and details.
Study Monitors
Study Monitors are generally fish health professionals with experience in diagnosing and treating fish diseases, and the ability to monitor overall fish health with respect to ongoing fish culture practices. A study monitor will be selected by each facility that is authorized to treat fish with CCP. A list of Study Monitors, along with addresses and phone numbers, can be found in Appendix II. Study Monitors are responsible for supervision of the trials, adherence of the Investigator to the Study Protocol, and inspection of the site.
Special equipment and materials
Most of the equipment and materials required for this study (with the exception of the CCP itself) are already available at each participating fish hatchery. In recent years, induced final gamete maturation has become a fairly common occurrence at many broodstock facilities. Fish hatchery managers (i.e., Investigators) are well trained and well equipped to handle these situations (see Appendix IIIb). If any additional equipment or materials are required, they will be provided by the Study Monitors (See Section VII.B. Items needed for sample collection, observations, etc.).
Administrator of the drug
CCP will be administered directly by the assigned Investigator (typically a fish hatchery manager) or under the Investigator's direct supervision (see Appendix IIIa for names). CCP will be maintained in a secure location, and only the Investigator or a person under his/her direct supervision will have access.
Drug accountability records
See protocol Section VII.A.6. Accountability for details and the following forms will be used as guides for data collection: Forms CCP-W, CCP-1, CCP-2, CCP-3, and CCP- 4N.
Recording observations
The Investigator or a person under his/her direct supervision will be responsible for implementing the Study Protocol, making observations, collecting samples, and recording data during the clinical field trials. After the data have been collected and recorded on the forms, the Investigator will send the data to the Study Monitors who will review the information and ensure that all required data is provided. The Study Monitors will in turn send the data to the Study Director. The Study Director will analyze and summarize the data and prepare a report that will be submitted to the FDA. Note: If the Study Monitor does not think all required information has been provided, or forms have not been satisfactorily completed, he/she should contact the Investigator and rectify the situation before forwarding the package to the Study Director.
Data storage
The Investigator is responsible for complete and accurate data collection, and must complete all required data forms (see Section XIII). The Investigator should forward all completed forms to the Study Monitor for review. Study Monitors should carefully check each set of data for accuracy and completeness. If a form is incomplete or inaccurate, it should be returned to the Investigator. If a form is complete and accurate, it should be forwarded to the Study Director at the AADAP Office. Note: data that is entered through the online INAD database will be archived in the database. These archived forms will be available as long as the study participant accounts remain open.
Data analysis will be completed by the Study Director located at the AADAP Office. Data from the treatment year will be summarized through tabulation and appropriate statistical analysis. INAD reports will be prepared and submitted to the FDA as required. This submission may include a request for an extension of the INAD based on the data collected during that year. When sufficient data are collected, the entire INAD data set will be summarized in a final report for submission to support a full NADA.
A signed copy of the Study Protocol must be retained by each Investigator. At any time before the study begins, desired changes in the Study Protocol should be brought to the attention of the Study Director. The desired changes will be fully described in the form of an amendment along with the reason for the change. The amendment will be signed by the Sponsor (or its representative) and forwarded to FDA for review. Copies of the signed amendment will be attached to each copy of the Study Protocol. Investigators will be liable for non-compliance violation if drugs are used without a Study Protocol or in a manner different than specified in the Study Protocol, if forms are not filed on time, or if the study data are not properly collected, maintained, and reported. The Study Monitor is responsible for ensuring that all INAD procedures are being followed as defined by the Study Protocol.
Deviations from the established Study Protocol occasionally cannot be avoided. If deviations occur, the Study Monitor should be notified immediately. Protocol deviations should be fully documented and should be accompanied by a written explanation of what happened, why, and what steps were taken to mitigate the deviation. Deviation statements should be documented on Form CCP-3 in the Description of Results section and in the Study Deviation field.
The contents of this document do not have the force and effect of law and are not meant to bind the public in any way. This document is intended only to provide clarity to the public regarding existing requirements under the law or agency policies.
Chaudhuri, H. 1976. Use of hormones in induced spawning of carps. J. Fish. Res. Bd. Can. 33:940-947.
