CMS-10174 - Supporting Statement A_
CMS-10174 - Supporting Statement A_.docx
Collection of Prescription Drug Data from MA-PD, PDP and Fallout Plans/Sponsors for Medicare Part D Payments (CMS-10174) - IRA
OMB: 0938-0982
Supporting Statement (Part A) Collection of Prescription Drug Event Data
From Contracted Part D Providers for Payment CMS-10174, OMB 0938-0982
In December 2003, Congress enacted the Medicare Prescription Drug, Improvement, and Modernization Act of 2003 (P.L. 108-173), referred to as the Medicare Modernization Act (MMA). The Medicare Prescription Drug Benefit program (Part D) was established by section 101 of the MMA and is codified in section 1860D-1 through 1860D-41 of the Social Security Act (hereinafter, “the Act”). Effective January 1, 2006, the Part D program established an optional prescription drug benefit for individuals who are entitled to Medicare Part A and/or enrolled in Part B. In general, coverage under the prescription drug benefit is provided predominately through private at-risk Prescription Drug Plans (PDPs) that offer drug-only coverage, Medicare Advantage (MA) plans that offer integrated prescription drug and health care coverage (MA-PD plans), or through Cost Plans that offer prescription drug benefits.
The Patient Protection and Affordable Care Act, as amended by section 1101 of the Health Care and Education Reconciliation Act of 2010, establishes the Coverage Gap Discount Program (CGDP) by adding sections 1860D-14A and 1860D-43 of the Act. Effective January 1, 2011, the CGDP provides manufacturer discounts to applicable Medicare beneficiaries receiving applicable covered Part D drugs in the coverage gap phase of the benefit.
Section 9008 of the Patient Protection and Affordable Care Act (ACA; P.L. 111–148), as amended by section 1404 of the Health Care and Education Reconciliation Act of 2010 (HCERA; P.L. 111–152), imposes an aggregate annual fee on certain manufacturers of branded prescription drugs (please refer to section 9008(e)(2) of the ACA for a definition of branded prescription drugs). CMS is required to provide dollar amounts of sales of branded prescription drugs under the Medicare Part D program on a yearly basis to the Secretary of the Treasury in order to determine the fee amount to be paid by each manufacturer.
The Inflation Reduction Act (IRA), signed into law on August 16, 2022 (P.L. 117-169), makes a number of changes to the Part D program. Changes that impact the information in this collection requirement are as follows:
Eliminates the deductible for adult vaccines recommended by the Advisory Committee on Immunization Practices (ACIP) and eliminates coinsurance or other cost-sharing for these vaccines beginning January 1, 2023. § 1860D-2(b)(8) of the Act. For 2023 only, Medicare pays Part D sponsors a temporary retrospective subsidy (Inflation Reduction Act Subsidy Amount, or IRASA) for this reduction in cost sharing and deductible, which must equal the difference between the beneficiary cost sharing for the ACIP- recommended adult vaccine under a Part D plan’s 2023 benefit design (submitted by sponsors to CMS prior to the passage of the IRA) and the applicable statutory maximum cost sharing limit created by the IRA. § 1860D-15(h) of the Act.
Eliminates the deductible for covered Part D insulin products and limits the beneficiary cost sharing for these insulin products beginning January 1, 2023. § 1860D-2(b)(9) of the
Act. For 2023 only, Medicare pays Part D sponsors a temporary retrospective subsidy (IRASA) for this reduction in cost sharing and deductible, which must equal the difference between the beneficiary cost sharing for the covered insulin product under a Part D plan’s 2023 benefit design (submitted to CMS prior to the passage of the IRA) and the applicable statutory maximum cost sharing limit created by the IRA. § 1860D-15(h) of the Act.
Provides for lower prices for certain high-priced single source drugs through a drug price negotiation process established by the Secretary, whereby a maximum fair price (MFP) is set for negotiation-eligible drugs beginning January 1, 2026. Part E of Tile XI of the Act.
Requires a manufacturer of a Part D rebatable drug to pay a rebate to the Medicare Prescription Drug Account in the Federal Supplementary Medical Insurance Trust Fund if the drug’s annual manufacturer price in an applicable period (a 12-month period beginning October 1, 2022) exceeds the drug’s inflation-adjusted payment amount, which is based on the drug’s benchmark period manufacturer price, increased by the Consumer Price Index for All Urban Consumers (hereinafter referred to as the “Part D inflationary rebate”). This provision requires that the calculations take into consideration and exclude drugs units that for which the manufacturer has provided a discount under section 340B of the Public Health Service Act. § 1860D-14B of the Act.
Redesigns the Part D benefit by eliminating the cost-sharing in the catastrophic phase in 2024; capping annual out-of-pocket costs for prescription drugs under Part D at $2,000 beginning in 2025; reducing the government reinsurance in the catastrophic phase of Part D coverage for applicable drugs from 80 percent to 20 percent and for non-applicable drugs from 80 percent to 40 percent beginning in 2025; eliminating the coverage gap phase and coverage gap discount program, and replacing it with a Manufacturer Discount Program (MDP) that requires manufacturers to provide a 10 percent discount in the initial phase, and a 20 percent discount in the catastrophic phase on applicable drugs beginning in 2025; and creating a new Selected Drug Subsidy Program, under which Medicare covers the portion the manufacturer would otherwise pay under the MDP in the initial coverage phase of the benefit for the years in which a MFP applies for the drug. §§ 1860D-2(b), 1860D-15(b), 1860D-14A, 1860D-14C, 1860D-14D of the Act.
