CAHPS Inspection Guideliness
CAHPS Inspection Guidelines_Version1.1_Jan2023.docx
Comprehensive Aquaculture Health Program; Use of MI-CO Application
CAHPS Inspection Guideliness
OMB:
Comprehensive Aquaculture Health Program Standards (CAHPS)
CAHPS Program Guidance
Decision Trees
USDA APHIS VS Strategy and Policy
17 Jan 2023

Table of Contents
Introduction
Guiding Principles
Premises and Population Definitions
CAHPS Achievements
CAHPS Pillars
Early Detection Systems (EDS)
Official Surveillance (OS)
Pathogen-based Reductions in Sampling
Premises Freedom (PF) Status for Named Pathogens
Risk-based Reductions in Sampling
(pending) Considerations for Zones
Decision Support Tools
Checklists
Decision Trees and Tables
Introduction
The new National Aquaculture Health Plan & Standards (NAHP&S) provides guidance on official health inspection options including the Comprehensive Aquaculture Health Program Standards (CAHPS). NAHP&S provides a framework for facilities wishing to obtain official recognition of a strong health infrastructure and risk-based confidence in health inspections. Premises participating in CAHPS implement five “CAHPS pillars” which work to protect and support livestock health through systems for risk evaluation and mitigation, early disease detection, aquatic animal health oversight, communication, and response, as well as surveillance strategies. CAHPS premises that also have a 2+ year history of compliance with these pillars, evidence to support named pathogen absence, and strong risk mitigations, may be eligible for CAHPS Global - Premises Freedom status for named pathogens, and reductions in traditional sampling via risk-based testing.
This guidance describes criteria that State or Federal Authorities or their designates may use to verify CAHPS compliance and pathogen-specific health status attestations. CAHPS participants may propose different methods better suited to their premises, aquatic animal species, life stage and production type, and pathogen(s) of concern, to the APHIS Aquaculture Health Team for consideration. However, the following guidelines are meant to provide uniform benchmarks.
This guidance document is intended to support CAHPS field inspections. However, it can also support design of premises-specific biosecurity, surveillance, and disease response plans that will help Premises achieve their stated aquatic animal health and CAHPS inspection goals. It is arranged in two parts.
Guiding Principles. This section provides background and supporting details for key concepts (e.g., CAHPS pillars, early detection systems, surveillance, risk evaluation) used in CAHPS assessment. It also links to corresponding Decision Support Tools.
Decision Support Tools. This section outlays decision trees and tables meant to standardize and ease the field inspection process. The tools are color-coded by type: blue for decision tools to guide the site visit, green for decision tools to accompany laboratory consult, and orange for tools that will use gathered results to generate sampling, status, or related recommendations. A mobile app (under development) will navigate data collection, automate resulting decisions, and track key data and recommendations for CAHPS status reports.
1. Guiding Principles
1.1 Population Description
This CAHPS inspection guidance is designed to evaluate individual premises. However, a single management may oversee multiple systems holding aquatic animal groupings with different certification needs. If epidemiologically linked (i.e., connected in ways that might allow pathogen exchange), these groupings are considered a single population with a uniform classification, and health status, under CAHPS. If epidemiologically distinct (i.e., separated in ways that prevent pathogen exchange), these groupings are considered separate populations, each with its own classification and health status (independently sampled, tested, assessed) under CAHPS. The Population Description decision tree (Figure 1) lays the foundation for all subsequent CAHPS assessments. It relies on the following information that is to be maintained by the Aquatic Animal Health Team (AAHT), and available for review to support CAHPS participation.
Descriptions (and maps) of premises structures, including
Property location and boundaries
Locations and types of structures on the property
Locations, numbers, and types of aquaculture systems on the property
Number and type of holdings in each system
Direction of water, personnel, and (if applicable) vehicle, flow throughout the premises
Nearest facilities or waterbodies with similarly susceptible aquatic animal populations
Descriptions of animal populations, including
Aquatic animal species on the property
Life-stages of each species on the property
Physical groupings (by structure and system) of each species/life-stage on the property
Additional information that distinguishes groupings (e.g., year-class, parentage, or end-use)
Pathways for pathogen exchange, including
Linkages between aquatic animal groupings on the premises
Linkages with aquatic animal groupings on other premises (i.e., contact networks)
1.2 CAHPS Levels
Following enrollment, three CAHPS participation options are available (Diagrams 1-3). These options are designed to support a variety of aquaculture structures and business objectives.
(1) CAHPS-Farm
(2) CAHPS-National
(3) CAHPS-Global, including the option for Premises Freedom status
CAHPS Farm is structured to support premises interested in program participation for marketing or health management improvement purposes. CAHPS National is structured to support varied certification demands of inter-state trade requirements. CAHPS Global is structured to support the most rigorous demands of international trade partners, including premises freedom declarations at 95% confidence and 2% detection prevalence levels.
Each level demands annual inspection and continuous implementation of all 5 CAHPS Pillars. However, surveillance and biosecurity expectations vary by CAHPS level, business objective and pathogens of concern. CAHPS enrollees can achieve any given level via a standard or grandfather track. Assurances from the standard enrollment track derive from two trial years of CAHPS, or equivalent, involvement. The grandfather enrollment track is a fast track designed for premises with a strong health history and pre-existing relationship with an APHIS-accredited veterinarian.
The logic guiding inspections, and related decision support tools, is outlined in the following sections.