Donaldson, E.M., and G.A. Hunter. 1983. Induced final maturation, ovulation, and spermiation in cultured fish. Pages 351-403 in W.S. Hoar, D.J. Randall, and E.M. Donaldson, editors. Fish physiology, volume 9. Part B. Academic Press, New York.
Goetz, F.W. 1983. Hormonal control of oocyte maturation and ovulation in fishes. In: Fish Physiology Vol IX, Part B. Eds. W.S. Hoar, D.J. Randall and E.M. Donaldson. Academic Press, New York. pp. 117-169.
Hasler, A.D., Meyer, R.K., and H.M. Field. 1939. Spawning induced prematurely in trout with the aid of pituitary glands of the carp. Endocrinology. 25:978-983.
Hasler, A.D., Meyer, R.K., and H.M. Field. 1940. The use of hormones for the conservation of muskellunge, Esox masquinongy immaculatus Garrad. Copia pp. 43-46.
Hoar, W.S. 1969. Reproduction. In: Fish Physiology Volume III. Eds. W.S. Hoar and D.J. Randall. Academic Press, New York and London. pp.1-72
Houssay, B.A. 1931. Action sexuelle de l'hypophyse sur les poissons et les reptiles. C.R. Seances Soc. Biol. Ses Fil. 106:377-378
Pickford, G.E., and J.W. Atz. 1957. The Physiology of the Pituitary Gland of Fishes. New York Zoological Society, New York. pp. 613
Von Ihering, R. 1937. A method for inducing fish to spawn. Prog. Fish Culturist. 34:15-16.
Appendix I. Sponsor Contact Information for CCP INAD #8391
Sponsor: Dr. Marilyn Blair, U.S. Fish and Wildlife Service, Aquatic Animal Drug Approval Partnership (AADAP) Program
Phone: (406) 994-9904
Fax: (406) 582-0242
Email: [email protected]
Sponsor Address: 4050 Bridger Canyon Road, Bozeman, MT 59715
Study Director: Ms. Bonnie Johnson
Aquatic Animal Drug Approval Partnership
(AADAP) Program
Phone: (406) 994-9905
Fax: (406) 582-0242
Email: [email protected]
Principal Clinical Field
Trial Coordinator: Ms. Paige Maskill
Aquatic Animal Drug Approval Partnership
(AADAP) Program
Phone: (406) 994-9911
Fax: (406) 582-0242
Email: [email protected]
Appendix II. Study Monitors for CCP INAD #8391
Note: This information will be provided directly to CVM
Appendix IIIa. Facilities and Names of Investigators
Participating under CCP INAD #8391
Note: This information will be provided directly to CVM and Argent Aquaculture
Appendix IIIb. Sample of Knowledge Required for Position of Hatchery Manager (i.e. Investigators)
Professional knowledge of all facets of fishery biology as well as the ability to apply new scientific findings, developments, and advances toward the resolution of critical propagation problems involving the rearing a variety of fish species under a variety of water quality conditions, water temperatures, water chemistry, etc.
Knowledge of general bacteriology, parasitology, and water chemistry sufficient to treat fish for various diseases.
Skill in interpreting biological observations and ability to draw sound conclusions from available data.
Skill in developing and coordinating available resources to ensure effective management and utilization of manpower, equipment, and funds relative to established priorities and needs.
Skill in coordination of sometimes divergent resource issues to obtain common objectives, including interaction with other Federal, State, Tribal, and private agencies/facilities.
Knowledge of and skill in the use of effective management and supervisory techniques to provide support, guidance, and motivation to hatchery staff.
Appendix IV. Safety Data Sheet (SDS) for CCP INAD #8391
SAFETY
DATA SHEET
SECTION
1: Identification
1.1 Product Name
Product
Name: Common Carp Pituitary Extract (Acetone Dried
Powder)
Product Item Number: C-CARP-CCP
Brand: Argent
Aquaculture
1.2 Product uses
Specific use:
USFWS INAD 8391
1.3 Company name:
Argent
Aquaculture LLC
8547 152nd Ave. NE
Redmond,
WA 98052
United State of America
Telephone:
425-606-0933
Fax: 425-605-4505
1.4 Emergency
telephone number
Emergency Telephone No:
425-605-0933
SECTION
2: Harzard Identification
2.1
Classification of the substance or mixture
Not a hazardous
substance or mixture.
2.2 GHS Label elements,
including precautionary statements
Not a hazardous
substance or mixture.
