CMS-10569 Supporting Statement A EQRS CY 2025
CMS-10569 Supporting Statement A EQRS CY 2025.docx
Data Collection for Quality Measures Using the Consolidated Renal Operations in a Web-Enabled Network (CROWNWeb) (CMS-10569)
OMB: 0938-1289
Supporting Statement – Part A
Data Collection for Quality Measures Using the End-Stage Renal Disease Quality Reporting System (EQRS)
Background
Pursuant to section 1881(h) of the Social Security Act (the Act) as amended by section 153(h) of the Medicare Improvements for Patients and Providers Act (MIPPA), the Centers for Medicare and Medicaid Services (CMS) established the End-Stage Renal Disease (ESRD) Quality Incentive Program (QIP) starting in 2011. The ESRD QIP is the first value-based purchasing program established by CMS, and it is aimed at promoting patient health by providing a financial incentive for renal dialysis facilities to deliver high-quality care.
In implementing the ESRD QIP, CMS believes that a successful quality incentive program will promote the delivery of high-quality health care services in the renal dialysis facility setting. Under section 1881(h)(2) of the Act, the Secretary is required to specify quality measures for evaluating the quality of care ESRD patients receive at renal dialysis facilities. While the Act outlines few mandatory measure topics, the Secretary is authorized to adopt measures on specified areas or medical topics determined appropriate by the Secretary (§ 1881(h)(2) of the Act). The ESRD QIP began in calendar year (CY) 2011 with an initial set of three quality measures and has increased and refined the measure set over the intervening years through notice and comment rulemaking.
In order to score facility performance on quality measures, CMS must be able to collect data on these measures. CMS collects these data from multiple sources, including Medicare claims and other tools such as the In-Center Hemodialysis Consumer Assessment of Healthcare Providers and Systems (ICH CAHPS) and the Centers for Disease Control and Prevention’s (CDC) National Healthcare Safety Network (NHSN) Dialysis Event Protocol. To further expand the measures used to evaluate the quality of care provided to ESRD patients in renal dialysis facilities, CMS also collects data using the ESRD Quality Reporting System (EQRS), formerly known as the Consolidated Renal Operations in a Web-Enabled Network (CROWNWeb) system. Because of the complexity of the existing systems and because of the need to comply with the protections for private or confidential data, CROWNWeb was implemented in phases starting in February 2009. CROWNWeb went into production nationally on June 14, 2012 and brought together all of CMS’ information systems that collect, maintain, and report on data about ESRD patients and provided electronic reporting tools for use by renal dialysis facilities. On November 9, 2020,1 we launched the EQRS, which contains the functionalities of the three legacy ESRD Systems, including CROWNWeb, in one global application, and aims to provide ongoing support to the ESRD user community to foster accurate and timely monthly data submission. This migration eliminates the need for multiple user accounts, and will in the long-term also improve the overall user experience and reduce burden due to enhanced navigation features.
The ESRD QIP is updating this PRA package under OMB control number 0938–1289 to ensure that it remains specific to reporting and validating EQRS data for the payment year addressed in the CY 2025 ESRD PPS proposed rule (i.e. Payment Year (PY) 2027).
Data Collection for ESRD QIP Measures
In selecting measures for adoption into the ESRD QIP measure set, CMS strives to achieve several objectives. First, the measures should consider national priorities such as those established by the Department of Health and Human Services’ Meaningful Measures Framework. Second, the measures should be tailored to the needs of improved quality in the renal dialysis facility setting; thus, the measures selected are most relevant to renal dialysis facilities. Finally, the burden of measure compliance on renal dialysis facilities should be weighed against the potential for improvements in patient health and well-being resulting from the measure’s collection.
Many measures currently finalized in the ESRD QIP are extracted from Medicare claims and therefore require no additional effort on the part of dialysis facilities to report.2 However, some quality data relevant to the care received by ESRD patients cannot be derived from Medicare claims or other administrative forms. For these measures, dialysis facilities are required to submit data via a web-based tool such as EQRS or the CDC’s NHSN system. The burden associated with submitting measure data to the NHSN Bloodstream Infection Modules3 and for the In-Center Hemodialysis Consumer Assessment of Healthcare Providers and Systems survey (ICH CAHPS)4 are already captured under previously approved packages. The burden associated with the COVID-19 Vaccination Coverage Among Healthcare Personnel (HCP) reporting measure that will require measure data to be submitted through the NHSN is accounted for under OMB control number 0920-1317 (expiration date March 31, 2026). Therefore, this package is specific to the burdens associated with ESRD QIP measure data submitted via EQRS.
