0920-1355_SSA Eval Technical Assistance Hub Overdose Prevention_10.08.24 (003)
0920-1355_SSA Eval Technical Assistance Hub Overdose Prevention_10.08.24 (003).docx
[NCIPC] Evaluation of the Overdose Data to Action Technical Assistance Hub
OMB: 0920-1355
Supporting Statement: Part A
Evaluation of the Division of Overdose Prevention Technical Assistance Center
September 9, 2024
Point of contact:
Taylor Jennings, MPH
Division of Overdose Prevention
Centers for Disease Control and Prevention
National Center for Injury Prevention and Control
Table of Contents
Summary Table
Section A: Justification for Information Collection
A. 1 Circumstances Making the Collection of Information Necessary
A. 2 Purpose and Use of Information Collection
A. 3 Use of Improved Information Technology and Burden Reduction
A. 4 Efforts to Identify and Use of Similar Information
A. 5 Impact of Small Businesses or Other Small Entities
A. 6 Consequences of Collecting the Information Less Frequently
A. 7 Special Circumstances Relating to the Guidelines of 5 CFR 1320.5(d)2
A. 8 Comments in Response to the Federal Register Notice and Efforts to Consult Outside the Agency
A. 9 Explanation of Any Payment or Gift to Respondents
A. 10 Protection of the Privacy and Confidentiality of Information Provided by Respondents
A. 11 Institutional Review Board (IRB) and Justification for Sensitive Questions
A. 12 Estimates of Annualized Burden Hours and Costs
A. 13 Estimates of Other Annual Cost Burden to Respondents or Record Keepers
A. 14 Annualized Cost to Federal Government
A. 15 Explanation for Program Changes or Adjustments
A. 16 Plans for Tabulation and Publication and Project Time Schedule
A. 17 Reason(s) Display of OMB Expiration Date is Inappropriate
A. 18 Exceptions to Certification for Paperwork Reduction Act Submissions
List of Attachments
OD2A in States and OD2A: LOCAL Recipient List
Authorizing Legislation
Technical Assistance Feedback Form (Individual)
3a. Screenshot Individual Technical Assistance Feedback Form
Technical Assistance Feedback Form (Universal)
4a. Screenshot Universal Technical Assistance Feedback Form
Annual Technical Assistance Survey
5a. Screenshot Annual Technical Assistance Survey
Implementation Feedback Form
6a. Screenshot Implementation Feedback Form
Annual Technical Assistance Survey Email Invitation and Reminder
Focus Group Email Invitation and Reminder
Focus Group Session Script
60 Day FRN
Public Comment
Privacy Determination
Research Determination

SUMMARY
TABLE
Goal
of the study
The
goal of this study is to continue monitoring and evaluating the
effectiveness and impact of technical assistance (TA) provided to
Overdose Data to Action (OD2A) in States and OD2A: LOCAL program
recipients funded to implement opioid surveillance and prevention
efforts in their jurisdictions. Specifically, the evaluation will
gather real-time information to improve ongoing provision of TA and
to demonstrate the overall impact of OD2A TA delivery.
Intended
use of the resulting data
The
information obtained through this evaluation will allow TA providers
to assess OD2A recipients’ experience and utility of knowledge
and resources gained through individual TA support, peer-to-peer
sessions, and other group trainings. Ultimately, the evaluation data
will inform
subsequent rounds of TA and allow TA providers to make necessary
adjustments to the overall TA strategy for continuous quality
improvement.
This will ensure recipients have the support necessary to implement
strategies that will improve opioid surveillance and prevention
policies and practices within their communities.
Methods
to be used to collect
The
evaluation consists of two web-based surveys designed to collect
process and outcome measures about TA access, utilization, and
outcomes across all 90 OD2A in States and OD2A: LOCAL recipient
programs. The Technical
Assistance (TA) Feedback Form will be administered to collect
immediate feedback following individual TA encounters and group
events such as webinars and in-person trainings. The Annual TA
Survey will be distributed three times to assess
satisfaction with overall TA provided and the extent to which TA
supports informed implementation of OD2A strategies. The
Implementation Feedback Form will collect feedback three to six
months after the delivery of substantial TA to ascertain how it
impacted implementation.