The PRA requirements referenced in this submission, as reflected in the regulations at 42 CFR Part 423 and the statutory provisions described above, assist in the implementation of the provisions of the Act to effectuate and monitor the Medicare Prescription Drug Benefit and support the continued administration of the program. The existing approved package is being revised to update burden estimates based on the expansion of the prescription drug event (PDE) record file layout, updates to existing fields on the PDE record, and the addition of new fields on the PDE record. This expansion is being implemented to accommodate future business needs, including but not limited to, updates necessary for the IRA and anticipated updates to the National Council for Prescription Drug Programs (NCPDP) Telecommunications Standard. We are making changes to existing fields and adding new fields to assist Medicare Part D sponsors in submitting complete and accurate data for payment, to be compliant with CMS regulations and statutory provisions, and based on the feedback received from our stakeholders. We are also
revising the package to update burden estimates based on the number of contracts as well as the growth of Medicare beneficiaries enrolled in Part D.
In this collection of information request, CMS is notifying users that it will also use data reported under sections 1860D-15(c)(1)(C) and (d)(2) to implement the Medicare Drug Negotiation Program and to calculate inflation rebates as required by the Inflation Reduction Act of 2022, as well as to fulfill our statutory obligations under the Social Security Act. This includes the use of PDE data to fulfill obligations or operationalize any future legislative changes to the Social Security Act that impact the Part D program.
Our fundamental goal is to have the least burdensome data submission requirements necessary to acquire the data needed for accurate Medicare Part D payment and appropriate program oversight. We believe that claims data provide the most reliable approach to ensuring that payment calculations are accurate. In the absence of claims level data, we will not be able to determine that reinsurance and risk corridor payments are accurate, fallback plans have been paid accurately, low income subsidies are appropriate, the CDGP reconciliation is accurate, the MDP reconciliation is accurate, or the IRASA and Selected Drug Subsidy have been paid accurately.
This claims level information is reported by the Part D sponsors to CMS on the PDE record. We limit our data collection to those critical data elements that are necessary for the accuracy of payment-related calculations, in addition to other elements for validation of the PDE record, quality monitoring, and program integrity and oversight, all of which are necessary for ensuring accurate payment. By focusing on the critical data elements, the ability of plans to collect and submit accurate and complete data for the purpose of payment calculations will be optimized.
Our view is that in order to fulfill the statutory requirements of the Act, we will need the following data categories. Note that the examples of the fields in a given category are examples only and are not intended to be all inclusive or limit CMS’ ability to add or remove fields in the future. Additional fields will likely be added to operationalize certain provisions of the IRA.
Entity identification (for example, record ID, sequence no., submitter ID, contract number, and PBP ID)
Beneficiary information (for example, Medicare Beneficiary Identifier (MBI), cardholder ID, patient residence, date of birth, and gender)
Event identification information (for example, claim control number, prescription service reference number, prescription origin code, paid date, non-standard format code, pricing exception code, date original claim received, claim adjudication began timestamp, submission clarification codes, submission type codes, LTPAC dispense frequency, adjustment/deletion code, date of service, DAW/product selection code, and fill number)
Drug and Quantity identification information (for example, product service ID, and compound code, originally prescribed quantity, and days supply)
Cost information (for example, ingredient cost, dispensing fee, vaccine administration fee or additional dispensing fee, and sales tax)
Payment Breakout information (for example, catastrophic coverage code, total gross covered drug cost accumulator, true out-of-pocket (TrOOP) accumulator, deductible accumulator, patient pay amount, gross drug cost below and above out-of-pocket threshold (GDCB and GDCA), low-income cost sharing subsidy amount, covered D plan paid (CPP) amount, non-covered plan paid (NPP) amount, estimated remuneration at POS amount (ERPOSA), pharmacy price concessions at POS, reported gap discount, reported manufacturer discount, government pay/subsidy amount, and other TrOOP amount)
Prescriber information (for example, prescriber ID qualifier, and prescriber ID)
Service Provider information (for example service provider ID qualifier, service provider ID, and pharmacy service type)
Benefit Design information (for example, drug coverage status code, beginning benefit phase, ending benefit phase, brand/generic code, tier, and formulary code)
In addition to data for interim payments (i.e., direct subsidies), we will need these data on 100 percent of prescription drug claims for appropriate risk adjustment, reconciliation of reinsurance and low-income subsidies, calculation of risk sharing payments or savings, and program auditing. The PDE data submitted for claims falling in the coverage gap phase are used for the CGDP, including invoicing the manufacturers for the discount, for years in which the CGDP applies; and PDE data for applicable drugs in the initial phase and the catastrophic phases will be used for the MDP, including invoicing the manufacturers for the discount, for years in which the MDP applies. Consistent with section 9008 of the ACA, the data are used in the annual report provided to the Secretary of the Treasury. PDE data will also be used for the assessment and improvement of quality of care, the drug price negotiation process and the determination of the MFP for negotiation-eligible drugs, and the Part D inflationary rebate process.
Sections 11001 through 11004 of the Inflation Reduction Act of 2022 establish a Medicare Drug Negotiation Program for high-expenditure drugs. Section 11102 of the Inflation Reduction Act of 2022 establishes a Part D inflation rebate by manufacturers of certain single source drugs and biologicals with prices increasing at a rate faster than the rate of inflation. CMS will use data reported under sections 1860D-15(c)(1)(C) and (d)(2), in part, to rank drugs by total expenditures under Part D in order to select drugs for negotiation and to identify units to calculate inflation rebates.
We also intend to use the PDE data submissions from Part D sponsors to fulfill our statutory obligations under the Social Security Act. This includes the use of PDE data to fulfill obligations or operationalize any future legislative changes to the Social Security Act that impact the Part D program.
The sections of the Act that provide the statutory authority for data submission in the prescription drug benefit program are the following:
Payments – sections 1860D-11(g)(5), 1860D–14, 1860D-15, 1860D-22, and 1860D–14D.
Data submission – sections 1860D-12(b)(3)(D), 1860D–15(c)(1)(C), 1860D–15(c)(2)(C),
1860D–15(d)(2), 1860D–15(f), 1860D-14A(c)(1)(C), and 1860D-14C(c)(3).
Manufacturer Inflationary rebates – section 1860D–14B.
Drug Price Negotiation Program – Part E of Title XI.