2.3 Hazards not otherwise
classified (HNOC) or not covered by GHS.
None.
SECTION
3: Composition / Information on Ingredients
3.1
Substances
No components need to be disclosed according to
the applicable regulations.
SECTION
4: First-Aid Measures
4.1 Description of
first-aid measures
If inhaled
Move person
to fresh air. If not breathing, give artificial respiration.
mouth to an unconscious person. Rinse mouth with water.
In
case of contact with skin
Wash with soap and lots of
water.
In case of eye contact
Flush eyes with
water as precaution.
If swallowed
Never give
anything by mouth to an unconscious person. Use water to rinse
mouth.
4.2 Most important symptoms and effects, acute
and delayed.
The most important known symptoms and effects
are described in Section 2.2 and section 11.
4.3
Indication of any immediate medical attention and special treatment
needed.
No data available.
SECTION
5: Fire-Fighting Measures
5.1
Extinguishing media
Use water spray, alcohol-resistant
foam, dry chemical or carbon dioxide.
5.2 Special
hazards arising from the substance or mixture.
Nature of
decomposition products is not known.
5.3 Advise for
firefighters
Wear self-contained breating apparatus of
firefighting if necessary.
5.4 Further
Information.
No data available.
SECTION
6: Accidental Release Measures
6.1
Personal precaution, protective equipment and emergency
procedures
Avoid dust formation. Avoid breathing product
powder particles, vapors, mist or gas.
For personal protection
see Section 8.
6.2 Environmental precautions
Do
not let product enter drains.
6.3 Methods and
materials for containment and cleaning up
Sweep up and
collect residual with damp disposable paper towel. Keep in suitable,
closed containers for disposal.
6.4 Reference to
other sections
For disposal refer to Section 13.
SECTION 7: Handling
and Storage
7.1
Precautions for safe handling
Provide appropriate exhaust
ventilation at places where any product powder / dust may be formed.
7.2 Conditions for safe storage, including any
incompatibilities
Keep container tightly closed in a dry
and well-ventilated place.
Recommended long term storage
refrigerate or freeze -20 deg.C .
Storage Class (TRGS 510) Non
Combustible Solid.
7.3 Specific end use.
See
Section 1.2.
SECTION
8: Exposure Controls / Personal Protection
8.1
Control parameters
Components with workplace control
parameters
Contains no substances with occupational
exposure limit values.
8.2 Exposure
controls
Appropriate engeineering controls
Follow
general industrial hygiene practices.
Personal
protective equipment.
Eye and face protection
Use proper equipment for eye protection as tested and approved
under government standards (NIOSH (US) or EN 166(EU).
Skin
protection
Handle product with gloves. Inspect gloves for
integrity before use. Follow safe removal technique to take off
gloves and avoid skin contact with contaminated glove surfaces.
Dispose of gloves after use in accordance with applicable laws and
good laboratory practices. Wash and dry hands.
Body
Protection
Select and wear body protection in relation to
its type, and to the concentration, and amount of any associated
dangerous substances or equipment, and to the specific workplace.
The type of protective equipment must be selected according to the
concentration and amount of the dangerous substance at the specific
workplace.
Respiratory
protection
Respiratory protection is not required but
recommended when product powder is handled. Use type N95 (US) or
type P1 (EN 143) dust masks. Use respirators and components tested
and approved under appropriate government standards such as NIOSH
(US) or CEN (EU).
Control of
environmental exposure
Do not let product enter drains.
SECTION
9: Physical and Chemical Properties
9.1
Information on basic physical and chemical
properties
Appearance White tan powder
Odor No
Data Available
Odor Threshold No Data Available
pH No
Data Available
Melting point Decomposes
Freezing
point No Data Available
Initial boiling point No Data
Available
and boiling range No Data Available
Flash
point No Data Available
Evaporation rate No Data
Available
Flammability No Data Available
Upper / lower
No Data Available
flammability
or explosive
limits
Vapor pressure No Data Available
Vapor density No
Data Available
Relative density No Data Available
Water
solubility No Data Available
Partition coefficient No Data
Available
n-octanol / water
Autoignition temp. No Data
Available
Decomposition temp. No Data Available
Viscosity No
Data Available
Explosive properties No Data Available
Oxidizing
properties No Data Available
SECTION
10: Stability and Reactivity
10.1
Reactivity
No data available.