The
subpopulation to be studied
The
OD2A in States and OD2A: LOCAL recipient program staff and partners
will participate in the data collection tools for this study.
How
data will be analyzed
The
survey responses will
be compiled and analyzed to measure descriptive statistics including
means and percentages. Additionally, analyses will be summarized by
recipient type (e.g. state-level, county level), organization, or
mode of TA delivery. Survey findings will be used to generate
feedback reports and lessons learned reports to share with TA
providers, CDC staff, and other program stakeholders.
SUMMARY TABLE
Goal of the study
Intended use of the resulting data
Methods to be used to collect
The subpopulation to be studied
How data will be analyzed
Section A: Justification for Information Collection
This is a revision request for the currently approved “Evaluating the Overdose Data to Action TA Hub,” OMB# 0920-1355, expiration date 11/30/2024 for three years. The Centers for Disease Control and Prevention (CDC) requests approval of this revision to support the evaluation of technical assistance (TA) provided for the Overdose Data to Action (OD2A) in States and OD2A: Limiting Overdose through Collaborative Actions in Localities (LOCAL) programs. OD2A-S and OD2A: LOCAL are cooperative agreements funded in 2023 to focus on comprehensive and interdisciplinary opioid overdose prevention efforts in 49 state health departments, 39 localities, Puerto Rico, and Washington D.C. (Attachment 1). Each program consists of two required components– a surveillance component and a prevention component. OD2A recipients implement a combination of activities across nine states strategies and eight local strategies within these components to gain access to high quality, complete, and timelier data on opioid prescribing and overdoses and to use those data to inform prevention and response efforts in their jurisdictions.
In the previously approved iteration of this ICR, the information collected surrounding OD2A (version 1.0) recipient feedback on their experiences receiving TA, proved invaluable in the process of improving TA delivery and overall providing more useful TA. The feedback provided in the original data collection instruments was also used to improve the TA Strategy of the updated iterations of OD2A (including OD2A-S and OD2A: LOCAL) and their recipients. With the information that was collected in the previously approved ICR, CDC can more effectively deliver TA to an almost-doubled recipient group across two programs instead of one and ensure that continuous improvement in TA. Further information gathering through two new instruments proposed in this ICR (the Implementation Feedback Form and the Focus Group script), will enhance TA perspectives and needs to utilize the DOP TA Center resource effectively and responsibly. There is an increase in annual burden from 222 (previously approved) to 388, primary increase in burden comes from the increase in program recipients from 60 in OD2A to 90 across OD2A in States and OD2A: LOCAL and the two instruments being added to this request.
Training and technical assistance (TA) is essential to building knowledge and strengthening the capacity of recipients to implement and evaluate OD2A program strategies. CDC developed and deployed a TA hub (hereafter referred to as the DOP TA Center) to deliver comprehensive technical assistance and training to support the successful implementation and evaluation of surveillance and prevention activities. The DOP TA Center is designed to enhance the efficiency, coordination, and effectiveness of TA efforts by streamlining and centralizing the provision of overdose surveillance and prevention TA. TA to OD2A recipients is divided into four different levels with multiple modes of TA delivery and involves a wide range of TA providers including CDC staff, internal and external subject matter experts (SMEs) and program partners as well as Tanaq and ICF staff. The four TA levels below are used to direct the process for engaging stakeholders to support program recipients and triage appropriate resources to support their needs.
TA Levels
Level 1. Individual or direct TA provided by CDC State Support Team: DOP OD2A Project Officers, Prevention Science Officers, Surveillance Science Officers. These individuals serve as the first line of TA for OD2A recipients.
Level 2. Individual or direct TA provided by DOP Strategy Leads, DOP SMEs.