The CY 2025/PY 2027 ESRD QIP
The CY 2025 ESRD Prospective Payment System (PPS) proposed rule proposes updates to program requirements for the CY 2025/PY 2027 ESRD QIP. During CY 2025/PY 2027, we will continue collecting data for the follow measures using EQRS:
Clinical Depression Screening and Follow-Up Clinical Measure (88 FR 76451 through 76453): Facility reports in EQRS one of four conditions for each qualifying patient treated during performance period.
Facility Commitment to Health Equity Reporting Measure (88 FR 76437 through 76446): Facilities will receive two points each for attesting to five different domains of commitment to advancing health equity for a total of ten points.
Hemodialysis Vascular Access: Long-Term Catheter Rate Clinical Measure (82 FR 50777 through 50778): Measures the use of a catheter continuously for 3 months or longer as of the last hemodialysis treatment session of the month. Facilities report in EQRS the vascular access type.
Hypercalcemia Reporting Measure (87 FR 67250 through 67251): Proportion of patient-months with 3-month rolling average of total uncorrected serum calcium greater than 10.2 mg/dL.
Medication Reconciliation for Patients Receiving Care at Dialysis Facilities Reporting Measure (83 FR 57008 through 57010): Percentage of patient-months for which medication reconciliation was performance and documented by an eligible professional.
Screening for Social Drivers of Health Reporting Measure (88 FR 76466 through 76476): Percentage of patients at a dialysis facility who are 18 years or older screened for all five health-related social needs (HRSNs) (food insecurity, housing instability, transportation needs, utility difficulties, and interpersonal safety).
Screen Positive Rate for Social Drivers of Health Reporting Measure (88 FR 76476 through 76480): Percentage of patients at a dialysis facility who are 18 years or older screened for all five HRSNs, and who screened positive for one or more of the following five HRSNs: Food insecurity, housing instability, transportation needs, utility difficulties, or interpersonal safety.
In the CY 2025 ESRD PPS proposed rule, we are proposing updates to measures in the ESRD QIP measure set beginning with PY 2027. Beginning with PY 2027, we are proposing to replace the Kt/V Dialysis Adequacy Comprehensive clinical measure, on which facility performance is scored on a single measure based on one set of performance standards, with a Kt/V Dialysis Adequacy measure topic, which would be comprised of four individual Kt/V measures and be scored based on a separate set of performance standards for each of those measures. We are also proposing to remove the National Healthcare Safety Network (NHSN) Dialysis Event reporting measure from the ESRD QIP measure set beginning with PY 2027.
Table A. Measures Collected via EQRS in CY 2025
Consensus-Based Entity (CBE) Goal |
CBE Endorsement Number |
Measure Title |
Data Collected |
Clinical Care |
CBE #2978 |
Hemodialysis Vascular Access: Long-Term Catheter Rate Clinical Measure |
Vascular Access Type |
Clinical Care |
CBE #1454 |
Hypercalcemia |
Uncorrected serum calcium |
Clinical Care |
Based on CBE #0323, #0321, #2706, and #1423 |
Kt/V Dialysis Adequacy Measure Topic |
Kt/V values for adult hemodialysis (HD) Kt/V, adult peritoneal dialysis (PD) Kt/V, pediatric HD Kt/V, and pediatric PD Kt/V |
Clinical Care |
N/A |
Clinical Depression Screening and Follow-Up |
One of four clinical depression screening and follow up conditions |
Safety |
CBE #2988 |
Medication Reconciliation for Patients Receiving Care at Dialysis Facilities Reporting Measure |
|
Health Equity |
N/A |
Facility Commitment to Health Equity |
Facility attests affirmatively to elements in the following 5 domains:
|
Health Equity |
N/A |
Screening for Social Drivers of Health |
Number of eligible patients who were screened for all five health-related social needs (HRSNs):
|
Health Equity |
N/A |
Screen Positive Rate for Social Drivers of Health |
Number of eligible patients with ‘Yes’ or ‘No’ (non-missing) screening responses for each of the five HRSNs. |
EQRS Data Validation for the ESRD QIP
One of the critical elements of the ESRD QIP’s success is ensuring that the data submitted to calculate measure scores and facility Total Performance Scores (TPS) are accurate. We began a pilot validation study program for the ESRD QIP in CY 2013. That validation study has continued in subsequent years. In the CY 2019 ESRD PPS final rule, we finalized a policy to make the CROWNWeb validation study a permanent element of the Program rather than a continued pilot study (83 FR 57001 through 57003). Making the CROWNWeb validation study permanent did not alter the methodology that we employ to validate CROWNWeb data and signals the importance that we place on accurate and complete quality data to participating ESRD facilities. Although we have transitioned from CROWNWeb to EQRS, we continue the validation using the data that was previously submitted to CROWNWeb and is now submitted to EQRS. Specifically, we will continue sampling the same number of records (approximately 10 per facility) from the same number of facilities (300 facilities). If a facility is randomly selected to participate in the validation but does not provide us with the requisite medical records within 60 calendar days of receiving a request, then we will deduct 10 points from the facility’s TPS.