Section 1.3 CAHPS Pillars
Section 1.4 Early Detection Systems
Section 1.5 Official Surveillance
Section 1.6 Pathogen-based Reductions in Sampling
Section 1.7 Premises Freedom Status (for named pathogens)
Section 1.8 Risk-based Reductions in Sampling
(pending) Section 1.9 Considerations for Zones

Diagram 1. CAHPS Farm requirements and achievements. Structured to support premises interested in program participation for marketing or health management improvement purposes.

Diagram 2: CAHPS National requirements and achievements. Structured to support varied certification demands of inter-state trade requirements.

Diagram 3: CAHPS Global requirements and achievements. Structured to support the most rigorous demands of international trade partners, including premises freedom declarations at 95% confidence and 2% detection prevalence levels.
1.3 CAHPS Pillars
Who/What?
All CAHPS Premises must implement each of the five CAHPS Pillars (Figure 2).
Aquatic Animal Health Team (AAHT)
Risk Evaluation (Figure 3) and Mitigation (Figures 8A-8E)
Surveillance, General Tenets1 (Figures 4-7)
Disease Investigation and Reporting
Response and Recovery
Why?
CAHPS Pillars are the foundation for CAHPS health management strategies and assertions. They ensure the awareness, readiness, and relationships necessary to prevent, assess, and respond to pathogen threats. They also provide the oversight, surveillance history, and structure critical for health verification and credibility of health verification claims.
How?
The site-specific details of each pillar are designed by the Aquatic Animal Health team. Figure 2 provides an overview of the CAHPS Pillars. Additional functionality is addressed in Figures 3-8.
1.4 Early Detection System (EDS)
Who/What?
EDSs are required of all CAHPS participants, regardless of level or track. EDS is producer-led and AAHT-designed to detect general changes in population health. Based on routine observations or screenings, EDS is typically supervised by the producer under the direction of the AAHT. EDSs are NOT affected by pathogen or risk-based reductions. Rather they are maintained as a continuous surveillance foundation.
Why?
EDS capitalize on low-cost, routine points of contact between animals and staff to gather information that might deviate from normal, and signify a problem, if a pathogen were introduced. Though typically designed around known pathogens, EDS are also often general enough to signal the introduction of new or emerging pathogens. A key is their frequent or time-targeted, reflexive application. Benefits include:
Early detection affords early response (while the disease is relatively contained), potentially reducing long-term impacts to the farm, region, and trade relationships.
EDS can detect known, as well as emerging, pathogens.
CAHPS Participants with Premises Freedom status may use EDS results to bolster confidence in premises freedom, potentially reducing Official Surveillance (OS) testing requirements.
How?
Figures 5 & 6 guide assessment of the sufficiency of EDS.
There are two types of EDS: observational (Figure 5) and screening (Figure 6). Observational EDS monitors for changes in mortality and morbidity rates, feed consumption, growth, appearance, swimming, or other behaviors. Trained observers monitor key traits (in-person or via remote technology), and report immediately to management any that exceed pre-defined thresholds. Unexplained deviations above threshold trigger prompt investigation by an aquatic animal health professional and qualified laboratory.
Pathogens unlikely to manifest clinically need a different approach. Screening EDS, like observational, is farm-led. Also known as routine moribund sampling, it consists of periodic submission of moribunds, even when morbidity/mortality levels are below investigation thresholds. Sampling may coordinate with routine tasks (mortality collection) or events requiring extra staffing (e.g., grading or spawning). Tests typically screen for multiple pathogens (e.g., necropsy, histology, culture, multiplex assays), and may incorporate new approaches to disease detection, (e.g., telemedicine or environmental testing). Again, unexplained findings trigger formal investigation by a health professional and qualified laboratory.
Though designed for early detection, CAHPS-National and Global premises may use EDS alongside OS for confidence substantiation (Figure 12, Pathogen-based Reductions), assuming: (1) the pathogen must be detectable by the system described, (2) the frequency and extent of “sampling” (e.g., timing and units observed or screened) must be captured2, and (3) positive and negative results must be tracked.
1.5 Official Surveillance (OS)
Who/What?
Official, a.k.a. Active, Surveillance (OS) is required for all CAHPS-National and CAHPS-Global participants. OS is NOT required for CAHPS-Farm participants. OS is investigator-led, regularly scheduled, and geared to detect specific pathogens present at or above selected prevalence thresholds (a.k.a., design prevalence, DP). OS uses approved laboratories, approved assays, and standardized testing methods (e.g., APHIS VS Sampling and Pooling Guidance). Under CAHPS, OS is conducted at least twice per year, sampling is supervised by an AAHT-designated veterinarian or aquatic animal health professional and testing for WOAH and NLRAD pathogens is conducted at an APHIS-approved laboratory or laboratory compliant with ISO testing standards for the named pathogen. Pending official channels for non-export approvals, CAHPS-National participants may use laboratories and assays approved by the Diagnostic Working Group considering other criteria such as history, publications, or internal validation studies.
Why?
OS is a common source of data for health assertions related to trade. Typically based on pathogen-specific laboratory tests with known or presumed high accuracy, OS can detect pathogens present at low levels, with or without clinical symptoms. However, as it is resource-intensive and intermittent, OS is typically NOT well-designed for early detection. As such, OS should always be coupled with EDS.
How? Figure 7 assesses the sufficiency of OS.