Level 3. Individual or direct TA provided by an SME external to DOP, such as an SME from another CDC division, or a CDC partner and/or an Tanaq funded SME (Includes direct TA encounters).
Level 4. TA provided by CDC or Tanaq to a group of recipients. For example, webinars, site visits, peer exchanges, and virtual meetings.
The OD2A TA approach incorporates an evidence- and practice-based framework and principles to guide prioritization, design, and development. As shown in Exhibit 1, there are four core components involved in the TA framework, each building on the preceding and creating a feedback loop over time: Assess OD2A Recipient Capacity (Phase 1); Plan and Design TA and the TA Hub (Phase 2); Coordinate, Deliver, and Track TA (Phase 3); and Evaluate and Report (Phase 4). Phase 4 allows the unique opportunity to focus on monitoring, evaluation, and continuous quality improvement activities and ensure a flexible responsive TA approach to evolving needs during the OD2A program implementation cycle.
Exhibit 1: OD2A TA Center Framework

Understanding TA delivery and how it is used to support the OD2A programs is a critical component of the framework, ensuring that recipient needs are met and that TA services result in meaningful change. There are two overarching purposes of evaluating OD2A TA: to gather real-time information to improve ongoing provision of TA and to demonstrate the overall outcomes of TA. Evaluation of TA provided through the DOP TA Center will focus on outcomes that align with the provision of TA, including:
Alignment of TA to OD2A recipients’ needs
Increase in access to TA
Increase in use of TA
Recipient use of TA providers and reported changes in implementation (or plans for implementation)
The OD2A TA Center evaluation plan is comprised of two primary components:
Extant Data Sources
Implementation needs assessment via systematic document review
Online DOP TA Center analytics and reports
CDC and recipient feedback via meetings and ad hoc calls
Primary Data Collection
Technical Assistance Feedback Form
Annual Technical Assistance Survey
Implementation Feedback Form
Community of Practice Focus Groups
This revision request is for approval of the data collection instruments to be administered as part of the primary data collection component for this evaluation. This data collection will help inform Phase 4 of the OD2A TA Framework (Evaluate and Report) and is critical for completing the feedback loop that drives the overall TA approach (Exhibit 1). CDC is authorized to collect the data described in this request for evaluation and quality improvement purposes through Section 301(a) of the Public Health Service Act (Attachment 2).
Effective TA is built on a foundation of continuous quality improvement using data and feedback from the OD2A TA recipients. The information obtained through the primary data collection tools will allow TA providers to assess recipients’ experience and utility of information and resources gained through individual TA support, peer-to-peer sessions, and other group trainings. Additionally, data collection will inquire about additional program support needs, which will be used to inform and modify subsequent training and TA plans. This will ensure recipients have the support necessary to implement strategies that will ultimately improve opioid surveillance and prevention policies and practices within their communities. Information gathering through the two new instruments proposed in this ICR (the Implementation Feedback Form and the Focus Group script), will enhance TA perspectives and needs to utilize the DOP TA Center resource effectively and responsibly.
The evaluation activities will address questions organized along a continuum beginning with TA planning and ending with TA outcomes, as demonstrated in the Exhibit 2 below.
Exhibit 2. Monitoring and Evaluation Questions
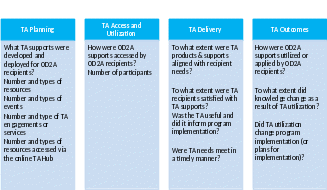
To answer these process and improvement questions and continually improve the program, CDC developed two questionnaires. The surveys contain both process and outcome measures about TA access, utilization, delivery, and outcomes. The TA Feedback Form, Implementation Feedback Form, Focus Group script, Annual Technical Assistance Survey are included in Attachments 3-9. Below is a description of the primary data collection instruments and the uses of the information collected.