The regulations set forth in this requirement for submitting PDE data are codified in 42 CFR Part 423 – Voluntary Medicare Prescription Drug Benefit. There are a number of places in which statutory provisions in Part D reference specific sections in Part C of Medicare (the MA program). The MA regulations appear at 42 CFR Part 422 Medicare Advantage Program. The major subjects applicable to the prescription drug data submission in Part 423 are as follows:
42 CFR 423.301 implements section 1860D-15 of the Act and the deductible and cost sharing provisions are addressed in section 1860D–14(a) of the Act. This section sets forth rules for the calculation and payment of our direct and reinsurance subsidies for Part D plans; the application of risk corridors and risk sharing adjustments to payments; and retroactive adjustments and reconciliations to actual enrollment and interim payments.
42 CFR 423.322 and 423.329(b)(3) implement sections 1860D-15(c)(1)(C), 1860D-15(c)(2)(C),
1860D-15(d)(2) and 1860D–15(f) of the Act. These provisions set forth the requirement that payments to a Part D sponsor are conditioned upon provision of information necessary to CMS to carry out payment.
42 CFR 423.771, 423.772, 423.780, 423.782, and 423.800 implement section 1860D-14 of the Act. This section sets forth rules for premiums and cost-sharing subsidies for low-income beneficiaries.
42 CFR 423.875 implements section 1860D-11(g) of the Act, and sets forth, but not limited to, the amount payable for a fallback prescription drug plan in accordance with § 423.871(e).
42 CFR 423.888 provides payment methods, including provision of necessary information, and implements section 1860D-22(a) of the Act, as amended by section 101 of the MMA. This section implements the statutory requirement that a subsidy payment be made to sponsors of qualified retiree prescription drug plans.
Subpart W of part 423 implements sections 1860D-14A and 1860D-43 of the Act. 42 CFR 423.2325 requires Part D sponsors to provide CMS with appropriate data on the applicable discounts provided by the Part D sponsors in a manner specified by CMS.
42 CFR 422.310 sets forth rules for submitting data that can be linked at the individual level to Part A and Part B data.
42 CFR 423.505(f)(3) implements section 1860D-12(b)(3)(D) of the Act to allow the Secretary to collect the same claims information collected under the authority of section 1860D-15 of the Act for purposes deemed necessary and appropriate by the Secretary, including reporting to the Congress and the public, conducting evaluations of the overall Medicare program, making legislative proposals to Congress, and conducting demonstration projects.
The information users will be pharmacy benefit managers (PBMs), third party administrators and pharmacies, and the PDPs, MA-PDs, Fallbacks, and other plans that offer coverage of outpatient prescription drugs under the Medicare Part D benefit to Medicare beneficiaries. The statutorily required data is used primarily for payment and is used for claim validation as well as for other legislated functions such as quality monitoring, program integrity and oversight. In addition, the PDE data are used to support operations and program development.
Annually, CMS publishes a Public Use File (PUF) that summarizes the annual Low-Income reconciliation amount, the Reinsurance reconciliation amount, the Risk-Sharing amount, and the Reconciliation amount (which is a sum of the 3 previously stated reconciliation amounts). The PDEs are one of the inputs used to determine these reconciliation amounts.
CMS has used PDE data to create summarized dashboards and tools, including the Medicare Part D Drug Spending Dashboard & Data, the Part D Manufacturer Rebate Summary Report, and the Medicare Part D Opioid Prescribing Mapping Tool. The data are also used in the Medicare Trustees Report. Due to the market sensitive nature of PDE data, external uses of the data are subject to significant limitations. However, CMS does analyze the data on a regular basis to determine drug cost and utilization patterns in order to inform programmatic changes and to develop informed policy in the Part D program.
CMS information users will leverage data reported under sections 1860D-15(c)(1)(C) and (d)(2) to implement the Medicare Drug Price Negotiation Program and calculate inflation rebates. We will also use the data reported to fulfill our statutory obligations under the Social Security Act. This includes the use of PDE data to fulfill obligations or operationalize any future legislative changes to the Social Security Act that impact the Part D program.
The Drug Data Processing System (DDPS) is the information system that collects, validates and stores PDE data received from PDPs, MA-PDs, Fallback and other plans offering coverage of outpatient prescription drugs under the Medicare Part D benefit. PDE records enter the DDPS through the Prescription Drug Front-End System (PDFS). The PDFS receives the PDE records at least monthly, once the plan sponsors or their PBMs or other third party administrators have received drug claims from pharmacies and completed their “in-cycle” events. Plan sponsors or their third-party submitters must submit PDE records electronically. The PDFS performs file format and face validity checks. Once the file has passed the front-end checks, it moves through the DDPS where detail-level edits are performed and the data are stored. The DDPS also receives corrected and adjusted PDE records.
The PDFS and DDPS consists of two levels of edits, front-end (face validity and file format) and detail-level (PDE validity and verification) edits. CMS provides a processing system that allows critical data fields to be edited before rejecting or returning a file for more information to allow, at a minimum, a one-time resubmission for corrected data.
The system also provides simplified reporting from CMS to plan sponsors. Plan sponsors will receive one complete report with all transactions and statuses listed. The report identifies which PDE records were accepted and which were not, along with reason codes. Data tracking is
improved by reporting complete results daily. Plan sponsors also receive periodic summary reports that aggregate the number of drug events submitted, rejected, and accepted.
Drug event data can be submitted via the Medicare Data Communications Network (MDCN) utilizing Internet Protocol (IP) and Secure File Transfer protocol (SFTP) or Systems Network Architecture (SNA) and Connect:Direct, or through the CMS TIBCO mail boxing system.
Production PDE data are submitted into the PDFS and DDPS. When data are submitted, each system applies various edit checks to the data and issues response reports to submitters describing data that was accepted, rejected, and any errors that were or may be present in the data, so that plans can manage, correct and resubmit their data as necessary.
The DDPS edits records at the detail level, checking event-level information on the beneficiary, drug, costs, and other items. CMS maintains a complete, up-to-date listing of edits at https://www.csscoperations.com/internet/csscw3.nsf/DIDC/FGSMOX8LWK~Prescription%20D rug%20Program%20(Part%20D)~References.