Justification
Need and Legal Basis
Section 1881(h)(2) of the Act requires that the Secretary specify measures for each year of the program and with each successive year of the ESRD QIP, CMS has increased the sophistication and scope of the Program’s measure set. While Medicare claims can be an appropriate data source for some measures, claims do not represent the entirety of the ESRD population and are also limited in the depth of information available. For these reasons, in furtherance of our obligations under section 1881(h)(2) of the Act, we have specified several measures utilizing data reported by renal dialysis facilities using the EQRS system described below. These collections are authorized under 42 CFR 494.180(h) of the Conditions for Coverage of End-Stage Renal Disease Facilities, which requires renal dialysis facilities to furnish data and information (both clinical and administrative) electronically to CMS at intervals specified by the Secretary. CMS proposes and finalizes data reporting requirements for the ESRD QIP through notice and comment rulemaking.
While the ESRD QIP was not solely intended as a cost saving program, below we show the Program’s estimated payment reductions in recent years. We note that the estimated payment reductions for PY 2027 have been updated from the estimates in the CY 2024 ESRD PPS final rule, due to updated information about the total number of facilities expected to receive a payment reduction and the estimated impact of policies in the CY 2025 ESRD PPS proposed rule on facilities.
PY 2027; $14,647,335 (CY 2025 ESRD PPS proposed rule)
PY 2026; $15,990,524 (88 FR 76500)
PY 2025; $32,457,692.52 (87 FR 67297)
PY 2024; $17,104,030.59 (86 FR 62011)
PY 2023; $5,548,652.69 (87 FR 67297)
PY 2022; $0 (86 FR 62011)5
PY 2021; $32,196,724 (83 FR 57061)
PY 2020; $31,581,441 (81 FR 77960)
PY 2019; $15,470,309 (80 FR 69074)
PY 2018; $11,576,214 (79 FR 66257)
PY 2017; $11,954,631 (79 FR 66255)
Information Users
Section 1881(h) of the Act requires the Secretary, generally, to adopt a set of quality measures and to assess the quality of care provided by renal dialysis facilities using those measures. CMS and others use these data to monitor and assess the quality and type of care provided to ESRD patients. Specifically, CMS uses these data to calculate performance scores on certain measures included in the ESRD QIP measure set (described in detail below) and conducts a validation each year to ensure that those data are accurate.
CMS will make available to renal dialysis facilities their scores on individual measures and their total performance score for their use in internal quality improvement initiatives. CMS will also make available to facilities information on the performance of other facilities on individual measures and their total performance score. Most importantly, facility performance on individual measures and their TPS is available to beneficiaries, as well as to the public, to assist them in making decisions about their health care. Although we provide participating facilities the results of validation for their facility, as well as a comparison of their results to facilities that are a similar size and all the facilities in the study, beneficiaries and the public do not have access to validation results. CMS intends to use information on facility performance on measures and their TPS as well as validation results to direct its contractors to focus on areas of improvement and to develop quality improvement initiatives. CMS uses the validation to independently sample and test the reliability and validity of the clinical data submitted electronically in EQRS against providers’ source medical records, and to encourage facilities to accurately report data to EQRS.