Technical Assistance Feedback Form
The TA Feedback Form is a web-based survey that will be used to measure participant knowledge gain, intended application, and experience after each TA encounter implemented by external partners and SMEs (Level 3 and 4 TA). The TA Feedback Form will be administered following the close of each appropriate TA activity (e.g., Level 3 or Level 4 TA webinar, in-person event, virtual training, direct consultation).
The TA Feedback Form will gather OD2A recipient feedback regarding the perceived quality and effectiveness of each relevant TA activity. Survey items include a mix of close-ended questions to rate aspects of the TA provided as well as open-ended questions to gather detailed feedback about satisfaction and usefulness of the TA encounter and recommendations for improvement. In addition, the survey asks participants to indicate other TA items to address in the future. The survey also collects the role/profession and recipient jurisdiction of the respondent. Results from the TA Feedback Form will be presented in TA Feedback Reports shared with CDC following in-person and virtual trainings. The TA Feedback Reports will highlight requests for future TA support in addition to a summary of OD2A recipient feedback regarding the usefulness and quality of the TA provided. Additionally, TA Feedback Form results will inform adjustments in order to improve quality and utility in subsequent rounds of TA.
Annual Technical Assistance (TA) Survey
The Annual TA Survey will include both qualitative and quantitative questions to assess satisfaction with overall TA and the extent to which TA supports informed implementation of OD2A strategies. The survey will be conducted three times (annually over the OMB approval period) with recipient leadership and with individuals from all OD2A recipient jurisdictions who participated in TA activities (levels 1-4). Since OD2A programs are 5-year cooperative agreements, the survey will be conducted in winter 2024-25, and twice again each following year over the 3-year OMB period. The survey will be automated for each respondent to reflect the TA that has been received or accessed by the OD2A recipient.
The findings from these questionnaires will enable CDC and program stakeholders to improve delivery of TA and guide decision-making about future TA resource allocations. An Evaluation and Lessons learned report will be generated to maximize the application and benefit of the compiled feedback. The report will share program successes, a summary of TA activities, participants, barriers encountered, TA outcomes, and recommendations for improving TA and sustaining knowledge gains.
Implementation Feedback Form
This new instrument will collect information about OD2A recipients’ experiences receiving substantive TA that can will impact their work on the OD2A cooperative agreement for which they are funded. Recipients who receive significant or substantive TA that entails a high level of effort and involvement with the TA provider (either internal to CDC or external) will be invited to complete this form. The information collected in this form will inform future TA delivery and guide decision-making about future TA resource allocations. The findings will illuminate how the recipients receiving substantial levels of TA through the DOP TA Center are implementing the knowledge and resources gained through this TA delivery.
Focus Group
This new instrument will collect information about OD2A recipients’ experiences receiving technical assistance in any of the DOP TA Center Communities of Practice (CoPs). The information collected in these hour-long focus groups will support alignment between TA needs and TA delivery in the tailored CoP sessions delivered throughout the project period. The Focus Groups will also support DOP TA Center subject matter experts in delivering tailored TA in their respective CoPs. The TA team will also send a recruitment email to anticipated participants with a follow-up email to encourage participation and provide the necessary details.
Data collection instruments will be administered via the Web to (1) minimize the burden to respondents and to the government, (2) maximize convenience and flexibility, (3) maximize participation and engagement, and (4) ensure the quality and utility of the information collected.
Data will be collected by web-based surveys through a link shared with respondents. Use of Web-based surveys decreases respondent burden by allowing for direct transmission of the instrument to and from survey respondents – as compared to alternative methods such as paper format. In addition, data entry and quality control mechanisms built into the Web-based system will reduce errors that might otherwise require follow-up, thus reducing burden compared to a hardcopy administration. Respondents will also be able to complete the survey at a time and location that is convenient for them. The web-surveys associated with the DOP TA Center evaluation will recruit respondents to participate through an e-mail invitation that includes the Web-site URL to complete the survey, which will further increase the ease of responding. The Web-based questionnaires offer the following advantages for burden reduction:
Easy and secure access for respondents, decreasing the burden of reporting program activities.