10.2 Chemical
stability
Stable under recommended storage conditions and
identified uses.
10.3 Possibility of hazardous
reaction
No data available.
10.4 Conditions
to avoid
Store dry do not expose to moisture before
use.
10.5 Incompatible materials
No data
available.
10.6 Hazardous decomposition products
No
data available.
In case of fire se Section 5.
SECTION
11: Toxicological Information
Information
on toxicological effects
Acute
toxicity
Inhalation: No data available.
Dermal: No
data available.
Skin
corrosion and irritation
No data available.
Serious
eye damage and eye irritation
No data
available.
Respiratory or skin sensitization
No
data available.
Carcinogenicity
IARC: No
component of this product present at levels greater than or equal to
0.1% is identified as probable, possible or confirmed human
carcinogen by IARC.
ACGH!: No component of this product present at levels greater than or equal to 0.1% is identified as a carcinogen or potential carcinogen by ACGIH.
NTP: No component of this product present at levels greater than or equal to 0.1% is identified as a known or anticipated carcinogen by NTP.
OSHA: No
component of this product present at levels greater than or equal to
0.1% is on OSHA’s list of regulated carcinogens.
Reproductive
toxicity
No data available.
Specific target
organ toxicity – Single exposure
No data
available.
Specific target organ toxicity –
Repeated exposure
No data available.
Aspiration
hazard
No data available.
Additional
information
RTECS: Not available.
SECTION
12: Ecological Information
Toxicity
No data
available.
Persistence and degradability
No
data available.
Bio-accumulative potential
No
data available.
Mobility in soil
No data
available.
Results of PBT and vPvB assessment
PBT
/ PvB assessment is not available as chemical safety assessment is
not required.
Other adverse effects
No data
available.
SECTION
13: Disposal Considerations
Waste
treatment methods
Product
Unused, in accordance
with Section 1.2; or regulatory approved disposal
process.
Contaminated packaging
Regulatory approved
disposal process.
SECTION
14: Transport Information
DOT USA
Not
dangerous goods.
IMDG
Not dangerous
goods.
IATA
Not dangerous goods.
SECTION
15: Regulatory Information
SARA
302 Components
No chemicals in this product are subject to
the reporting requirements of SARA Title III Section 302.
SARA
311/312 Hazards
No SARA Hazards.
SARA 313
Components
This product does not contain any chemical
components with known CAS numbers that exceed the threshold
reporting levels established by SARA Titel III Section
313.
Massachusetts Right To Know Components
No
components are subject to the Massachusetts Right To Know
Act.
Pennsylvania Right To Know Components
Common
Carp Pituitary Extract (Acetone Dried Powder).
New
Jersey Right To Know Components
Common Carp Pituitary
Extract (Acetone Dried Powder).
California Proposition
65 Components
This product does not contain any chemicals
known to State of California to cause cancer, birth defects, or any
other reproductive harm.
SECTION
16: Other Information
Argent Aquaculture
LLC. SDS intended for use by Facilities and Investigators under INAD
8391.
The information in this SDS is considered
current and reliable, but is not all inclusive and shall be used
only as a guide. We encourage users to conduct their own testing
best suited for their purposes under their conditions. This SDS does
not represent a guarantee of the content or properties of the
product. The data is provided without any warranty, expressed or
implied, regarding its correctness or accuracy. Since the conditions
for use, handling, storage and disposal are beyond Argent
Aquaculture’s control, it is the responsibility of the user to
determine safe conditions for use and to assume liability for loss,
damage, or expenses arising from improper use. No warranty expressed
or implied regarding the product described herein will be created by
or inferred from any statement or omission. Argent Aquaculture shall
not be held liable for any damage resulting from handling, contact
or use of the product. Various agencies may have specific
regulations concerning the transportation, handling, storage, use or
disposal of this product which may not be reflected in the SDS. The
user should review these regulations to ensure full
compliance.
Created: January 16, 2014 Last Updated: May
22, 2020
Appendix V. Investigational Label for CCP INAD #8391
1. Investigational label for tests in vitro and in laboratory research animals [511.1(a)]:
"Caution. Contains a new animal drug for investigational use only in laboratory animals or for tests in vitro. Not for use in humans."
