SSA_FINAL_w/changes (24AB)
SSA_NIOSH_Lab with VR Pkg_Final.docx
[NIOSH] Direct Reading Methodologies, Sensors, and Robotics Technology Assessment in Lab/Simulator-based Settings
SSA_FINAL_w/changes (24AB)
OMB: 0920-1441
Direct Reading Methodologies, Sensors, and Robotics Technology Assessment
in Lab/Simulator-based Settings
GENERIC Information Collection Request
OMB Control Number: 0920-1441
Expiration Date: 09/30/2027
CDC/NIOSH
Supporting Statement A
Emily Haas, PhD
Associate Director for Science
Tel. 412.386.4627
April 25, 2024
Table of Contents
1. Circumstances Making the Collection of Information Necessary 3
2. Purpose and Use of Information Collection 5
3. Use of Improved Information Technology and Burden Reduction 8
4. Efforts to Identify Duplication and Use of Similar Information 9
5. Impact on Small Businesses or Other Small Entities 9
6. Consequences of Collecting the Information Less Frequently 10
7. Special Circumstances Relating to the Guidelines of 5 CFR 1320.5 10
8. Comments in Response to the Federal Register Notice and Efforts to Consult Outside Agencies 10
9. Explanation of Any Payment or Gift to Respondents 11
10. Protection of the Privacy and Confidentiality of Information Provided by Respondents 12
11. Institutional Review Board (IRB) and Justification for Sensitive Questions 13
1opl2. Estimates of Annualized Burden Hours and Costs 14
13. Estimates of Other Total Annual Cost Burden to Respondents and Record Keepers 18
14. Annualized Costs to the Government 18
15. Explanation for Program Changes or Adjustments 19
16. Plans for Tabulation and Publication and Project Time Schedule 19
17. Reason(s) Display of OMB Expiration Date is Inappropriate 19
18. Exceptions to Certification for Paperwork Reduction Act Submissions 19
List of Exhibits
List of Attachments
Attachment A – Occupational Safety and Health Act of 1970
Attachment B – Mine Safety and Health Act of 1977
Attachment C – 60 Day Federal Register Notice
Goal
of the study:
The goal of this GENERIC information collection request is to
enable CDC/NIOSH to assess the effectiveness of a sub-set of
automated direct reading methodologies, sensor technologies, and
robotics technologies (hereafter referred to as “technologies”)
that are used to protect worker safety and health. Standards
development organizations and safety and health associations
routinely update technological standards to keep up with research
and development efforts. The National Institute for Occupational
Safety and Health (NIOSH) is requested to assess and study the
performance, safety, and worker interactions with new or updated
technologies. The projects under this information collection will
enable NIOSH to respond to these requests in a resource-efficient
fashion, catalyzing improved worker safety and health as workplaces
experience and integrate new, novel technologies. These studies
primarily occur in NIOSH laboratory settings, including virtual
reality simulated space. No studies proposed under the auspices of
this generic IC intend to produce results that can be generalized
beyond the scope of each study.
Intended
use of the resulting data:
The resulting data will benefit the federal government and U.S.
workforce to inform research, development, and technology
integration and implementation recommendations to advance the
nation’s Future of Work needs. Data collected will seek to:
1) identify the validity and reliability and general performance of
direct reading methodologies, sensors, and robotic technologies; 2)
identify the impact of technologies on worker and organizational
outputs and decision making; 3) assess the perceived knowledge,
attitudes, and skills to assess risks associated with the use and
integration of technologies among workers; and 4) identify barriers
faced while using, interacting with, adopting, and maintaining
technologies to prevent unintended safety and health consequences.
Data will assist in the ongoing research and development of
resonant technologies along with complementary practices for
integrating new technologies in the workplace to reduce unintended
outcomes. Through this data collection, ultimately the federal
government will be able to efficiently react to the Future of Work
needs of workers across the country thereby fulfilling CDC/NIOSH’s
mission.
Methods
to be used to collect:
Methods to collect information from participants will include
health screenings; demographic information; psychometrically
supported surveys/interviews of user experiences and their
perceptions of direct reading, sensor, or robotics technologies
(before, during or after use); direct physiological measurements of
response to the technologies; biological measures of physiological
responses; anthropometric measures of body size and shape; measures
of wearable sensors’ fit; and measures of the body’s
movement through space (biomechanics). Sampling methods for
laboratory- and virtual reality-based studies will vary across
projects depending on the technology being assessed, methods used,
and industry/population of interest. Methods will be described in
detail for each individual project but will likely entail
measurement data collected via the technology of interest and
transferred to a computer and/or via an electronic survey completed
in the lab setting. All measurements will be conducted within a
controlled laboratory environment setting by trained laboratorians
and researchers.
The
subpopulation to be studied:
Study participants will include persons from the general population
who are generally healthy, thus are safe to participate in all
study procedures. Data collection may focus on technologies
ubiquitous to the industry being studied, new to the industry being
studied, or novel to any industry. Respondents will be recruited
via a variety of avenues (e.g., email, flyers, advertisements) and
are expected to vary in gender, age, races, ethnicities,
rural/urban locations, and/or specific regions.