Instant publication of survey results, with no printing, labeling, or postage costs, no lost paperwork, and no misprints.
Automatic sequencing of questions based on responses to previous questions, eliminating problems of inapplicable questions.
Error-checking to ensure the integrity of responses before they are submitted for review.
These questionnaires are not duplicated by other survey efforts or program monitoring activities. Additionally, as this program began in Fiscal Year 23, there are no existing data collected on TA provided to the CDC OD2A in States and OD2A: LOCAL recipients in these new cooperative agreements or on the intermediate-term after TA delivery (such as that which will be collected from the Implementation Feedback Form and Focus Groups) that can be used to generate data that are similar to the information collected under this clearance. There are rare instances of studies evaluating TA provision on a program level and there are no other planned efforts to assess TA provided to the CDC OD2A recipients across either program. This data collection does not duplicate any information currently being collected on the OD2A program such as the OD2A Annual Progress Report (APR) – OMB Control # 0920-1283, which focuses on the OD2A recipients’ progress in implementing overdose surveillance and prevention activities, or the Cross-Site Evaluation of OD2A program OMB Control # 0920-1330, which evaluates OD2A recipients’ success in implementing their activities. The purpose of this proposed data collection is to evaluate the technical assistance provided to OD2A in States and OD2A: LOCAL recipients through CDC TA supports and the impact of this assistance on their implementation efforts.
Most of the data for this evaluation will be collected from state and local administrators, program staff, and partners affiliated with the OD2A recipients’ program. The data for this evaluation will be collected from individuals involved with public agencies, such as the Department of Health and Human Services, Department of Public Health, Department of Safety, and local health districts.
It is important to gather real-time feedback to inform the ongoing TA provision that is required to support the OD2A recipients. The collection of information for the TA Feedback Form will be ongoing within 2 weeks of a relevant TA activity and the Annual TA Survey is scheduled to occur three times during the evaluation period. Less frequent data collection will hamper the evaluation impact and the ability to inform the OD2A TA strategy. The information collected by this proposed data collection will directly inform subsequent TA efforts and is critical for improving the effectiveness of the TA delivered. The TA Feedback Forms will provide near real-time data on the effectiveness of the TA that is delivered to OD2A jurisdictions and on the TA needs that exist, and will be used to continuously improve the content, format, and delivery of the technical assistance. Results from the Annual Technical Assistance Survey will provide a comprehensive understanding of jurisdictions’ TA needs and how well we’ve addressed those needs through technical assistance, and critical information around any gaps or inefficiencies in our TA approach. These data will allow us to maximize the reach and impact of our technical assistance efforts. Without this timely feedback on the effectiveness of the TA that is being delivered to OD2A jurisdictions, it is impossible to quickly adapt to changing needs and deliver the assistance in a way that works best for this audience. Without this information, it would not be possible to learn about the practices that promote effective TA, as well as address recipient TA needs in a short timeframe. Less frequent data collection would result in data gaps and would negatively impact our ability to understand TA needs across all OD2A jurisdictions, how to provide the most effective assistance, and how to maximize our TA resources for the purpose of improving the implementation of the OD2A program.
This request fully complies with the regulation 5 CFR 1320.5.
This request fully complies with the regulation 5 CFR 1320 .5 Comments in Response to the Federal Register Notice and Efforts to Consult Outside the Agency
A 60-day Federal Register Notice was published in the Federal Register on May 7, 2024, Volume 89, Number 89, pp 38150 (Attachment 10). CDC received one non-substantive public comments (Attachment 11).
Efforts to Consult Outside the Agency:
The TA Feedback Form and Annual TA Survey were developed based on instruments previously approved by OMB (CDC Workplace Health Promotion Resource Center OMB#0920-1151) for other resource center evaluation effort.
No material or financial incentives will be provided to respondents for completing the questionnaires.