Use of Information Technology
As noted previously, CMS developed EQRS to reduce the burden to renal dialysis facilities of submitting data to CMS. This system brings together all of CMS’ information systems that collect, maintain, and report on data about ESRD patients and provides electronic reporting tools for use by renal dialysis facilities. Renal dialysis facility users are required to open an account under their CMS Certification Number and are then able to complete the necessary data submission.
Duplication of Efforts/Similar Information
The information to be collected is not duplicative of similar information collected by the Centers for Medicare and Medicaid Services.
Small Businesses
Information collection requirements were designed to impose minimal burdens on small renal dialysis facilities subject to the ESRD QIP, and to facilitate the collection and reporting of required data. Specifically, the EQRS was created to allow small renal dialysis facilities to enter data via a web-based application rather than using paper-based data submissions or employing a full electronic health record, which can be prohibitively expensive for these facilities.
Less Frequent Collection
Measures developers employ clinical and statistical knowledge during the measure development process to determine the optimal schedule for collecting measure data. These data are then collected on the schedules provided in Table B to best evaluate the care provided to ESRD patients. Without this frequency of information collection, CMS would be unable to assess the correlations between the endpoints collected and the health and well-being of ESRD patients treated by the renal dialysis facilities participating in the ESRD QIP.
Table B. Measure Collection Schedule/Frequency
Measure Title |
Measure Collection Schedule/Frequency |
Hypercalcemia |
Monthly |
Kt/V Dialysis Adequacy Measure Topic |
Monthly |
Clinical Depression Screening and Follow-Up |
Annually |
Hemodialysis Vascular Access Type: Long-Term Catheter Rate Clinical Measure |
Monthly |
Medication Reconciliation for Patients Receiving Care at Dialysis Facilities (MedRec) Measure |
Monthly |
Facility Commitment to Health Equity Measure |
Annually |
Screening for Social Drivers of Health Measure |
Annually |
Screen Positive Rate for Social Drivers of Health Measure |
Annually |
Special Circumstances
There are no special circumstances.
Federal Register Notice/Outside Consultation
The CY 2025 ESRD PPS proposed rule’s publication, serving as the 60-day Federal Register notice, was published on July 5, 2024 (89 FR 55760).
Payment or Gift to Respondent
Dialysis facilities are required to submit measure data to CMS as part of the Conditions for Coverage of End-Stage Renal Disease Facilities (see 42 CFR 494.180(h)). No additional payments or gifts will be given to respondents for compliance with the reporting requirements of the ESRD QIP measures submitted via EQRS.
Confidentiality
CMS adheres to all confidentiality-related statutes, regulations, and agency policies. All information collected under ESRD QIP will conform to all applicable Federal laws and regulations and Federal, HHS, and CMS policies and standards as they relate to information security and data privacy. The laws and regulations that may apply include, but are not limited to: The Privacy Act of 1974; the Federal Information Security Management Act of 2002; the Computer Fraud and Abuse Act of 1986; the Health Insurance Portability and Accountability Act of 1996; the EGovernment Act of 2002, the Clinger Cohen Act of 1996; the Medicare Modernization Act of 2003, and the corresponding implementing regulations. OMB Circular A–130, Management of Federal Resources, Appendix III, Security of Federal Automated Information Resources also applies. Federal, HHS, and CMS policies and standards include but are not limited to: All pertinent National Institute of Standards and Technology publications; the HHS Information Systems Program Handbook and the CMS Information Security Handbook.
SORN #: 09-70-0520 – ESRD Program Management and Medical Information System (PMMIS) published 6/17/2002 (67 FR 41244), updated 5/8/2007 (72 FR 26126), and revised 6/26/2009 (74 FR 30606).
Sensitive Questions
There are no questions of a sensitive nature being collected as part of this quality assessment.