How
data will be analyzed:
The data will be analyzed using various methods to be further
defined for individual projects submitted under this generic
information collection. In addition to submission of the
instruments utilized, all collections submitted under this generic
pathway will include a full Supporting Statement Part A that
describes the tool/method/intervention under development,
identifies the targeted respondent populations, includes a
justification for any incentives offered, assesses applicability of
the Privacy Act and includes a complete Privacy Impact Assessment
if necessary, and an accompanying Supporting Statement Part B if
any statistical methods are employed for sampling or analyses in
the study. For each package that may fall under the auspices of
this generic information collection, NIOSH does not claim that the
organizations and respondents will be statistically representative
of the entire working population and is not claiming
generalizability of results.
1. Circumstances Making the Collection of Information Necessary
The Centers for Disease Control and Prevention (CDC), National Institute for Occupational Safety and Health (NIOSH), is requesting approval of a new generic umbrella package for a period of three years under the project titled, “Direct Reading Methodologies, Sensors, and Robotics Technology Assessment in Lab/Simulator-based Settings.” This study is being conducted by NIOSH. Under the OSH Act of 1970 (29 USC 669 Section 20(a)(1)) (Attachment A), NIOSH has the responsibility to conduct research related to innovative methods, techniques, and approaches dealing with occupational safety and health problems. The requested generic information collection package focuses on a sub-set of automated direct reading methodologies, sensors, and robotics technologies that are used and continuously updated in the workplace. NIOSH is often requested to assess and study the accuracy and safety of new or updated technologies as well as the potential impact of these technologies interacting with workers. These studies primarily occur in laboratory settings, including virtual reality simulated space.
NIOSH operates within the CDC as a federal institute specifically dedicated to generating new knowledge in the field of occupational safety and health and responsible for transferring that knowledge into practice for the betterment of workers. To achieve the Institute’s mission, NIOSH conducts scientific research, develops guidance and authoritative recommendations, and disseminates information. Given NIOSH’s mission to develop new knowledge, the Institute is uniquely positioned to evaluate potential benefits and risks relative to occupational safety and health issues of the 21st century workplace, work, and workforce – also discussed as the Future of Work (FOW). Areas requiring detailed attention and advancement include research and development in artificial intelligence, robotics, and technologies (Tamers et al., 2020).
NIOSH has established alliances and partnerships with other federal agencies and external partners to collaborate and share technical knowledge to improve awareness around workplace hazards and appropriate safeguards as it relates to technology (Occupational Safety and Health Administration [OSHA], 2019; National Safety Council, 2024). NIOSH has also created Centers charged with leading and coordinating FOW efforts, with a focus on technology assessment and integration in the workplace that revolves around emerging recommendations and standards in advancing automation. Data collection will occur in laboratory space, including those equipped with virtual reality that simulate the applications and use of direct reading methodologies, sensor and automation technologies, and robotics technologies.
While the creation of alliances, partnerships, and Centers has broadened NIOSH’s approach to worker safety, health, and well-being and reduced redundancy, there is still a need to keep up with research as it relates rapid technological innovations, such as robotics and artificial intelligence, that are changing the nature of work. Direct reading, sensor, and robotics performance requirements and test methods are specified in a variety of voluntary consensus standards with no federal regulations currently in existence. Common voluntary consensus standards and tangential efforts include:
(1) American National Standards Institute (ANSI) safety standard R15.08-1-2020 and R15.08-2-2023. These standards address rapid changes underway in the industrial mobile robot segment, with a focus on keeping workers safe in a shared and dynamic work environment. As indicated, there is no OSHA standard in this area. However, OSHA refers to and relies on R15.08 as the primary standard applicable to robot systems.
(2) International Organization for Standardization (ISO) 10218-1 and ISO 10218-2 are standards that discuss the robots and robotic devices and safety requirements for industrial robots. These safety standards are organized in hierarchies based on technology types.
(3) ISO 13482:2014 and ASTM International Technical Committee on Exoskeletons and Exosuits (ASTM F48) focuses on exoskeletons or wearable robots and been tasked to develop performance standards for exoskeletons.
(4) American Industrial Hygiene Association (AIHA) has several working groups to explore how the performance of wearable sensors and technologies can be standardized and accredited in lab space. AIHA also developed a technical framework on the use of direct reading instruments to guide standardization in this space.
NIOSH has tried to adapt to conduct research more quickly and efficiently as technologies advance by using virtual reality simulations where human subjects can interact with simulated robots, as an example. However, the use of virtual reality settings, among other lab-based assessments, still require the need to develop and submit new PRAs each time NIOSH is called upon to assess the safety and health implications of new direct reading methodologies, sensors, and robotic technologies. Both the data collection mechanisms and information collected from human subjects in these settings are generally consistent across NIOSH studies and are expected to be largely consistent with the sample data collection instruments provided in Appendix B. Specifically, it is just the technology system and output being generated from that technology (e.g., worker response to an industrial robot versus worker response to an exoskeleton) that differs.