The Office of the Chief Information Officer at the CDC has determined that the Privacy Act does not apply to this information collection request. Some personally identifiable information (PII) already collected and publicly available will be used, including the respondents’ name, official role, organization, state, and date of interview. All information will be kept on secure, encrypted, password protected servers accessible only to specific project team members. The Privacy Impact Assessment (PIA) for this evaluation is attached (Attachment 12).
Information will be collected from stakeholders who oversee, implement, and support the program activities under OD2A, including state agency administrators, program staff, local health department staff, and evaluators. Data collection will include the TA utility, preferences, needs, and interests for the optimal delivery of TA content across a diverse group of stakeholders who are directly involved in building or sustaining opioid surveillance and prevention program activities. Although the name and work email address of the contact persons receiving the email with the survey link will be stored, this information will be stored separately from the survey responses and will be destroyed after administration.
Data collection does not involve the collection of sensitive, personal, and/or personally identifiable information, we are using secondary data with PII to contact participants. Respondents to the TA Feedback Form, Implementation Feedback Form, Focus Groups, and Annual TA Survey will be recruited and sent an invitational email prior to participation in the information collection.
Three of the DOP TA Center evaluation surveys involve use of web-based data collection methods (all instruments excluding the Focus Group). The website does use cookies, and access to the web-based questionnaire will use a link to an anonymous survey. CDC’s contracting company will be the only organizations to collect, store, and maintain individual identifiable information. The electronic file linking the TA recipient and their email will be securely stored. All information will be stored in a password protected system and only accessible to evaluation staff. IT servers and data rooms have additional security. All hard drives on the server are encrypted.
An active consent process will be implemented that informs participants of the purpose of the evaluation, describes what participation entails, and addresses maintenance of privacy. All respondents who participate in the web-based surveys will be required to complete an electronic consent form prior to beginning the survey. Participation by all respondents is completely voluntary. Respondents will be assured that (1) their participation is voluntary (2) their responses will be kept privately and only seen by Tanaq, and (3) that there are no personal risks or benefits to them related to their participation.
IRB Approval
The CDC National Center for Injury Prevention and Control’s OMB and human subject research officer has determined that IRB approval is not needed for this non-research project (Attachment 13).
Sensitive Questions
Activities do not involve the collection of information of personal or sensitive nature.
A.12 Estimates of Annualized Burden Hours and Costs
This collection includes the Implementation Feedback Form, Focus Group sessions, and the Annual Technical Assistance Survey. Also, a subset of individuals from OD2A in States and OD2A: LOCAL recipient locations that participate in TA activities will receive an email notification and a link with instructions for accessing the surveys. The TA Feedback Form will take approximately 5 minutes to complete. There are two versions of the TA Feedback Form that will be employed to collect this information. One version will be used if the TA delivered is considered “individual,” meaning the TA is directed to a small group of people; The other version will be used for “universal” TA, which includes webinars and panel sessions. Each version will be used to collect information on recipient satisfaction and their perceptions of applicability. The forms will take the same amount of time to complete. Based on experience with satisfaction surveys an 18% response rate is expected for the TA feedback Form. Given this anticipated response rate, the estimated number of completed responses is 2,469, which will include 1,235 unique individuals completing either version of the TA Feedback Form twice on average each year.
The Annual TA Survey will be conducted three times over the three-year data collection period and each survey will take 10 minutes to complete, as it has been streamlined from the previous iteration that took 13 minutes. The Annual TA Survey will be distributed to up to 10 individuals from each recipient (900 individuals) for each administration. With a response rate of 18%, it is expected to receive a total of 162 completed surveys at each of the three administrations.
In conjunction with the Annual TA Survey, a group of email notifications, composed of one invitation email and two reminders to complete the survey, will be sent to up to 10 individuals from each recipient (900 individuals) for each survey administration, for a total of 2,700groups of email notifications over the three-year project period. Together, the invitation email and the two reminders will take 2 minutes to read for each individual invited to complete the survey.