OS tests a sample (versus census) of animals from the population of interest (Figure 4). Sampling is often distributed in proportion to size of key population groupings (e.g., by species, age, or water system) and sub-groupings. However, if sub-groupings (e.g., moribund animals, or certain cages) are of heightened susceptibility, exposure risk, or consequence, their targeted sampling is appropriate and encouraged.
OS assay selection is determined in consult with the APHIS-approved laboratory responsible for testing. Selected assay(s) should have high (e.g., ≥ 85%) diagnostic sensitivity, and the laboratory should meet expectations described by the National Aquaculture Health Plan and Standards (NAHP&SP). Methods for tissue selection, processing, pooling, and shipping are set in consultation with the selected laboratory.
CAHPS-Global participants typically design OS to maintain 95% confidence about 2% DP in keeping with WOAH’s Aquatic Animal Code defaults and common expectations for international trade. This means twice per year assessment of 175 animals (Table 1), or higher number if pooling (Table 2), assuming an un-pooled diagnostic test sensitivity of 85% or better (Figure 9), which is also adjusted if pooling; and ensures 95% probability of detection should the pathogen(s) occur at or above 2% prevalence. CAHPS-National status allows flexibility for higher DP targets in keeping with inter-State trade requirements.
Confidence statements applied to individual surveys (e.g., lot-based, movement, or batch testing) are transient. Premises Freedom is a longer-running status, due to added demands that introduction pathways are managed or secure (Figure 8). This heightened security affords CAHPS-Global participants eligibility for risk-based refinements to OS (Section 1.8) without impact to Premises Freedom claims. CAHPS-Global participants may also consider pathogen-based reductions (Section 1.6). CAHPS-National participants, with fewer requirements for introduction pathways, are eligible for pathogen-based but not risk-based reductions. All modifications to OS apply only to the post-enrollment period.
1.6 Pathogen-Based Reductions in Sampling
Who/What?
CAHPS-Global participants are eligible for pathogen-based reductions in sampling following their enrollment period. This is also true of CAHPS-National participants using confidence (e.g., 95% confidence about 2% DP), rather than counts (e.g., 60 animals), as the output for their sampling designs. Pathogen-based reductions fine-tune OS sample sizes to adjust for information gained through EDS.
As the quality of EDS data depends heavily on producer and animal health professional cooperation, this option is only available to premises that have established trust through 2+ years of CAHPS compliance. Note that pathogen-based sampling reductions do not apply to EDS, but rather rely on continuous EDS.
Why?
Convention dictates that premises freedom declarations should rest on repeated rounds of OS. While sensible during initial stages of health evaluation, the expense of testing multiple pathogens separately is often unsustainable. Further, because OS is typically based on pathogen-specific tests and is designed to find pathogens explicitly on its radar and testing schedule, it may miss those that are new or emerging, or those that are introduced between scheduled surveys. Instead, CAHPS supports a re-balancing of surveillance for active participants: shifting some emphasis away from recurrent individual pathogen surveys, and towards early detection systems that are more general and responsive. By crediting EDS and revising (where appropriate) DP targets, CAHPS-National and Global participants can improve efficiency3 and performance of their surveillance systems.
How?
Figures 10-13 calculate pathogen-based reductions.
Observational EDS Credit (Figure 10),
Screening EDS Credit (Figure 11), and
Official Surveillance Test Balance (Figure 12).
Optimally balanced surveillance, employing all three systems (Observational EDS, Screening EDS, OS), can reduce sampling burden while improving surveillance capacity. A rule of thirds4 simplifies their ideal contributions to health oversight. When appropriate (Figures 10, 11), CAHPS allows Observational and Screening EDS to each contribute up to 1/3 the surveillance evidence desired per pathogen. The balance is attributed to OS (note, pooling may alter the balance per Tables 1,2). Methods are shown in Figures 11 &12. Screening EDS credit requires advance formal approval through AAHT consult with APHIS ASEP.
Premises Freedom (PF) Status for Named Pathogens
Who/What?
This section describes requirements for premises aiming to achieve or maintain CAHPS-Global status. A core component is the Pathways Assessment Tool which assesses risk mitigation options to support freedom claims, as well as eligibility for risk-based sampling (Section 1.8).
Why?
Premises Freedom claims build on a 2+ year running history of named pathogen absence defined by twice annual surveillance assessments achieving 95% confidence re 2% design prevalence. However, Premises Freedom status also requires reason to believe the named pathogen(s) will remain absent into the future. Premises Freedom status thus demands demonstration that the premises has (1) met all requirements of a CAHPS-Global standing (including OS and continuous EDS), (2) effectively managed pathogen introduction risks, and (3) designed ongoing (risk-based) surveillance to maintain confidence at 95% through time.
The Pathways Assessment Tool provides a means to rapidly gauge pathogen introduction risks on aquaculture premises. Results support biosecurity, premises freedom, and risk-based sampling evaluations and designs. The Pathways Assessment Tool provides both qualitative and quantitative appraisals, and as such will meet the needs (for biosecurity design and/or risk estimation) of many CAHPS Participants. However, CAHPS Participants may also, or instead, choose to periodically conduct a formal risk assessment for more detailed advice.
How?
Figure 8a-8e decision trees form the Pathways Assessment Tool. Figures 13 and 14 apply the results toward (named pathogen) Premises Freedom status.
