Annual review and product listing update of registration
Establishment Registration and Product Listing for Manufacturers of Human Blood and Blood Products and Licensed Devices
CBER On-Line Login Page
Annual review and product listing update of registration
OMB: 0910-0052
Document [docx]
Download: docx | pdf
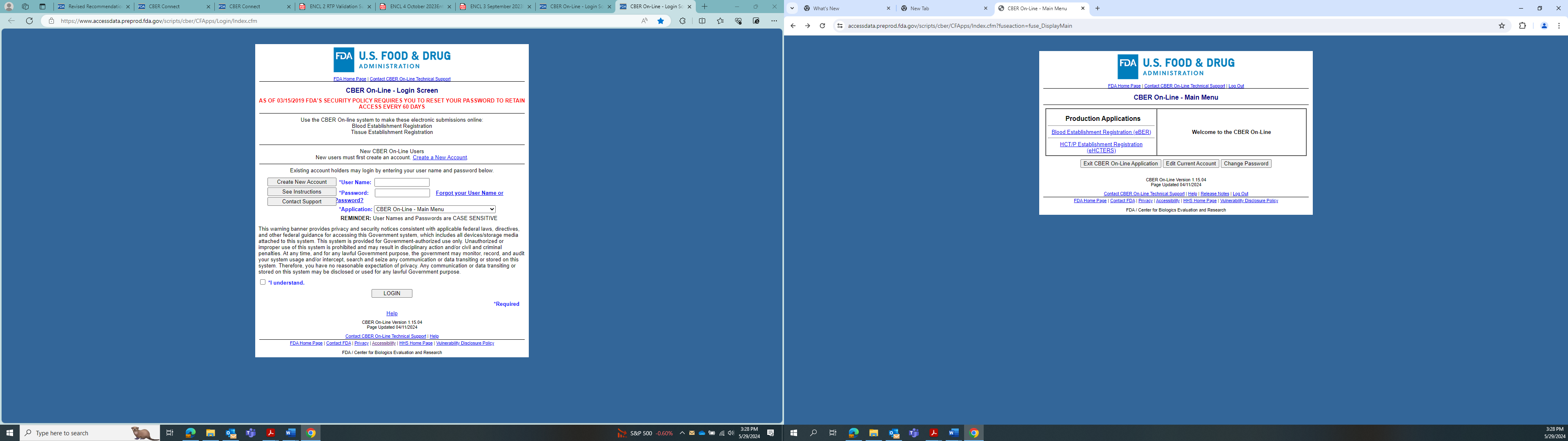
After user has logged in using username and password
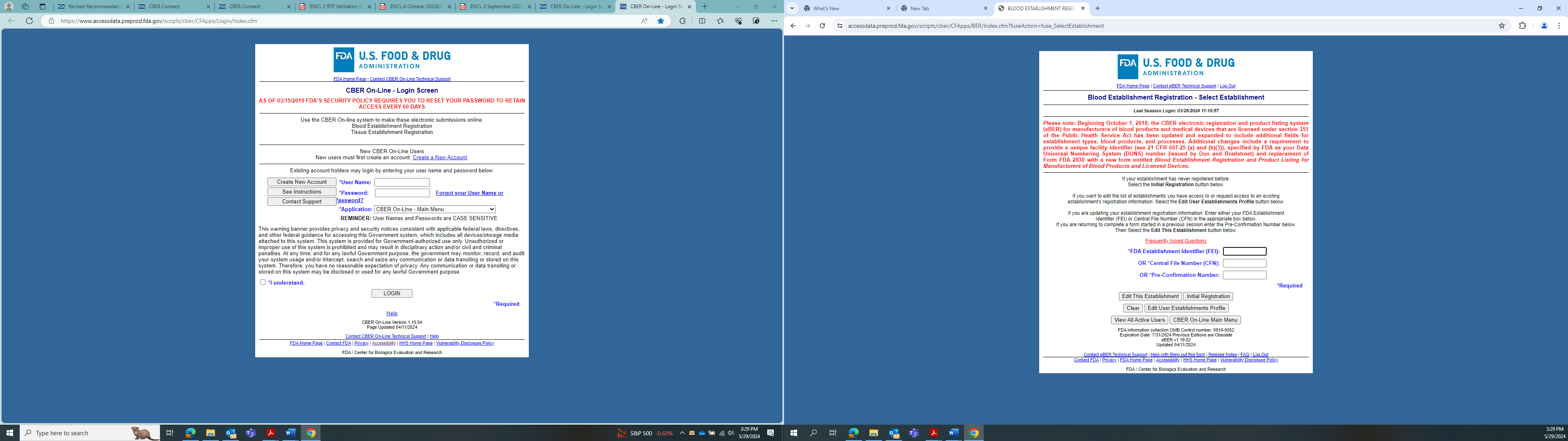

OMB Control Number and expiration date visible on page prior to user entering FEI Number associated with their establishment.
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | Perry, Jaime |
| File Modified | 0000-00-00 |
| File Created | 2024-07-21 |
© 2026 OMB.report | Privacy Policy