2. Investigational label for use in clinical field trials [511.1(b)]:
"Caution. Contains a new animal drug for use only in investigational animals in clinical field trials. Not for use in humans. Edible products of investigational animals are not to be used for food unless authorization has been granted by the U.S. Food and Drug Administration or by the U.S. Department of Agriculture."
Appendix VIa. Fish Species Treated under CCP INAD #8391
All finfish
Appendix VIb. Table of Facilities and Fish Stocks Treated under CCP INAD #8391
Note: This information will be provided directly to CVM
All data must be entered through the online INAD database:
The following forms are to be used as a guide for collecting data that will be entered
into the online INAD database. Any paper forms that are submitted to AADAP will be
sent back to the study participants.
Form CCP-W: Worksheet for Designing Clinical Field Trials under CCP INAD 8391
INSTRUCTIONS
1. Investigator must fill out Form CCP-W for each trial conducted under this INAD before actual use of CCP.
2. Investigator should forward a copy of CCP-W to the Study Monitor for review.
3. After review, the Study Monitor should forward a copy to the AADAP Office for review and assignment of a
Study Number.
SITE INFORMATION
Facility |
|
||
Address |
|
||
|
|
||
Investigator |
|
||
Reporting Individual (if not Investigator) |
|
||
Phone |
|
Fax |
|
FISH CULTURE AND DRUG TREATMENT INFORMATION
Fish species to be treated |
|
|||||
Average fish size (in) |
|
Average fish weight (gm) |
|
|||
Number of treated males |
|
Number of treated females |
|
|||
Number of control males |
|
Number of control females |
|
|||
Anticipated date of treatment |
|
Estimated total amount of drug for proposed treatments (mg) |
|
|||
Intended CCP dosage (mg/kg) |
|
Female |
|
Male |
Method of administration (IP or IM injection) |
|
Number of injections per fish |
|
Female |
|
Male |
Injection Interval (hrs) |
|
Drug manufacturer |
Argent Aquaculture |
Drug lot number |
|
|||
STUDY DESIGN: Provide a brief description of your planned study. The description should include the reason you feel fish should be treated, the treatment dates, the number of fish that will be treated, and if the fish are a threatened or endangered species.
Study designed by |
|
DISPOSITION OF TREATED FISH (Human Food Safety Considerations):
|
Estimated time (days) from last treatment day to first possible harvest for human consumption. |
|
|
|
Investigator should initial here to indicate awareness that fish disposition must be in compliance with FDA-mandated withdrawal times as described in Section XV of the Study Protocol. |
WORKER SAFETY CONSIDERATIONS:
|
Investigator should initial here to indicate that all personnel handling drug have read the Safety Data Sheet for CCP and have been provided protective equipment, in good working condition, as described in the SDS. |
Date Prepared: |
|
Investigator: |
|
|
|||
Date Reviewed: |
|
Study Monitor: |
|
Form CCP-1: Report on Receipt of Drug - Guide for Reporting Investigational New Animal Drug Shipments for Poikilothermic Food Animals
INSTRUCTIONS
1. Investigator must fill out Form CCP-1 immediately upon receipt of CCP.
2. Investigator should forward a copy of Form CCP-1 to the Study Director at the AADAP Office
The sponsor, U.S. Fish and Wildlife Service, submits a notice of claimed investigational exemption for the shipment or delivery of a new animal drug under the provisions of Section 512 of the Federal Food, Drug, and Cosmetics Act. The following information is submitted to FDA:
|
Name of Drug |
CCP |
INAD Number |
8391 |
|
|||
|
Proposed Use of Drug |
To induce gamete maturation in a variety of fish species. |
|
|||||
|
Date of CVM Authorization Letter |
March 6, 2020 |
|
|||||
|
Source of Drug |
Argent Aquaculture |
|
|||||
|
Date of Drug Receipt |
|
Amount of Drug Received (mg) |
|
|
|||
|
Drug Lot Number |
|
Study Worksheet Number |
|
|
|||
|
Name of Investigator |
|
|
|||||
|
Address of Investigator |
|
|
|||||
|
Location of Trial |
|
|
|||||
|
Approximate Number of Treated Animals |
|
|
|||||
|
Study Protocol Number |
8391 |
|
|||||
|
Approximate dates of trial (start/end) |
|
|
|||||
|
Species, Size, and Type of Animals |
|
|
|||||
|
Maximum total dose |
25 mg/Kg body weight |
|
|||||
|
Methods of Administration |
IP or IM Injection |
|
|||||
|
Withdrawal Period |
Zero days |
|
|||||
Date Prepared: |
|
Investigator: |
|
|||||
Date Reviewed: |
|
Study Monitor: |
|
|||||
Date Reviewed: |
|
Sponsor: |
|
|||||
Form CCP-2: Drug Inventory Form
For Use in CCP Clinical Field Trials Conducted under CCP INAD 8391 INSTRUCTIONS
1. Investigator should initiate a new form CCP-2 immediately upon receipt of each shipment of Common Carp Pituitary.
Each lot number of CCP may be used for multiple treatment regimens.
Qty of CCP from Reporting
previous page (mg) Facility individual
Date |
Amount of new CCP received (mg) |
Lot number of CCP received |
Study Number |
Amount of CCP used in treatment (mg) |
CCP transferred (mg) |
CCP discarded (mg) |
CCP remaining on hand (mg) |
Inventory by (Initials) |
|
|
|
|
|
|
|
|
|
|
xxxx |
xxxx |
|
|
|
|
|
|
|
xxxx |
xxxx |
|
|
|
|
|
|
|
xxxx |
xxxx |
|
|
|
|
|
|
|
xxxx |
xxxx |
|
|
|
|
|
|
|
xxxx |
xxxx |
|
|
|
|
|
|
|
xxxx |
xxxx |
|
|
|
|
|
|
|
xxxx |
xxxx |
|
|
|
|
|
|
|
xxxx |
xxxx |
|
|
|
|
|
|
|
xxxx |
xxxx |
|
|
|
|
|
|
|
xxxx |
xxxx |
|
|
|
|
|
|
Date Prepared: |
|
Investigator: |
|
Date Reviewed: |
|
Study Monitor: |
|
STUDY NUMBER |
|
Form CCP-3: Results Report Form For Use in CCP Clinical Field Trials conducted under
CCP INAD 8391
INSTRUCTIONS
1. Investigator must fill out Form CCP-3 no later than 10 days after completion of the study period. Attach lab reports and other information.
2. If CCP was not used under the assigned Study Number, contact the Study Director at the AADAP Office to close-out the study.
3. Investigator should forward a copy of Form CCP-3 to the Study Monitor. Within 10 days of receipt, the Study Monitor should forward a copy to the Study Director at the AADAP Office.
SITE INFORMATION
Facility |
|
Reporting Individual |
|
FISH CULTURE AND DRUG TREATMENT INFORMATION
Drug lot number |
|
Total amount drug used (mg) |
|
Fish species treated |
|
Water temperature (oF) |
|
Drug dosage – males (mg/kg body wt) |
|
Drug dosage - females (mg/kg body wt) |
|
Average fish weight (gm) |
|
Average fish length (in) |
|
Number of treated males |
|
Number of treated females |
|
Number of control males |
|
Number of control females |
|
Treatment date(s) |
|
|
|
Number of injections per males |
|
Number of injections per females |
|
Injection Interval (hrs) |
|
Method of administration (IM or IP injection) |
|
Spawning/evaluation interval (time from treatment until spawning) |
|
Spawning/evaluation date(s) |
|
Hormone Results Record
INSTRUCTIONS
“Ripe” females are those fish that have ovulated or released their eggs; “ripe” males are those fish that are actively spermiating. “Non-ripe” fish are the converse after treatment.
Motility Score based on a scale of 0 - 4 (see Study Protocol Section XII).
Use Additional copies of this form for additional treatment days
Be sure the facility name is written here:
|
TREATED FISH - Females |
CONTROL FISH - Females |
|||||||||||
Date Treated |
Date Evaluated |
# of Fish |
# Ripe |
# Non-Ripe |
% Ripe |
% Eye-Up |
% Hatch |
# of Fish |
# Ripe |
# Non-Ripe |
% Ripe |
% Eye-up |
% Hatch |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
TREATED FISH - Males |
CONTROL FISH - Males |
||||||||||||
Date Treated |
Date Evaluated |
# of Fish |
# Ripe |
# Non-Ripe |
% Ripe |
Milt/ fish (mL) |
Motility Score |
# of Fish |
# Ripe |
# Non-Ripe |
% Ripe |
Milt/ fish (mL) |
Motility Score |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
RESULTS: Describe in detail treatment results. Was treatment successful? If treatment did not appear to be successful, explain why not? Were there any mitigating environmental conditions that may have impacted treatment results? Were there any deviations from the Study Protocol?