The Pathways Assessment Tool is a series of decision trees which categorize mitigations for key introduction pathways: water, animal movement, cohort separation, vectors (non-human), vectors (human), feed, and fomites. The decision trees grade context and biosecurity practices to gauge each pathway’s degree of mitigation of risk. Figure 13 calculates a Risk Mitigation Score from the results. Figure 14 and Table 3 use this Score to determine the site’s suitability for (named pathogen) Premises Freedom designations5, and (per Section 1.8) risk-based sampling.
Risk-Based Reductions in Sampling
Who?
CAHPS-Global participants are eligible for risk-based reductions in OS sampling. Suitability for risk-based reductions on a premises is pathogen-specific: reductions may apply to certain pathogens but not others. Note that risk-based reductions do NOT apply to EDS, but rather depend on continuous operation of EDS (and other CAPHS pillars, including biosecurity to mitigate introduction risks).
Why?
CAHPS compliance coupled with risk mitigation safeguards population health, affording historical testing retained value through time. Allowing historical data to retain value in accordance with risk reduces the amount of OS testing needed to maintain (named pathogen) Premises Freedom status in the future. Risk-based savings expand on those already afforded through pathogen-based reductions (Section 1.6). Risk-based reductions in OS sampling benefit CAHPS-Global participants by
reducing the test burden and costs of OS, and
affording flexibility to better tailor surveillance to site-specific concerns, such as early detection of endemic or emerging threats.
Note that risk-based reductions adjust sampling without impacting confidence. For example, premises claiming 95% confidence about a 2% detection prevalence (DP) will maintain that claim whether using baseline or risk-based sampling strategies.
How?
Figure 8 decision trees form the Pathways Assessment Tool. Figures 13, 15, and Table 3 apply the results towards risk-based sampling reductions.
Risk-based OS sampling reductions hinge on estimates of the risks of (named) pathogen introduction. The Pathways Assessment Tool (Figure 8) scores inspection results (Risk Score) for easy conversion to an introduction risk estimate (Figure 13 and Table 3). This estimate then guides risk-based reductions in OS sampling (Figure 15)6,7 for premises rated managed or secure in all pathways. Note that pooling may alter these targets (Tables 1, 2).
Decision Support Tools
This section outlays decision tools designed to support and standardize CAHPS Inspections. A mobile app (MiCO) is under development to aid field navigation of the decision suite and related data collection.
2.1 Checklists
Checklists orient field inspections to their targeted level (CAHPS-Farm, CAHPS-National, or CAHPS-Global). They also indicate the decision tools (Figures and Tables) required at each step.
2.1.1 Prerequisites: CAHPS Enrolled (CAHPS-E) Requirements
To enroll in CAHPS, a premises must have an engaged AAHT and have committed to a CAHPS Level (Farm, National, Global) and track (standard, grandfather). They then have 3 yrs to advance to targets.
2.1.2: CAHPS-Farm Checklist
CAHPS-Farm status is awarded to premises that achieve the following
CAHPS Pillars (Figure 2)
Aquatic Animal Health Team
Risk Characterization and Mitigation (Figure 3)
Surveillance, Early Detection System (Figures 4-6)
Disease Investigation and Reporting
Response and Recovery
History of compliance with the above, provided by EITHER
≥2 years of annual inspections (“standard track”), OR
1 inspection (“grandfather track”), plus ≥2 years collaboration with an APHIS accredited veterinarian
2.1.3: CAHPS-National Checklist
CAHPS-National status is awarded to premises that achieve the following
CAHPS-Farm Requirements
Official Surveillance Sufficiency (Figure 7), see also
Representative Sampling (Figure 4), and
Diagnostic Sensitivity Estimates (Figure 9)
History of compliance with the above, documented by
≥2 years of annual inspections (“standard track”), OR
1 inspection (“grandfather track”), plus
≥2 years collaboration with an APHIS accredited veterinarian, and
≥2 years of active surveillance meeting OS sufficiency standards above
Premises with CAHPS-National Status may opt for
Pathogen-based Sampling Reductions (Figure 12), see also
Observational EDS Credit (Figure 10)
Screening EDS Credit (Figure 11)
2.1.4: CAHPS-Global Checklist
CAHPS-Global status (Figure 14) is awarded to premises that achieve the following
CAHPS-National Requirements
Managed Introduction Risks (MIR)
All pathways rated managed or secure (Figure 8)
History of compliance with the above, documented by
≥2 years of annual inspections (“standard track”), OR
1 inspection (“grandfather track”), plus
≥2 years collaboration with an APHIS accredited veterinarian, vouching for
≥2 years of OS and MIR as described above
CAHPS-Global Premises may also opt for
CAHPS National Achievements, and
Premises Freedom application (see Checklist 2.1.5)
2.1.5: Premises Freedom Checklist
Note that PF status for each named pathogen is assessed separately.
Premises Freedom status (Figure 14) is awarded to CAHPS Global participants that achieve the following
CAHPS-Global Requirements
2+ years of all-negative test results for the named pathogen(s)
Risk Mitigation Score ≥ 2 (Figure 13)
History of compliance with the above, documented by
≥2 years of annual inspections (“standard track”), OR
1 inspection (“grandfather track”), plus
≥2 years collaboration with an APHIS accredited veterinarian, vouching for
≥2 years of OS and MIR as described above
Premises with Premises Freedom status may also opt for
CAHPS Global Achievements, and
Risk-based Sampling Reductions (Figure 15)
2.2 Decision Trees and Tables
Decision tools are provided as a series of Figures and Tables. Decision tool names are color coded to indicate whether they are intended for use by the CAHPS Inspector during the site visit (blue), in advance consult with laboratory or APHIS VS Aquaculture representatives (green), or in the background (orange) as a computational metric.