Once DDPS performs all necessary edits, the accepted PDE records are forwarded to the Integrated Data Repository (IDR). The IDR stores PDE records and accumulates summary data for payment reconciliation. The Payment Reconciliation System (PRS) creates a beneficiary/plan record for each beneficiary enrolled in a plan during the payment year and calculates reconciliation payments at the beneficiary and plan level. Specific reports issued from the IDR inform plans of their year-to-date financial values in preparation for reconciliation. CMS calls these management reports.
An outside contractor manages the data submission process for CMS and maintains a Customer Service and Support Center (CSSC) that provides customer service and support to data submitters.
All data (100%) is collected by CMS electronically.
See section 12 of this Supporting Statement under Collection of Prescription Drug Event Data for an illustration of the dataflow.
This information collection does not duplicate any other effort and the information cannot be obtained from any other source.
The collection of information will have a minimal impact on small businesses or other small organizational entities since the applicants must possess an insurance license and be able to accept risk. Generally, state statutory licensure requirements effectively prevent small organizations from accepting the level of risk needed to provide the pharmacy benefits required in the Medicare Prescription Drug Benefit program.
In the April 27, 2006, Instructions on Requirements for Submitting Prescription Drug Event Data, CMS indicated that PDE records must be submitted to CMS electronically at least once a month. In addition to this guidance, CMS has issued more specific guidance on submission of original PDE records and corrections. The October 6, 2011 Health Plan Management System (HPMS) memorandum titled, “Revision to Previous Guidance Titled ‘Timely Submission of Prescription Drug Event (PDE) Records and Resolution of Rejected PDEs,” states that original PDE records must be submitted within 30 days following Date Claim Received or Date of Service (whichever is greater); resolve rejected records and re-submit within 90 days following receipt of rejected record status from CMS; submit adjustments within 90 days of discovery; and submit adjustments and deletions within 90 days following discovery of issue requiring change.
CMS believes this frequency minimizes burden yet allows for reimbursement to proceed in a timely and accurate manner. CMS will begin an initial reconciliation 6 months after the end of a coverage year and, consistent with 42 CFR 423.346(a)(2) and the timeframes described within, will perform a global reopening of the reconciliation for that contract year.
There are no special circumstances that would require an information collection to be conducted in a manner that requires respondents to:
Report information to the agency more often than monthly;
Prepare a written response to a collection of information in fewer than 30 days after receipt of it;
Submit more than an original and two copies of any document;
Retain records, other than health, medical, government contract, grant-in-aid, or tax records for more than three years;
Collect data in connection with a statistical survey that is not designed to produce valid and reliable results that can be generalized to the universe of study;
Use a statistical data classification that has not been reviewed and approved by OMB; or
Include a pledge of confidentiality that is not supported by authority established in statute or regulation that is not supported by disclosure and data security policies that are consistent with the pledge, or which unnecessarily impedes sharing of data with other agencies for compatible confidential use.
The 60-day notice published in the Federal Register (88 FR 27896) on May 3, 2023.
One entity submitted comments. The comments can be found attached. After review of the comments, no action will be taken. Specifically, no requirements or documents have been revised nor have any burden estimates been revised as a result of the comments.
The 30-day notice published in the Federal Register (88 FR 45428) on July 17, 2023.
The table below is a summary of our specific communications and consultations with the industry since the package was last approved. The table breaks out events by Type, Date and Status.
Table 1: Communications with Industry
Type of Communication |
Dates |
Status |
Weekly Part D User Group Teleconferences |
Began February 9, 2005 |
Active (now held as-needed) |
NCPDP Workgroup |
August 2006 November 2006 November 2007 August 2008 May 2009 August 2012 – present |
Active |
PDE Training- Webinars |
June 2014 August 2014 February 2015 |
Training is no longer performed via Webinars, but through Computer Based Training |
PDE Training- Computer Based Training |
November 2015 December 2015 July 2016 December 2018 May 2021 |
Active |
HPMS memorandum - Revision to Previous Guidance Titled ‘Timely Submission of Prescription Drug Event (PDE) Records and Resolution of Rejected PDEs |
October 6, 2011 |
Active |
HPMS memorandum - Proposed Updates to the Prescription Drug Event (PDE) File Layout; Seeking Feedback |
May 31, 2022 |
Draft – comments requests |
HPMS memorandum - PDE Reporting Instructions for Implementing the Cost Sharing Maximums Established by the Inflation Reduction Act for Covered Insulin Products and ACIP-Recommended Vaccines for Contract Year 2023 |
September 26, 2022 |
Active |
HPMS memorandum - Reporting Estimated Remuneration Applied to the Point-of-Sale Price |
October 14, 2022 |
Active |
HPMS memorandum - New 2025 Prescription Drug Event (PDE) File Layouts (draft); Seeking Feedback |
November 1, 2022 |
Draft – comments requested |
New 2025 Prescription Drug Event (PDE) File Layouts (FINAL) |
April 18, 2023 |
Active |
Respondents will not receive any payments or gifts for responding to this information collection. Submitting information using the prescribed CMS format is one of the requirements for participation in the Medicare Part D drug benefit program.
The information provided by the plan or sponsor organizations regarding prescription drug events are protected and held confidential in accordance with 20 CFR 401.30. The information provided electronically and on forms will become part of the contracted organization’s computer history, microfilm, and hard copy records retention system as published in the Federal Register, Part VI, "Privacy Act of 1974 System of Records" on September 20, 1976 (HI CAR 0175.04).
All electronic claims or drug events sent from pharmacies to PBMs or other third party administrators and from them to plans constitute HIPAA-covered transactions. Any plan or sponsor organizations that utilize an electronic format for their drug data collection will need to convert to ANSI X12.
Other than the information noted above in section 10 above, there are no questions of a sensitive nature. Specifically, the collection does not solicit questions of a sensitive nature, such as sexual behavior and attitudes, religious beliefs, and other matters that are commonly considered private.