Toxicity observations: Report any apparent drug toxicity including a description of unusual fish behavior.
OBSERVED WITHDRAWAL PERIOD OF TREATED FISH:
|
Number of days before human consumption. Ensure this time period meets the withdrawal period described in Section XV of the Study Protocol. |
NEGATIVE REPORT CCP analog was not used at this facility under this Study Number during the reporting period. The study will be closed out in the online INAD database.
Date Prepared: |
|
Investigator: |
|
|
|||
Date Reviewed: |
|
Study Monitor: |
|
Study Number:____________________ Page 1 of 1
Form CCP-4N: Necropsy Report Form
For Use in CCP Clinical Field Trials Conducted under INAD 8391
INSTRUCTIONS
1. Investigator must fill out Form CCP-4N for all fish that die or are euthanized during the study period. Use a new copy of Form CCP-4N for each individual fish.
2. This information will be reported in the Necropsy Report Data Entry table located in the Results Report Form in the online database.
Date _____________ Fish Species/ID ________________________ Fish Length (cm) ________
Evaluator(s): __________________________________
Body surface: normal excess mucus irregular color other ____________________
Dermal lesion: none hemorrhagic other _________________________________
closed open
Location: dorsal caudal ventral lateral cranial
base of fin - Pectoral (right), Pectoral (left), Adipose, Dorsal, Anal, or Caudal
Gills: normal pale hemorrhagic other ____________________________
Liver: normal pale mottled other _________________________________
Spleen: normal pale enlarged other _______________________________
Kidney: normal pale swollen other _________________________________
____________________________________________________________________________________
Notes and comments of gross pathologies on other organs and tissues.
eyes ________________________________ stomach _______________________________
body cavity ___________________________ gastrointestinal tract ______________________
gall bladder ___________________________ gas bladder _____________________________
adipose tissue ___________________________ musculature ____________________________
implant site___________________________________________________________________________
other________________________________________________________________________________
____________________________________________________________________________________
Investigator: ____________________________ Date: _______________
NOTICES
Paperwork Reduction Act
In accordance with the Paperwork Reduction Act (44 U.S.C. 3501 et seq.), the U.S. Fish and Wildlife Service collects information necessary to permit the use of an investigational new animal drug to generate data to support a new animal drug approval (NADA) as part of the Fish and Aquatic Conservation fish health network. Your response is voluntary, but is required to obtain or retain a benefit. According to the Paperwork Reduction Act of 1995, an agency may not conduct or sponsor and a person is not required to respond to a collection of information unless it displays a currently valid OMB control number. OMB has approved this collection of information and assigned Control No. 1018-####.
ESTIMATED BURDEN STATEMENT
We estimate public reporting for this collection of information to average 4 hours, including time for reviewing instructions, gathering and maintaining data, and completing and reviewing the form. Direct comments regarding the burden estimate or any other aspect of the form to the Service Information Clearance Officer, Fish and Wildlife Service, U.S. Department of the Interior, 5275 Leesburg Pike, MS: PRB (JAO/3W), Falls Church, VA 22041-3803, or via email at [email protected]. Please do not send your completed form to this address.
FREEDOM OF INFORMATION ACT STATEMENT
Information provided to the Service is generally subject to release to the public under the Freedom of Information Act (FOIA). Certain information, however, may be subject to withholding if the Service determines that the information is a trade secret and/or commercial or financial information that is privileged or confidential. To the extent you are submitting business information that falls into one of these categories, you must clearly mark this information as "Business Confidential" in order for the Service to assess the applicability of FOIA Exemption 4. Any information provided by you that is not marked as “Business Confidential” will be considered releasable to the public under the FOIA [43 CFR 2.26 – 2.33].
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | Peter McKenzie |
| File Modified | 0000-00-00 |
| File Created | 2023-08-23 |
© 2026 OMB.report | Privacy Policy