Decision Tools, Site Visit
Figure 1: Population Description
Figure 2: CAHPS Pillars
Figure 3: Risk Evaluation
Figure 4: Representative Sampling
Figure 5: Early Detection System, Observational
Figure 6: Early Detection System, Screening
Figure 7: Official Surveillance
Figure 8: Pathways Assessment
A: Water
B: Animals
C: Feed
D: Non-human Vectors
E: Fomites and Humans
Decision Tools, Laboratory or APHIS Consult
Figure 9: Diagnostic Sensitivity Estimates
Figure 10: Observational EDS Credit
Figure 11: Screening EDS Credit
Table 1: Baseline Sample Size
Table 2: Pooling-adjusted Sample Size
Decision Tools, Computational
Figure 12: Pathogen-based Sampling Reductions
Figure 13: Risk Score
Figure 14: Premises Freedom Eligibility
Figure 15: Risk-based Sampling Eligibility
Table 3: Risk-based Sample Size
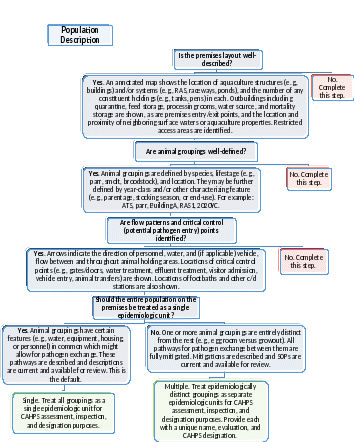
Figure 1: Population Description. Complete this decision tree to determine the number of distinct populations (epidemiologic units) that require separate evaluation and CAHPS designations on the premises.

Figure 2. CAHPS Pillars. This is a high-level overview of critical components of CAHPS health management systems. Whether their design and functionality substantiate CAHPS Farm, National, or Global levels, or Premises Freedom status, is assessed in decision trees that follow.
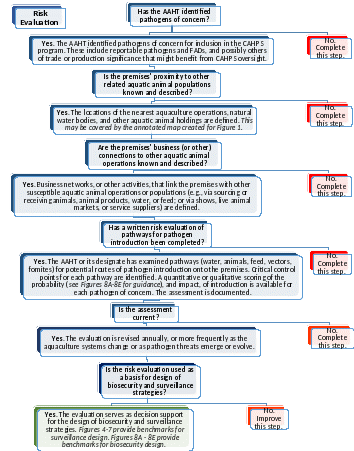
Figure 3. Risk Evaluation. Risk evaluations form the basis for biosecurity and surveillance designs. They are expected of all CAHPS participants.

Figure 4: Representative sampling. Complete this (and linked) Decision Trees to judge whether sampling efforts (observational or test-based) are likely to represent the larger population.
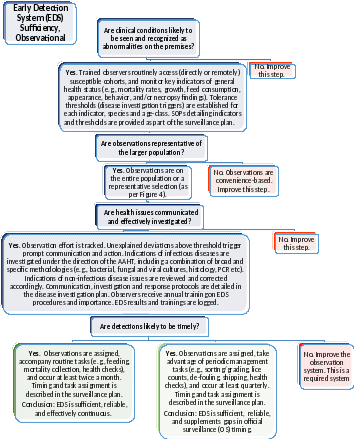
Figure 5: Early Detection System (EDS), Observational. Complete this Decision Tree to assess whether the observational system will contribute to early detection.
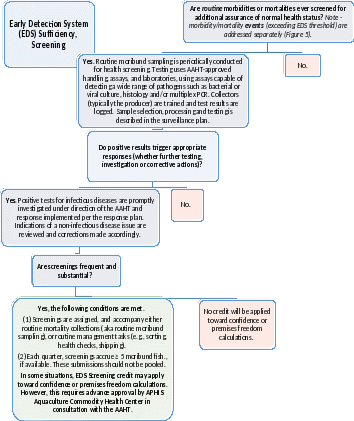
Figure 6: Early Detection System (EDS), Screening. Complete this Decision Tree to judge how well screening contributes to early detection. Green signifies a sound, rapid system. Routine moribund sampling requires fewer animals (they are credited at twice the value of apparently healthy fish) as higher pathogen prevalence can be expected for this subgroup. *If moribunds or fresh mortalities are not available (i.e., the site has few compromised animals), healthy fish are NOT required to meet the balance.

Figure 7: Official Surveillance Sufficiency. Complete this (and linked) Decision Trees to judge the sufficiency of official surveillance. Proxy data (e.g., sentinels, or – for eggs – spawning broodstock) may be necessary.

Figure 8A: Pathways Assessment Tool, Water. Complete this Decision Tree, for each named pathogen, to judge whether water-related introduction risks are effectively managed. If a criterion is met, indicate the water sources to which the criterion applies, describe the mitigations (or situation) involved, list the pathogens mitigated, and provide any justification. Elsewhere we can name water sources by (1) source, (2) structure(s) and systems (+/- holding) supplied, (3) mitigations, and 43) population grouping(s) or sub-grouping(s) supported.

Figure 8B: Pathways Assessment Tool, Animals. Complete this Decision Tree, for each named pathogen, to judge whether animal movement-related introduction risks are effectively managed.