Active Collection of Information and Associated Burden Estimates (Adjusted)
The burden placed on Part D sponsors (contracts) associated with submitted PDE data is predicated upon the following factors: (a) the amount of data that must be submitted; (b) the number of plans submitting data; and (c) the time required to complete the data processing and transmission transactions. The burden estimate is being revised to factor in the number of sponsors, number of beneficiaries, and number of PDE records for more recent contract years, in addition to the additional fields required on the PDE record from the Part D sponsors.
PDE Data Submission: The amount of data that must be submitted is a function of the number of prescription drug events per beneficiary and the number of data elements per event. Based on CMS data from February 2023, CMS estimates that an annual average of 50,028,700 Medicare beneficiaries enroll in Part D prescription drug coverage. The average number of PDEs per year is 1,499,064,780 based on data from 2019, 2020, and 2021. To compute the average number of PDEs per beneficiary, we divide the average number of PDEs per year by the average number of beneficiaries enrolled per year. This computation leads to an average of 30 PDEs per beneficiary per year.
Number of Part D Contracts (Respondents): The average number of Part D contracts per year is 856 (based on 2019, 2020, and 2021 data).
Time Required to Process Data: The third factor that contributes to the burden estimate for submitting PDE data depends upon the time and effort necessary to complete data transaction activities. Since our regulations require Part D sponsors to submit drug event data to CMS that can be linked at the individual level to Part A and Part B data in a form and manner similar to the process provided under § 422.310 (Part C), the data transaction timeframes will be based on risk adjustment (Part C) and prescription drug industry experiences. Moreover, our PDE data submission format (as well as the drug industry’s) will only support electronic formats. The drug industry’s estimated average processing
time for electronic data submission is 1 hour for 500,000 records. The risk adjustment estimated average annual electronic processing time cost per hour is $17.75.
All three factors are reflected in Table 1 and illustrate the relationship between these results and the burden estimate.
Table 2: Annual Cost to Respondents Estimate for Active Collection of Information
FIELD |
DESCRIPTION |
DATA |
NOTES |
A |
NUMBER OF RESPONDENTS |
856* |
856 is the annual average number of Part D contracts from 2019, 2020, and 2021 |
B |
NUMBER OF MEDICARE BENEFICIARIES ENROLLED IN PART D PER YEAR |
50,028,700** |
Average number of Medicare beneficiaries enrolled in Part D |
C |
AVERAGE NUMBER OF PART D BENEFICIARIES PER CONTRACT |
58,445 |
(B) divided by (A) |
D |
AVERAGE NUMBER OF PDES PER YEAR |
1,499,064,780** |
The average is based on annual average PDEs from 2019, 2020, and 2021 |
E |
FREQUENCY OF RESPONSE |
30 PDEs/per beneficiary per year |
average PDEs per beneficiary per year |
F |
NUMBER OF TRANSACTIONS PER HOUR |
500,000 |
Drug industry's estimated average processing volume per hour |
G |
TOTAL ANNUAL TRANSACTION HOURS |
2,998 |
(D) divided by (F) |
H |
AVERAGE ELECTRONIC COST PER HOUR |
$17.75 |
Based on $17.75 per hour, the risk adjustment estimated average annual electronic processing cost per hour |
I |
COST OF ANNUAL TRANSACTION HOURS |
$53,215 |
(H) multiplied by (G) |
J |
HOURS/RESPONDENT |
3.5023 |
Number of hours needed to process one contract’s PDEs |
K |
AVERAGE COST PER PART D BENEFICIARY |
$0.0011 |
(I) divided by (B) |
L |
ANNUAL COST TO RESPONDENTS |
$62.17 |
(K) multiplied by (C) or (J) x (H) |
*Data Source: Payment Reconciliation System Reports
**Data Source: CMS Integrated Data Repository
One-Time Burden Estimates Resulting from Expansion of the PDE File Layout and Addition of PDE Fields (New)
The expansion of the PDE file is the first increase in file length since the PDE file structure was implemented in 2006. Currently less than 5% of the file length is available on the PDE file layouts to add new fields and/or to expand existing fields. As we look ahead to the future, CMS will expand the PDE file layouts from their current 512-byte length to 1000 bytes, to be implemented effective January 1, 2025.
This expansion is being implemented to accommodate future business needs, including but not limited to, updates necessary for the IRA and anticipated updates to the NCPDP Telecommunications Standard. In addition, the lengths of some of the existing fields on the PDE file layouts will be increased, which requires a reorganization of the overall PDE file layouts.
The burden associated with this one-time requirement to update the PDE file layout to the expanded layout with new fields is related to updating the systems and files to accommodate such changes. Then, all Part D sponsors will be required to submit certification (CERT) test files to CMS prior to submitting production PDE files on January 1, 2025.
Number of Part D Respondents: The average number of Part D Contracts offering a Part D plan per year (Row (B)) is 856 (based on 2019 – 2021 internal CMS data).
Total Number of Responses: The total number of responses equals the total number of respondents, because for the one-time burden estimates, each entity will need to make system changes.
Labor Costs and Time Required: The hourly wage rate for this labor category was taken from the U.S. Bureau of Labor Statistics’ May 2021 National Occupational Employment and Wage Estimates for all salary estimates (https://www.bls.gov/oes/current/oes_nat.htm). The hourly wage is multiplied by a factor of 2 to account for fringe benefits and overhead (as shown in Table 2).
Table 3: Adjusted Hourly Wages Used in Burden Estimates1
Occupation Title |
Occupational Code |
Mean Hourly Wage ($/hr.) |
Fringe Benefits and Overhead ($/hr.) |
Adjusted Hourly Wage ($/hr.) |
Software Developers |
15-1252 |
58.17 |
58.17 |
116.34 |
Software Quality Assurance Analysts and Testers |
15-1253 |
46.97 |
46.97 |
93.94 |
Database Administrators |
15-1242 |
46.42 |
46.42 |
92.84 |
Computer Systems Analysts |
15-1211 |
49.14 |
49.14 |
98.28 |
In calculating the burden of this proposal, we must consider the following:
On average, for each of the 856 Part D Contracts, 2 software developers working at
$116.34/hr spend 20 hours performing system maintenance with an aggregate per contract dollar burden of $3,983,482.