In instances of longer-term technical assistance delivery (three to six months), the Implementation Feedback Survey will be delivered. This longer-term follow up survey will be administered to participants from group events or individual TA experiences that have clear intent to impart knowledge and build skills that can feasibly be applied to daily work efforts. It is estimated that this new instrument will be delivered to about 5 participants from 60 TA engagements over the course of the three-year project term; this is a total of 300 expected respondents. This instrument is expected to take roughly 15 minutes to complete for each respondent. We expect a total of 54 unique responses with the consistent response rate across past OD2A instruments of 18%.
To capture participants’ experiences attending various Communities of Practice held by the DOP TA Center, a subset of attendees will be invited to participate in Focus Groups. Approximately 20 sessions will be held each year for the project period (meaning 60 sessions are anticipated in total). About 30 people will receive the invitation to participate in each of the 20 sessions, meaning a total of 600 people will be invited to participate in a focus group on a CoP they engaged with. In addition to this initial invitation, those identified as potential Focus Group participants will receive a follow up email. The initial invitation and follow-up email consist of an email group and will take approximately 2 minutes for attendees to read. Given the previous response rate in OD2A recipient TA projects, it is expected that 18% of those 30 invitees per CoP will attend the session, yielding 5 attendees (respondents) on average per session. The Focus Group sessions will last 60 minutes.
Annualizing this data collection over three years results in an estimated annualized burden of 388 hours for all funded OD2A in States and OD2A: LOCAL recipients. Table A.12-2 provides annualized estimates of burden for a 3-year period. The response rates and amount of time required to complete the questionnaires is based on estimates from experience with previous data collections of similar scope and length. The primary increase in burden comes from the increase in program recipients from 60 in OD2A to 90 across OD2A in States and OD2A: LOCAL and two instruments being added to the project.
Table A.12-2 Estimated Annualized Burden to Respondents
Type of Respondents |
Form Name |
Number of Respondents |
Number of Responses per Respondent |
Average Burden Per Response (in hours) |
Total Burden (in hours) |
OD2A (OD2A in States and OD2A: LOCAL) Recipients |
TA Feedback Form Individual (Att. 3 & 3a) |
618 |
2 |
5/60 |
103 |
TA Feedback Form Universal (Att. 4 & 4a) |
617 |
2 |
5/60 |
103 |
|
Annual Technical Assistance Survey (Att. 5 & 5a) |
162 |
1 |
10/60 |
27 |
|
Implementation Feedback Survey (Att. 6 & 6a) |
18 |
1 |
15/60 |
4.5 |
|
Email invitation for Annual Survey (Att. 7) |
900 |
1 |
2/60 |
30 |
|
Focus Groups Email invitation (Att. 8) |
600 |
1 |
2/60 |
20 |
|
Focus Group Session Script (Att. 9) |
100 |
1 |
1 |
100 |
|
Total |
388 |
||||
Table A.12-3 provides estimates of the annualized cost to respondents for the collection of data. Cost estimates are based on average hourly rates for epidemiologists reported on the Department of Labor Statistics website listing the median hourly rate from 20231. Total estimated cost to respondents is $14,628.13 .
Table A.12-3 Annualized Costs to Respondents
Type of Respondents |
Form Name |
Total Burden (in hours) |
Avg. Hourly Wage Rate |
Total Cost |
OD2A Program Staff and Partners
|
TA Feedback Form Individual |
103 |
$37.75 |
$3,888.3 |
TA Feedback Form Universal |
103 |
$37.75 |
$3,888.3 |
|
Annual TA Survey |
27 |
$37.75 |
$1,019.25 |
|
Implementation Feedback Survey |
4.5 |
$37.75 |
$169.875 |
|
Email invitation for Annual Survey |
30 |
$37.75 |
$1,132.5 |
|
Focus Groups Email invitation |
20 |
$37.75 |
$755 |
|
Focus Group Session Script |
100 |
$37.75 |
$3,775 |
|
Total |
$14,628.13 |
|||
A.13 Estimates of Other Annual Cost Burden to Respondents or Record Keepers
No capital, start-up, or maintenance costs are involved.