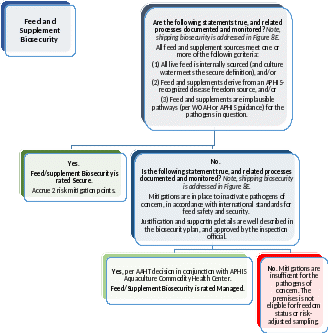
Figure 8C: Pathways Assessment Tool, Feed. Complete this Decision Tree, for each named pathogen, to judge whether animal movement-related introduction risks are effectively managed.
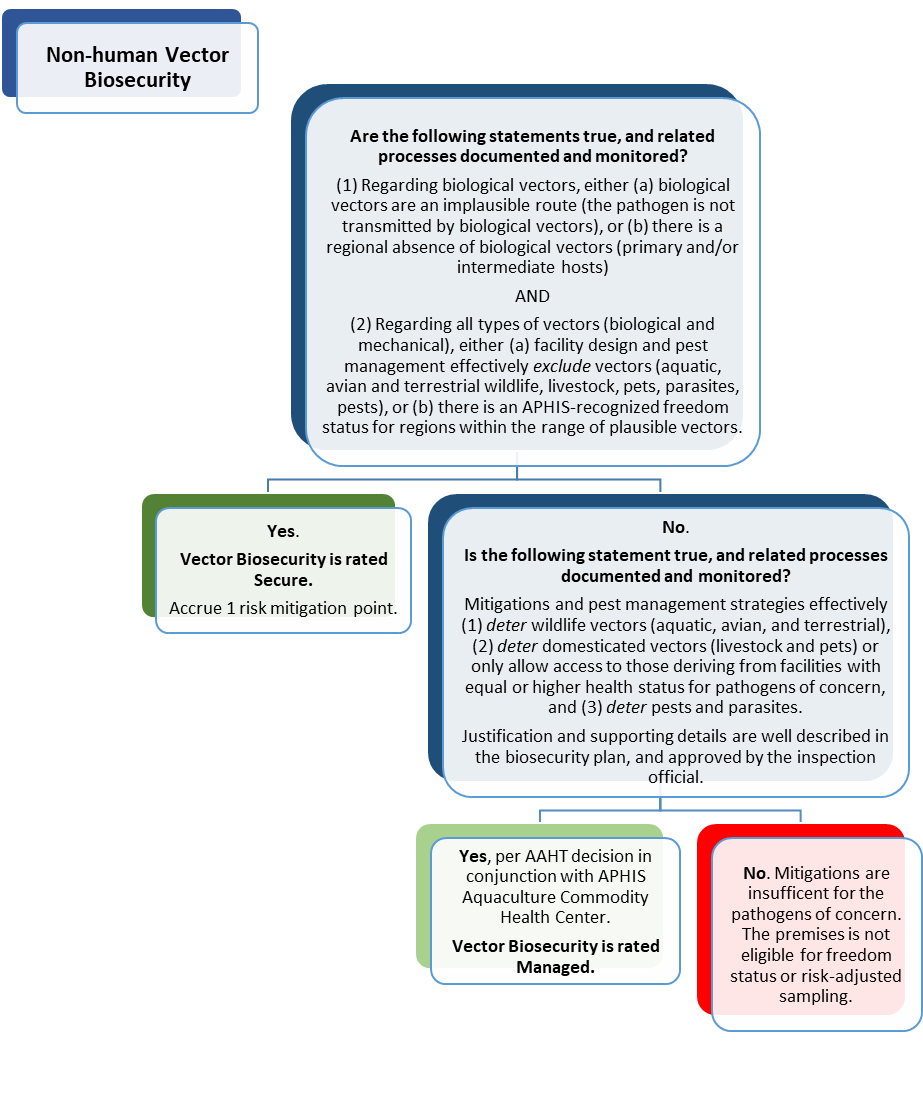
Figure 8D: Pathways Assessment Tool, Non-human Vectors. Complete this Decision Tree, for each named pathogen, to judge whether non-human vector-related introduction risks are effectively managed.

Figure 8E: Pathways Assessment Tool, Fomites. Complete this Decision Tree, for each named pathogen, to judge whether fomite-related introduction risks are effectively managed.
Figure 9: Diagnostic Sensitivity (Se). Complete this Decision Tree to estimate Se for each assay and named pathogen.
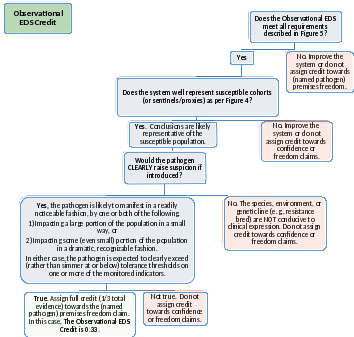
Figure 10: Observational EDS Credit. Complete this Decision Tree, for each named pathogen, to determine whether Observational EDS supports pathogen-based reductions in Official Surveillance sampling. Systems that score green provide 1/3 of the evidence required for (named pathogen) absence or premises freedom status. Pathogen absence refers to the immediate testing result; premises freedom is a longer lasting designation. Any confirmed positives preclude both claims. Highlighting shows information not yet captured in the app.