Based on internal CMS data, there are about 856 Part D Contracts. The burden of update requires that 2 software developers will each spend 20 hours performing necessary redesigns. Therefore, the aggregate burden across all 856 Part D contracts is 34,240 hours (2 software developers x 20 hours a developer x 856 Part D Contracts).
Thus, the total cost is $3,983,482 (34,240 hours x $116.34 wage/hr)
On average, for each of the 856 Part D Contracts, 1 Software Quality Assurance Analyst and Tester working at $93.94/hr spend 10 hours performing system maintenance with an aggregate per contract dollar burden of $804,126.
Based on internal CMS data, there are about 856 Part D Contracts. The burden of update requires that 1 Software Quality Assurance Analyst and Tester will spend 10 hours performing necessary redesigns. Therefore, the aggregate burden across
1 Source: Bureau of Labor Statistics, May 2021 Occupational Employment and Wage Statistics
all 856 Part D contracts is 8,560 hours (1 Software Quality Assurance Analyst and Tester x 10 hours an analyst/tester x 856 Part D Contracts).
Thus, the total cost is $804,126 (8,560 hours x $93.94 wage/hr)
On average, for each of the 856 Part D Contracts, 1 Database Administrator (DBA) working at $92.84/hr spend 10 hours performing system maintenance with an aggregate per contract dollar burden of $794,710.
Based on internal CMS data, there are about 856 Part D Contracts. The burden of update requires that 1 DBA will spend 10 hours performing necessary redesigns. Therefore, the aggregate burden across all 856 Part D contracts is 8,560 hours (1 DBA x 10 hours a DBA x 856 Part D Contracts).
Thus, the total cost is $794,710 (8,560 hours x $92.84 wage/hr)
On average, for each of the 856 Part D Contracts, 1 Computer Systems Analyst working at $98.28/hr spend 10 hours performing system maintenance with an aggregate per contract dollar burden of $841,277.
Based on internal CMS data, there are about 856 Part D Contracts. The burden of update requires that 1 Computer Systems Analyst will spend 10 hours performing necessary redesigns. Therefore, the aggregate burden across all 856 Part D contracts is 8,560 hours (1 Computer Systems Analyst x 10 hours an analyst x 856 Part D Contracts).
Thus, the total cost is $841,277 (8,560 hours x $98.28 wage/hr)
Total Costs: The total cost for all 856 Part D Contracts is $6,423,595 ($3,983,482 + $804,126 +
$794,710 + $841,277) (see Table 3 below).
Table 4: One-Time Burden Estimates – Expansion of PDE File Layout and Addition of PDE Fields
Estimated Number of Respondents |
Burden per Response (hours) |
Total Annual Burden (hours)/contract |
Wages / hr ($) |
Total Estimated Labor Cost ($) |
856 |
40 |
34,240 |
116.34 |
3,983,482 |
856 |
10 |
8,560 |
93.94 |
804,126 |
856 |
10 |
8,560 |
92.84 |
794,710 |
856 |
10 |
8,560 |
98.28 |
841,277 |
Total |
|
|
|
6,423,595 |
Information Collection/Reporting Instruments and Instruction/Guidance Documents
PDE Guidance on how to complete and submit a PDE. No changes were made to this guidance. (Available at: https://csscoperations.com/internet/csscw3.nsf/DIDC/GZEB 9OUQJ9~Prescription%20Drug%20Program%20(Part%20D)~References)
Revision to Previous Guidance Titled ‘Timely Submission of Prescription Drug Event (PDE) Records and Resolution of Rejected PDEs provides guidance on the timeframes for submitting a PDE.” No changes were made to this guidance. (Available at: https://www.hhs.gov/guidance/sites/default/files/hhs-guidance- documents/hpms_memo_pde_timeliness_clarification_68.pdf)
The following memorandum released on October 14, 2022, addresses how POS- remuneration must be applied and reported both through 2023 and from 2024 onwards and upcoming changes to PDE layout in 2024. This includes a change to the definition to field 40 and the addition of a new dollar amount field in 2025. Footnotes in the Memo explain the changes. No changes have been made to this guidance. “Reporting Estimated Remuneration Applied to the Point-of-Sale Price” (Available at: https://www.cms.gov/files/document/erposamemo508g.pdf)
“PDE Reporting Instructions for Implementing the Cost Sharing Maximums Established by the Inflation Reduction Act for Covered Insulin Products and ACIP-Recommended Vaccines for Contract Year 2023” released on September 26, 2022. (Available at: https://www.cms.gov/httpseditcmsgovresearch-statistics-data-and-systemscomputer-data- and-systemshpmshpms-memos-archive/hpms-memos-wk-5-september-26-30)
“New 2025 Prescription Drug Event (PDE) File Layouts (FINAL)” released on April 18, 2023, describes, after consideration of comments on the draft guidance, the final PDE file expansion and new file layout for contract year 2025. (Attached)
The MMA requires Medicare payment to sponsors of plans offering coverage of prescription drugs under Medicare Part D. The Act provided four summary mechanisms for paying plans:
direct subsidies
subsidized coverage for qualifying low-income individuals
federal reinsurance subsidies
risk corridor payments
The IRA mandates that Medicare pay Part D sponsors a temporary retrospective subsidy (hereinafter referred to as “IRA subsidy amount” or “IRASA”) for the reduction in cost sharing and deductible for contract year 2023, which must equal the difference between the beneficiary cost sharing for covered insulin products or ACIP-recommended adult vaccines under a Part D plan’s 2023 benefit design (submitted to CMS prior to the passage of the IRA) and the applicable statutory maximum cost sharing limit created by the IRA.