A.14 Annualized Cost to Federal Government
Cost will be incurred by the government in personnel time for overseeing the project. CDC time and effort for overseeing the data collection and answering questions posed by the contractor and OD2A recipients are estimated at 5% each for two CDC employees and 2% for another CDC employee. The cost to the federal government for oversight and project management is $13,000 (Table A.14-1).
The contractor’s costs are based on estimates provided by the contractor who will carry out the data collection activities. With the expected period of performance, the annual cost to the federal government from contractor and other expenses is estimated to be $182,034 (Table A.14-1). This is the cost estimated by the contractor, Tanaq, and includes the estimated cost of coordination with DOP, data collection and technical assistance, analysis, and reporting.
The total annualized cost to the government, including direct costs to the federal government and contractor expenses is $195,034.
Table A.14-1. Annualized and Total Costs to the Federal Government
Expense Type |
Expense Explanation |
Annual Costs (dollars) |
Direct Cost to the Federal Government |
||
CDC oversight of contractor and project |
CDC Supervisor labor costs |
$13,000 |
Subtotal, Direct Costs to the Government per year |
$13,000 |
|
Contractor and Other Expenses |
||
Data collection, analysis, and reporting |
Annual labor hours |
$182,034 |
Subtotal, Contract and Other Expenses per year |
$182,034 |
|
Total of all annualized expenses |
$195,034 |
|
A.15 Explanation for Program Changes or Adjustments
In addition to extending approval for instruments previously approved, there four new information collection instruments: TA Feedback Form - Universal Events: Implementation Feedback Form; Invitation to Complete the Annual Technical Assistance Survey (email); and Focus Groups included in this revision (the Implementation Feedback Form and Focus Group). The need for these instruments to be added was identified during the first iteration of the OD2A program and OD2A TA Hub version of this project; no intermediate-term feedback was being collected around how in-depth support ascertained by those receiving TA had impacted program work or been implemented after TA delivery. Additionally, the new Focus Group instrument is a new opportunity to collect in-depth feedback from Community of Practice and peer session participants in a way that not only aligns with the depth of TA they will have received in the CoP and engagement sessions but aligns also with the continuous improvement priorities of CDC. There is an increase in annual burden from 222 (previously approved) to 388, primary increase in burden comes from the increase in program recipients from 60 in OD2A to 90 across OD2A in States and OD2A: LOCAL and two instruments being added to the project.
A.16 Plans for Tabulation and Publication and Project Time Schedule
CDC may disseminate the outcomes of the study within the federal government and outside of it through the development of case studies, scientific presentations, peer-reviewed publications, and tools and resources developed for opioid surveillance and prevention programs. Additional dissemination channels may include publications that are commonly read and of interest to TA providers and administrators who regularly manage overdose prevention programs.
Figure A.16-1: OD2A TA Center Data Collection Timeline
Data Collection Activity |
Administration Frequency |
Technical Assistance Feedback Form |
Ongoing after OMB approval |
Annual Technical Assistance Survey |
Ongoing after OMB approval - Three times (mid-2025, -2026 and -2027) |
Implementation Feedback Form |
Ongoing after OMB approval |
Focus Group |
Ongoing after OMB approval |
A.17 Reason(s) Display of OMB Expiration Date is Inappropriate
The display of the OMB expiration date is not inappropriate.
A. 18 Exceptions to Certification for Paperwork Reduction Act Submissions
There are no exceptions to the certifications.
1 Bureau of Labor Statistics. Occupational Employment and Wages, Epidemiologists (bls.gov) Accessed February 2024.
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | Tony Richardson |
| File Modified | 0000-00-00 |
| File Created | 2024-10-30 |
© 2026 OMB.report | Privacy Policy