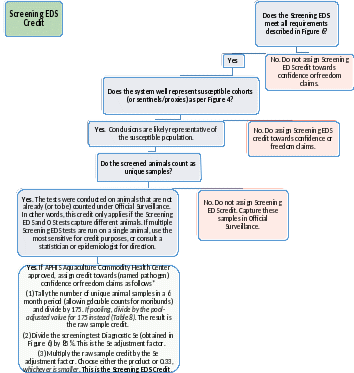
Figure 11: Screening EDS Credit. Complete this Decision Tree, for each named pathogen, to judge whether the Screening EDS supports pathogen-based reductions in Official Surveillance (OS) sampling. Screening systems that score green may provide up to 1/3 (0.33) of the evidence required. Any confirmed positives preclude (named pathogen) absence or premises freedom claims. Pathogen absence refers to the immediate testing result; premises freedom is a longer lasting designation with risk mitigation requirements. Screening EDS credit is capped at 2% DP regardless of the pathogen or CAHPS Participant status. This is intentional and meant to ensure that producer-led sampling supports but does not supersede OS. Highlighting shows information not yet captured in the app.
*These calculations are conservative assuming an original DP of 2% or higher and an original Se of 85% or higher. If the original DP is lower than 2%, the initial target sample size (e.g., 350 for 1% DP and 85% Se) should replace 175 in step 1. Similarly, if the original Se is lower than 85%, that number should replace 85% in step 2. In either case, consider consulting a statistician or epidemiologist to double-check the results.

Figure 12: Pathogen-based Sampling Reductions (aka, Official Surveillance (OS) Test Balance). Complete this Decision Tree, for each named pathogen, to determine new sample sizes for OS, following pathogen-based (DP and EDS) reductions. Using a rule of thirds, Participants supplement (rather than rely solely on) OS to support current pathogen absence or freedom claims. Confirmed positives preclude these claims. Optimally balanced systems gain up to a third of each assessment period’s evidence each from Observational and Screening EDS.
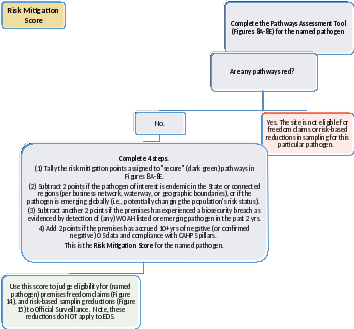
Figure 13: Pathways Assessment Tool, Risk Mitigation Score Calculation. Complete this Decision Tree to calculate a Risk Score for each named pathogen. Results will be used in later sections to judge eligibility for premises freedom designations and risk-based reductions in sampling. Highlighting shows information not yet captured in the app.
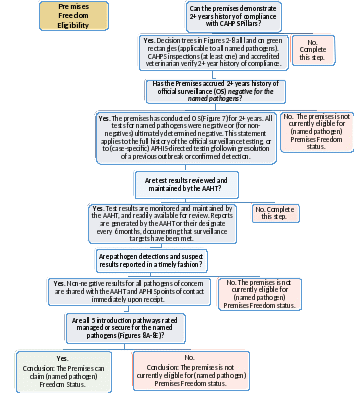
Figure 14: Premises Freedom Eligibility. Complete this Decision Tree, for each named pathogen, to determine eligibility for Active CAHPS Participant (named pathogen) Premises Freedom Status. Red indicates the site is not immediately eligible for Premises Freedom status for the pathogen in question. Highlighting shows information not yet captured in the app.
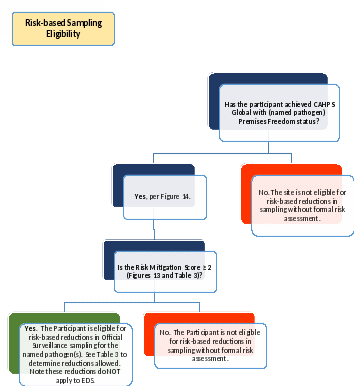 Figure
15: Risk-based Sampling Eligibility. Complete
this Decision Tree and Table 3, for
each named pathogen,
to determine eligibility for risk-based reductions in Official
Surveillance sampling.
Figure
15: Risk-based Sampling Eligibility. Complete
this Decision Tree and Table 3, for
each named pathogen,
to determine eligibility for risk-based reductions in Official
Surveillance sampling.
Table 1: Baseline sample size. Use this Table to estimate baseline Official Surveillance sample sizes for select diagnostic sensitivity (Se, from Figure 9) and design prevalence (DP) combinations. These estimates provide 95% confidence of (named) pathogen absence, for a given DP and test Se, assuming all-negative results, perfect specificity, and an infinite population size. Sample size calculators may provide better situation-specific estimates (https://epitools.ausvet.com.au/freedomss), e.g., for populations < 1000. If pooling multiple animals (or tissues) per test, adjustments to these estimates are required (see Table 2).
Design Prevalence |
100% Se |
85% Se |
80% Se |
70% Se |
50% Se |
25% Se |
1% |
298 |
351 |
373 |
427 |
598 |
1197 |
2% |
149 |
175 |
186 |
213 |
298 |
598 |
5% |
59 |
69 |
74 |
85 |
119 |
238 |
10% |
29 |
34 |
36 |
42 |
59 |
119 |
15% |
19 |
22 |
24 |
27 |
39 |
79 |
25% |
11 |
13 |
14 |
16 |
23 |
47 |
50% |
5 |
6 |
6 |
7 |
11 |
23 |
Table 2: Pooling adjusted sample size. Caution - not all samples and tests are eligible for pooling! Consult your diagnostic laboratory before pooling. If pooling is allowed, the typical pool size is 5. If animals are small and more than 5 are needed to meet the necessary tissue volume for testing, larger pool sizes may be approved. However, diagnostic Se (and thus total animals sampled) needs to be adjusted accordingly. See below for examples (in grey) from APHIS VS Official Sampling and Pooling Guidance for Shrimp Species (2022). Epitool’s pooled sample size calculators (https://epitos2aqzols.ausvet.com.au/ppfreedom, and https://epitools.fp7-risksur.eu/tools/index?toolId=40) can provide estimates8 for other scenarios (assuming pooling is APHIS Aquaculture Commodity Health Center approved, and pooled diagnostic Se is known).