Burden Estimates
This burden estimate includes measures which CMS is continuing to collect as part of the ESRD QIP and the ongoing EQRS (formerly CROWNWeb) data validation. As noted in section A.1. of this supporting statement, this estimate excludes burden associated the NHSN Bloodstream Infection measure topic and the ICH-CAHPS measure because the burden associated with these measures is captured under OMB control numbers 0920-0666 (NHSN) and 0938-0926 (ICH-CAHPS Survey), respectively. This estimate also excludes burden associated with the COVID-19 Vaccination Coverage among Healthcare Personnel (HCP) reporting measure because the burden associated with this measure is accounted for under OMB control number 0920-1317 (expiration date March 31, 2026). This burden estimate also excludes the burden associated with training facilities to use EQRS, which will continue to be accounted for in OMB Control Number 0938-0386. The burden associated with the NHSN BSI Data Validation is captured under OMB control number 0938-1340.
The assumptions used to compute the estimated burdens associated with submitting ESRD QIP measure data via EQRS and the ongoing EQRS data validation are described here.
We estimate the burden hours for reporting measure data using the EQRS system for CY 2025/PY 2027 to be 2,877,743 hours. We estimate that the total annual burden hours associated with the PY 2027 EQRS validation is 750.
a. Data Collection for ESRD QIP Measures Using EQRS
We have used the following equation to estimate the burden associated with these data collection and submission efforts.
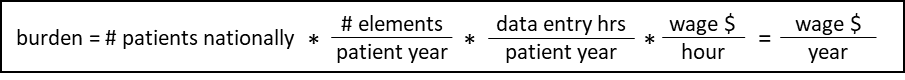
Table C. EQRS Data Collection Burden Estimate Elements
Burden Estimate Elements |
CY 2025/ PY 2027 |
Number of facilities6 |
7,833 |
Number of ESRD patients, nationally7 |
507,837 |
Number of data elements per patient |
136 |
The time spent for data entry and submission per element8 |
2.5 minutes |
Annual Burden Hours Nationally |
2,877,743 hours |
Median hourly wage of a Medical Records Specialist (Fringe benefit is calculated at 100%). |
$45.38 |
We estimate the number of patients per facility by calculating the mean number of patients per ESRD PPS-eligible facility nationwide, based on CY 2022 data, even though we recognize that the number of patients per renal dialysis facility is also highly variable, and may vary from month to month within a given facility. To estimate the total burden per facility, the mean number of patients per facility is then multiplied by the number of required elements per patient-year for each measure and the estimated time per element entry, as shown in Table D1. The estimated time per element entry for the EQRS measure is based on historical estimates previously finalized in the CY 2016 ESRD PPS final rule regarding the amount of time required to enter one data element for one patient (i.e. we assumed that it takes 2.5 minutes to report a data element, even though the time required is highly variable) (80 FR 69070).
To derive wage estimates, we used data from the U.S. Bureau of Labor Statistics’ (BLS) May 2022 National Occupational Employment and Wage Estimates.9 We anticipate that the labor required to collect and submit these data will be completed by either Medical Records Specialists or similar administrative staff. The median hourly wage of a Medical Records Specialist is $22.69. Fringe benefits and overhead are calculated at 100% using current HHS department-wide guidance on estimating the cost of fringe benefits and overhead. These are necessarily rough adjustments both because fringe benefits and overhead costs vary significantly from employer to employer and because methods of estimating these costs vary widely from study to study. Nonetheless, there is no practical alternative and we believe that these are reasonable estimation methods.
Using the assumptions described above, we estimate an hourly labor cost of $45.38 as the basis of the wage estimates for all collection of information calculations in the ESRD QIP. We also estimate the total annual burden for reporting measure data using the EQRS for CY 2025/PY 2027 to be $130,591,977 (2,877,743 hours x $45.38).