The IRA also mandates the Selected Drug Subsidy Program, in which Medicare pays Part D sponsors the portion the manufacturer would otherwise pay under the MDP in the initial phase of the benefit for the years an MFP applies for the drug.
In order to make payment in accordance with these provisions, CMS has determined to collect a limited set of data elements for 100 percent of prescription drug claims or events from plans offering Part D coverage. In determining these requirements, we have incorporated feedback from industry and other stakeholders obtained by formal and informal means including the rulemaking process, Open Door Forums and other consultation. We used four criteria in selecting the required data elements:
ability to pay plans timely and accurately;
minimal administrative burden on CMS, Part D sponsors, PBMs, pharmacies, and others;
legislative authority; and
validity and reliability of the data elements requested, to ensure that the information will
be useful.
The requirements for submitting PDE data provide that much of the data, especially financial fields, will be used primarily for payment. However, other data elements will be used for validation of the claims as well as for other functions such as quality monitoring, program integrity, and oversight.
We require that plans submit a PDE record for each dispensing event. The PDE record is a summary record that documents the final adjudication of the dispensing event. Since the pharmacy industry has an effective drug claims submission standard, which is electronically automated, we use the NCPDP version D.0 as the data format for PDE submissions, and we have added fields in anticipation of future updates to the NCPDP Telecommunications standard. PDE elements include data elements from the NCPDP billing transaction, data elements from the NCPDP billing response transaction, and CMS-defined data elements. Although a number of the statutory requirements of the Act are not available in the NCPDP data elements, we utilized the NCPDP format to construct the CMS-defined data elements to ensure minimal burden on plans.
The PDE File Layout that will reflect changes prior to 2025 implementation is available at https://csscoperations.com/internet/csscw3.nsf/DID/M7XCJKG0JI.
Collection of Prescription Drug Event Data
As a condition of payment, all Part D plans must submit data and information necessary for CMS to carry out payment provisions. Much of the data, especially dollar fields, will be used primarily for payment. However, some of the other data elements such as pharmacy and prescriber identifiers will be used for other legislated functions such as quality monitoring, program integrity, and oversight.
Every time a beneficiary fills a Part D prescription, plan sponsors must submit a summary record called the Prescription Drug Event (PDE) record to CMS. The PDE data is an extract of information from claims made by beneficiaries purchasing prescription drugs that are covered under Part D. The PDE record contains prescription drug cost and payment data that will enable CMS to make payment to plans and otherwise administer the Part D benefit. Specifically, the PDE record includes covered Part D drug costs above and below the out-of-pocket (OOP) threshold; distinguishes supplemental benefits from benefits provided under basic prescription drug coverage; and records payments made by Part D plans, other payers, and by or on behalf of beneficiaries. Plans must also identify costs that contribute towards a beneficiary’s True-Out-Of- Pocket (TrOOP) costs, separated into four categories: low-income cost-sharing subsidy amounts paid by the plan at the Point of Sale (POS), beneficiary payments, reported gap discount amount in the coverage gap phase (for years in which the CGDP applies), and all TrOOP-eligible payments made by qualified entities on behalf of a beneficiary, including IRASA.2 Plans must report the manufacturer discount in the initial and catastrophic phases (for years in which the MDP applies). PDE data also reflect how a plan has administered its Part D benefit package.
CMS uses the data to reconcile low-income cost-sharing subsidy and reinsurance payments, to
2 We define IRASA as the difference between the beneficiary cost sharing for the covered insulin, or ACIP- recommended vaccine, under the plan’s 2023 benefit design, and the applicable statutory maximum cost sharing ($35 for insulins and $0 for vaccines). The IRASA is available only for 2023. Per the statute, the amounts paid by the plan and reimbursed by Medicare through the retrospective subsidy must count as beneficiary incurred costs, meaning these costs count toward meeting the True-Out-of-Pocket (TrOOP) threshold.
implement risk sharing between the plan and the Federal government, to pay the IRASA for contract year 2023 only, and to pay the subsidy under the Selected Drug Subsidy Program. CMS also uses the data for the drug price negotiation process and the determination of the MFP for drug price negotiation-eligible drugs and Part D inflationary rebates, as well as to fulfill obligations or operationalize any future legislative changes to the Social Security Act that impact the Part D program.
In most situations, the Part D sponsor or a designated third-party processor on its behalf processes the claim that has been submitted electronically by the network provider (e.g., pharmacy or physician office) and determines the applicable cost sharing to be made by the beneficiary. Typically, the network providers provide billing transactions to plan sponsors in real-time and the claims processor can file the information to CMS promptly. In a limited number of situations, a beneficiary or other entity may submit a non-standard format claim such as a paper claim to a plan sponsor or its third-party processor. The plan/processor then creates a PDE record from the claim to submit electronically to CMS in a non-standard format (for example, there are special rules and exceptions for populating certain non-financial data elements).
The PDE record contains prescription drug cost and payment data that enable CMS to make payment to plans and otherwise administer the Part D benefit. Specifically, the PDE record includes covered drug costs above and below the OOP threshold; distinguishes enhanced alternative costs from the costs of drugs provided under the Basic Benefit; and records payments made by Part D plans, other payers, beneficiaries, or individuals on behalf of a beneficiary. Plans must also identify costs that contribute toward a beneficiary’s TrOOP limit.
Many electronic transactions take place between plans, pharmacies, and intermediaries when an enrollee fills a prescription. This process allows determination of patient cost-sharing at the POS by plan adjudication of the claim, and drives eventual plan payment to the pharmacy. The PDE record contains information that is vital for payment, quality oversight, and program integrity.
For each dispensing event, the plan sponsor must submit a PDE record. Most sponsors use a PBM or other third-party administrator to process incoming claims from pharmacies. Claims typically undergo several rounds of transactions between these parties before the plan sponsor finally adjudicates a claim for payment. The PDE is a summary record that documents the final adjudication of a dispensing event.