Species |
Baseline Sample Size (Table 1) |
Test Performance |
Example Pool-Adjusted Targets |
||||
Diagnostic Sensitivity un-pooled |
Pool Size: # samples/pool |
Diagnostic Sensitivity, pooled |
Pool Size |
Total Pools |
Total Animals |
||
All |
175 |
≥ 85% |
5 |
85% |
5 |
36 pools |
180 |
|
175 |
≥ 85% |
6-20 |
80% |
Complicated by life-stage combinations. See APHIS Sampling and Pooling Guidance. |
||
Shrimp |
175 |
≥ 85% |
21-50 |
70% |
|||
|
175 |
≥ 85% |
51-100 |
50% |
|||
Table 3: Risk-Based Sample Size. Use this chart to determine risk-based reductions in Official Surveillance sampling achieved for the (named) pathogen. Introduction risk is either estimated directly by formal risk assessment or indirectly by the Pathways Assessment Tool (Figures 8 & 13). Original sample size refers to baseline (e.g., enrollment period) sampling targets or their pathogen-based reduction equivalents. Round risk-based sample size up to nearest whole number. See Talbert et al. (in prep), or Cameron et al. (2014), for the theoretical basis to risk-based sampling. Note sampling reductions do NOT apply to EDS.
Risk Mitigation Score (Figure 13) |
Formal risk assessment required for further consideration? |
Introduction Risk/yr (assigned to risk mitigation score, or derived from formal risk assessment) |
Risk-based Surveys/yr |
Risk-based Sample Size for 95% Confidence (% of original sample size) |
Eligible for (named pathogen) Premises Freedom Status |
One or more red pathways |
Yes |
Risk assessment determines annual intro risk > 15% |
n/a |
100% |
No. Lot-based (i.e., batch or movement) testing is required. |
Risk assessment determines annual intro risk ≤ 15%. |
4 (increased) |
100% |
Only with increased survey frequency. Otherwise, lot-based testing is required. |
||
0-1 point |
No |
≤ 8.2% |
2 |
100% |
Yes |
2 points |
≤ 8.0% |
2 |
90.2% |
Yes |
|
3 points |
≤ 7% |
2 |
68.9% |
Yes |
|
4 points |
≤ 6% |
2 |
54.1% |
Yes |
|
5 points |
≤ 5% |
2 |
41% |
Yes |
|
6 points |
≤ 4% |
2 |
31.1% |
Yes |
|
7 points |
≤ 3% |
2 |
23% |
Yes |
|
8 points |
≤ 2% |
2 |
14.8% |
Yes |
|
9 points |
≤ 1% |
2 |
6.9% |
Yes |
|
10 points |
≤ 0.8% |
2 |
5% |
Yes |
1 Surveillance details are assessed in later sections. See Official Surveillance (Section 1.5) and Early Detection Systems (EDS, Section 1.4).
2 This need not be as difficult as it may sound. Using abnormal behaviors as an example, a written plan would detail who, when and where behaviors are to be observed, with logs simply recording whether observations occurred according to plan. Separate records would capture time, place, ID, and explanatory details of behaviors or results that trigger further investigation.
3 This approach is cost-efficient as it incorporates systems that screen for multiple pathogens, allowing alternatives to single-pathogen optimized tests. Here, OS lays the groundwork, while EDS provides continuous vigilance for named and emerging threats. As such, OS and EDS are complementary and key to best management of aquatic animal health.
4 1/3 is derived as follows. Observational EDS detection probability can be calculated as the product of probabilities that (1) the pathogen has had time to reach expected prevalence, (2) it exhibits clinical signs, (3) those signs are observed, and (4) they are recognized, communicated, and acted upon. Figure 5 ensures high probability of item 4. Figure 10 ensures a high probability of items 2 and 3. The remaining probability (time to spread) varies by pathogen. But, if we allow 1 month for an introduction to reach detection prevalence, an incursion in 5 of the previous 6 months between formal OS might be considered detectable by EDS (5/6 = 0.83). Applying a safety factor ensures this credit remains conservative at 33%. Similar logic applies to screening EDS.
5 Note that freedom status generally only applies to facilities with introduction risks < 9%/yr. However, premises with ≥ 9% annual risk may be able to retain freedom status by increasing sampling frequency. Consult with APHIS ASEP re applicability.
6 Methods are embedded in Table 3 and described in Talbert et al (in prep). Also see: FAO. 2014. Risk-based disease surveillance – A manual for veterinarians on the design and analysis of surveillance for demonstration of freedom from disease. FAO Animal Production and Health Manual No. 17. Rome, Italy.
7 Alternatively, CAHPS Participants may engage an epidemiologist to design a site-specific approach to risk-based surveillance.
8 Note that ‘pool size’ and ‘number of pools’ cells are flipped in the ‘calculate’ tab in the second (Risksur) calculator.
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | Gustafson, Lori L - APHIS |
| File Modified | 0000-00-00 |
| File Created | 2024-10-28 |
© 2026 OMB.report | Privacy Policy