Table D. CY 2025/PY 2027 EQRS Data Collection Burden Per Measure
Note: Numbers may not add up due to rounding
MEASURE REPORTING Renal Dialysis Facilities CY 2025 Measure Set |
Number of Facilities |
Number of Patients Nationally |
Average number of patients per facility |
Number of Elements per Patient-Year |
Estimated Time for Data Entry per Element (hours) |
Estimated Wage plus Benefits per Hour for Data Entry |
Annual Hour Burden per Facility |
Annual Burden per Facility |
Hemodialysis Vascular Access: Long-Term Catheter Rate Clinical Measure |
7,833 |
507,837 |
64 |
24 |
0.042 |
$45.38 |
64.8 |
$2,942.12 |
Hypercalcemia |
7,833 |
507,837 |
64 |
24 |
0.042 |
$45.38 |
64.8 |
$2,942.12 |
Kt/V Dialysis Adequacy Measure Topic |
7,833 |
507,837 |
64 |
36 |
0.042 |
$45.38 |
97.2 |
$4,413.18 |
Clinical Depression Screening and Follow-Up |
7,833 |
507,837 |
64 |
1 |
0.042 |
$45.38 |
2.7 |
$122.59 |
Medication Reconciliation for Patients Receiving Care at Dialysis Facilities Reporting Measure |
7,833 |
507,837 |
64 |
36 |
0.042 |
$45.38 |
97.2 |
$4,413.18 |
Facility Commitment to Health Equity Reporting Measure |
7,833 |
507,837 |
64 |
5 |
0.042 |
$45.38 |
13.5 |
$612.94 |
Screening for Social Drivers of Health Clinical Measure |
7,833 |
507,837 |
64 |
5 |
0.042 |
$45.38 |
13.5 |
$612.94 |
Screen Positive Rate for Social Drivers of Health Reporting Measure |
7,833 |
507,837 |
64 |
5 |
0.042 |
$45.38 |
13.5 |
$612.94 |
Table E. CY 2025/PY 2027 EQRS Total Data Collection Burden
Note: Numbers may not add up due to rounding.
Basis |
Number of Elements |
Annual Hour Burden |
Annual Burden |
Each Facility |
8,817 |
367.39 |
$16,672.03 |
National |
69,065,832 |
2,877,743 |
$130,591,977 |
b. EQRS Data Validation
We have used the following equation to estimate the burden associated with the ongoing EQRS data validation:
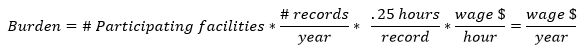
Table F. EQRS Data Validation Burden Estimate Elements
Burden Estimate Element |
CY 2025 (PY 2027) |
Number of facilities participating in the EQRS (formerly, CROWNWeb) data validation, annually |
300 |
Number of medical records per facility per year |
10 |
Time spent for record collection and submission per facility10 |
2.5 hours (approx. 0.25 hours per record) |
Hourly wage per hour engaged in data collection and submission11 |
$45.38 |
Under the EQRS data validation, we will randomly sample records from 300 facilities. Each sampled facility will be required to produce approximately 10 records. The burden associated with these validation requirements is the time and effort necessary to submit the requested records to a CMS contractor. We estimate that it will be take each facility approximately 2.5 hours in total, or 0.25 hours per medical record, to comply with this requirement. We therefore estimate that the total annual hourly burden for the ongoing EQRS data validation for CY 2025 to be 750 hours.
We anticipate that the labor required to collect and submit these data will be completed by either Medical Records Specialists or similar administrative staff. The median hourly wage of a Medical Records Specialist is $22.69 per hour. Fringe benefits and overhead are calculated at 100 percent. Therefore, using these assumptions, we estimate an hourly labor cost of $45.38 as the basis of the wage estimates for all collection of information calculations in the ESRD QIP. These are necessarily rough adjustments, both because fringe benefits and overhead costs vary significantly from employer to employer and because methods of estimating these costs vary widely from study to study. Accordingly, we estimate the total annual burden for the ongoing EQRS data validation for CY 2025 to be $34,035 (750 hours x $45.38).
Table G. CY 2025/PY 2027 EQRS Data Validation Burden
DATA VALIDATION Renal Dialysis Facilities CY 2025 |
Number of Facilities |
Number of Records per Year |
Estimated Time per Record |
Estimated Wage plus Benefits per Hour for Record Collection |
Annual Hour Burden per Facility |
Annual Burden per Facility |
EQRS Data Validation |
300 |
10 |
0.25 |
$45.38 |
2.5 |
$113.45 |
Table H. CY 2025/PY 2027 EQRS Total Data Validation Burden
Basis |
Annual Hour Burden |
Annual Burden |
Each Facility |
2.5 |
$113.45 |
National |
750 |
$34,035 |
The annual burden hours are 959,497 ((2,877,743 + 750)/3 years).
Capital Cost
There are no capital costs.