The following is an illustration of the PDE data flow:
Figure 1. PDE Data Flow
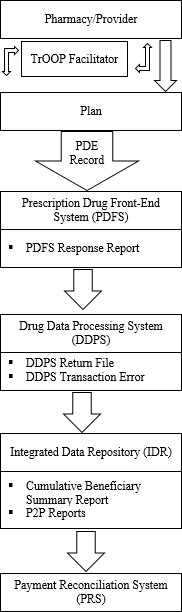
The following is a checklist of the PDE data flow:
The pharmacy, physician, or other provider submits a claim to the Part D plan sponsor.
If necessary, the pharmacy generates a secondary claim to any other payers via the TrOOP facilitator.
The Part D Plan submits data to CMS via the PDE record.
The Part D Plan successfully submits PDE records at least once a month to PDFS.
The PDE records are sent to PDFS where front-end edits are applied.
The Prescription Drug Front-End System (PDFS) response report indicates file acceptance or rejection. If any PDE records fail front-end edits, PDFS reports the failure on the PDFS Response Report.
After passing the PDFS checks, the file is submitted to Drug Data Processing System (DDPS) where detail editing is performed.
The DDPS Return File is returned daily and shows the disposition of all DET records and where errors occurred.
The DDPS Transaction Error Summary displays the count and rate for each error code found in the submitted data.
The Integrated Data Repository (IDR) sums LICS and calculates unadjusted reinsurance and risk corridor costs.
Management reports are generated in the IDR and provide a summary of net accumulated totals for all dollar fields.
Payment Reconciliation System (PRS) creates a beneficiary/plan record for each beneficiary enrolled in a plan during the payment year and calculates reconciliation payments at the beneficiary and plan level.
Any administrative and/or capital costs incurred will be recouped through the bidding process and secured through the reinsurance and/or risk corridors processes. The average number of Part D contracts per year is 856 (based on 2019, 2020, and 2021 data). These entities have sufficient capital assets in place to address reporting drug data. MA-PD plans also have sufficient capital assets in place to address drug data reporting.
CMS has estimated that the total cost of the PDE data submission activities utilizing the PDFS and DDPS will be approximately $13 million.
Table 5: Total Cost of PDE Data Submissions
|
PDFS |
DDPS |
Labor |
$1.8 Million |
$8.8 Million |
Infrastructure (or other Direct Costs) |
$1.4 Million |
$1.0 Million |
Total |
$3.2 Million |
$9.8 Million |
Active Collection of Information and Associated Burden Estimates (Adjusted)
Our active average number of Part D contracts per year was 739 (based on 2017, 2018, and 2019 data). In this 2023 iteration we have updated that figure to 856 (based on 2019, 2020, and 2021 data). The average number of PDE submissions per year decreased; the adjustment decreases our response figure by minus 173,310 responses (from 1,499,238,090 to 1,499,064,780).
Table 6: Associated Burden Estimates
|
No. Respondents |
Total Responses |
Total Annual Time (hr) |
Total Labor Cost ($) |
Active Burden |
739 |
1,499,238,090 |
2,998 |
53,215 |
Updated Burden |
856 |
1,499,064,780 |
2,998 |
53,215 |
Total Adjustment |
+117 |
-173,310 |
0 |
0 |
One-Time Burden Estimates Resulting from Expansion of the PDE File Layout and Addition of PDE Fields (New)
CMS will expand the PDE file layouts from their current 512-byte length to 1000 bytes, to be implemented effective January 1, 2025. This expansion is being implemented to accommodate future business needs, including but not limited to, updates necessary for the IRA and anticipated future updates to the NCPDP Telecommunications Standard. In addition, the lengths of some of the existing fields on the PDE file layouts will be increased, which requires a reorganization of the overall location of fields on the PDE file layout. We anticipate that this change would require Part D sponsors to make system changes related to updating the systems needed to accommodate the expanded layout and new fields on the PDE data collection form.
Table 7: Summary of Information Collection Requirements and Associated Burden Estimates
Citation |
Item |
Respondent |
Number of Respondents |
Number of Responses |
Total Responses |
Time per Respondent (hours) |
Total Time (hours) |
Total Estimated Labor Cost ($) |
Total Cost First Year ($) |
§§ 1860D-11(g)(5), 1860D-12(b)(3)(D), 1860D–14, 1860D-15, 1860D-22, 1860D- 14A(c)(1)(C), 1860D- 14C(c)(3), and 1860D– 14D of the Act |
Active Collection of Information (Adjusted) |
Part D contracts |
856 |
1,499,06 4,780 |
1,499,064,780 |
0.000002 |
2,998 |
53,215 |
53,215 |
§§ 1860D-11(g)(5), 1860D-12(b)(3)(D), 1860D–14, 1860D-15, 1860D-22, 1860D- 14A(c)(1)(C), 1860D- 14C(c)(3), and 1860D– 14D of the Act |
One-Time Burden Expansion of the PDE File Layout and Addition of New PDE Fields (New) |
Part D contracts |
856 |
856 |
856 |
70 |
59,920 |
various |
6,423,59 5 |
No requirements or documents have been revised nor have any burden estimates been revised as a result of the comments received on the 60-day notice.
The purpose of this data submission request is to support the payment of outpatient prescription drugs for beneficiaries who are members of Part D plans and who receive services under the Medicare Part D benefits program. There are no publication and tabulation dates.
The expiration date is displayed within the PRA Disclosure Statement and can be found on Reginfo.gov under OMB Control No. 0938-0982 (available at https://www.reginfo.gov/public/Forward?SearchTarget=PRA&textfield=0938- 0982&Image61.x=12&Image61.y=20).
CMS has no exceptions to Item 19, “Certification for Paperwork Reduction Act Submissions” of OMB Form 83-I.
Requirements for this data collection do not employ statistical methods.
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| File Title | CMS-10174 - CLEAN Supporting Statement A_PDE expansion and IRA |
| Author | CMS |
| File Modified | 0000-00-00 |
| File Created | 2023-07-29 |
© 2026 OMB.report | Privacy Policy