Cost to Federal Government
The cost to the Federal Government includes costs associated with the collection and validation of the data. The validation costs are an estimated $535,295 (FY) annually for the validation contract. For the claims-based measures, the cost to the Federal Government is minimal. CMS uses data from the CMS National Claims History system that are already being collected for provider reimbursement; therefore, no additional data will need to be submitted by dialysis facilities for claims-based measures. Additionally, the ESRD QIP program takes three CMS staff at the GS-13 Step 5 level ($117,516 annually per staff member), for an additional cost of $352,548. The total annual cost to the Federal Government is $887,843.
Changes to Burden
As discussed above, the ESRD QIP has consistently refined its measure set since the inception of the ESRD QIP in CY 2011. For CY 2025, we are proposing to replace the Kt/V Dialysis Adequacy Comprehensive clinical measure, on which facility performance is scored on a single measure based on one set of performance standards, with a Kt/V Dialysis Adequacy measure topic, which would be comprised of four individual Kt/V measures and be scored based on a separate set of performance standards for each of those measures. However, we are not proposing to update facility reporting requirements as part of that proposal, and therefore do not estimate any increased reporting burden associated with the proposed Kt/V Dialysis Adequacy measure topic. The annual burden hours specified in this PRA package (for the CY 2025 ESRD PPS proposed rule) for the 3-year OMB approval period decrease from the currently approved PRA package (associated with the CY 2024 ESRD PPS final rule), from 1,848,212 annual burden hours to 959,497 burden hours. That is because the annual burden hours in the currently approved PRA package associated with the CY 2024 ESRD PPS final rule include burden hours for both PY 2026 and PY 2027, reflecting 2.6 million hours in PY 2026 and 2.8 million hours in PY 2027 across all dialysis facilities averaged out over three years. Because the CY 2025 ESRD PPS proposed rule only discusses PY 2027 policies and proposals, this PRA package only reflects the 2.8 million burden hours for PY 2027. Averaged out over the three years, the annual burden hours are 959,497.
Publication/Tabulation Date
The goal of the data collection is to evaluate facility performance on measures in the ESRD QIP measure set for the given year in order to assess the payment reductions required under section 1881(h)(1) of the Act. These data are also made publicly available pursuant to section 1881(h)(6) of the Act and is used in other programs within CMS, such as public reporting of dialysis facility quality data on the CMS Care Compare website (formerly, Dialysis Facility Compare).
Expiration Date
CMS will display the expiration date on the collection instruments.
Explain any exceptions to the certification statement “Certification for Paperwork Reduction Act Submissions” of OMB form 83-I.
There are no exceptions to the certification statement “Certification for Paperwork Reduction Act Submissions” of OMB form 83-I.
2 For example, in the CY 2015 ESRD PPS final rule with comment period, CMS finalized 10 measures using Medicare claims as the primary data source.
3 The NHSN Bloodstream Infection measure is accounted for under OMB Control Number 0920-0666.
4 ICH CAHPS is accounted for under OMB Control Number 0938-0926.
5 In the CY 2022 ESRD PPS final rule, we finalized a special scoring methodology and payment policy for PY 2022 due to impacts of the COVID-19 public health emergency and EQRS system issues in CY 2020 (86 FR 61918 through 61919). Under this policy, we did not apply any payment reductions to ESRD facilities for PY 2022.
6 Total number of ESRD PPS facilities in the United States treating ESRD QIP-eligible patients.
7 Total number of patients treated at ESRD PPS facilities in the United States.
8 As stated in the CY 2016 ESRD PPS final rule (80 FR 69070), we estimate the amount of time required to submit measure data to EQRS (formerly CROWNWeb) to be 2.5 minutes.
9 https://www.bls.gov/oes/2022/may/oes292072.htm.
10 As stated in the CY 2020 ESRD PPS final rule (84 FR 60788), we estimate the amount of time required to submit measure data to CROWNWeb (now EQRS) to be 2.5 minutes.
11 https://www.bls.gov/oes/2022/may/oes292072.htm (Estimates are based on national median hourly wage).
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | Lobel, Devra |
| File Modified | 0000-00-00 |
| File Created | 2024-09-06 |
© 2026 OMB.report | Privacy Policy