The challenge is that direct reading methodologies, sensors, and robotic technologies change so rapidly that NIOSH research cannot keep up with the request to assess the safety and health risks of updated models and changing consensus standards in real time. By the time one sensor or robotic technology is assessed, the same manufacturer could be on a subsequent model or version that is already in the marketplace. In other words, by the time approvals are received and research is ready to commence, the marketplace has already advanced, and the research proposed is no longer as relevant. To protect the workforce and make accurate recommendations to manufacturers and employers, a generic package is critical.
Further, the previously mentioned standards development organizations (SDOs), and other safety and health associations routinely update voluntary standards in this rapidly changing area to keep up with research and development efforts. For example, ANSI’s R15.08 safety standards around industrial and mobile robots have substantially changed and been updated three times in five years. NIOSH is often not able to conduct timely research to inform these voluntary consensus standards or keep up with new technology developments. NIOSH researchers participate on these various technical SDO committees to (1) discuss known gaps in the standard, (2) identify research that is needed to address these gaps, and (3) prioritize and select those issues that will be addressed during the revision cycle (which happens every 3-5 years). Without an expedited PRA approval, NIOSH is unable to provide the data needed to SDOs and other partners to make evidence-based decisions regarding performance requirements for direct reading methodologies, sensors, and robotics technologies in the workplace.
Data collection for this project is authorized under the OSH Act of 1970 (29 USC 669 Section 20(a)(1)) (Attachment A).
2. Purpose and Use of Information Collection
NIOSH requests a generic information collection package for assessing the safety and health considerations of rapidly changing direct reading methodologies, sensors, and robotics technologies. Data collection will occur in laboratory space, including virtual reality space that simulates these various technologies. Data collection may also occur virtually via follow-up phone interviews or surveys.
This generic information collection request will allow the federal government to maintain a relevant and considerate scientific understanding of voluntary consensus standards, manufacturer prototypes, and workplace policies and practices that govern the performance; test methods; or use, design, or construction of direst reading methodologies, sensors, and robotics, allowing for robust protection to the United States workforce while ensuring low burden and high usability in the workplace that is subject to rapid technological updates. To achieve this goal, this package requests recurring information collection from human subjects which will directly examine the interaction between human subjects and direct reading methodologies, sensors, and robotics under varying boundary conditions. Data will be collected by NIOSH employees or contractors and will occur for finite testing periods on a non-routine basis.
As illustrated in Appendix B, the types of data collection may include demographics and anatomical and physiological measurement of human subjects and measurements of physiological, perceptual, and biomechanical responses to wearable direct reading, sensors, and robots as well as examining the human worker interaction with these technologies to ensure adequate protection and low user burden across a variety of use scenarios. The objective of this request specifically is to enable NIOSH to engage in these types of information collection activities in a time- and resource-efficient fashion, catalyzing improved worker health and safety. None of the studies proposed under the auspices of this generic IC intend to produce results that can be generalized beyond the scope of each study. In addition, this information is not intended to be used for wider policy development, budget formulation, or other public-facing purposes (for example, as evidence to support worker safety rulemaking) not described in this supporting statement. Lastly, the subject matter and data collection methods are expected to be free of any controversy or special circumstances that may warrant public comment or extended review by OMB. Any such surveys will be submitted through the normal clearance process.
NIOSH has a continuing need for a more comprehensive understanding of the impacts of constantly changing direct reading methodologies, sensors, and robotics to inform relevant performance standards and associated methods towards the goal of adequately protecting workers across various industries via new technologies as well as reducing the burden of using or interacting with these technologies on the workers themselves. CDC NIOSH’s Future of Work (FOW) agenda (NIOSH, 2021) further supports this need and use for a generic information collection. Specifically, this generic IC supports the following goals:
Goal 5: Mitigate worker safety and health challenges and leverage opportunities associated with robotics. The CDC NIOSH FOW Agenda (2021) discusses the increasing use of collaborative robots in the workspace, use of unmanned aerial vehicles (UAVs, or drones), an increasing number of human workers who wear robots (i.e., powered and non-powered exoskeletons and exosuits), and the operation of fully automated vehicles to transport materials.
As a specific example, the autonomous, industrial mobile robot market size was valued at USD 1.61 billion in 2021 and is predicted to reach USD 22.15 billion by 2030 (Next Move Strategy Consulting, 2022). The most recent data from the Bureau of Labor Statistics (BLS) show that in 2020, the manufacturing and transportation/warehousing industries accounted for 14% and 8% of the 2.7 million workplace injuries and illnesses, where 36% and 48% led to cases with days away from work, respectively (BLS, 2020). Emerging technology, such as industrial mobile robots, are being rapidly implemented and has introduced potential new sources for workplace hazards.
Despite the advantages, workers are at risk of physical or psychological harm due to increased exposure to hazardous situations as more businesses adopt collaborative and mobile robots (Murashov et al., 2016; Howard et al., 2018, 2020). Additionally, advancements in fully and partially automated vehicle technologies pose new and unforeseen safety challenges (American Society of Safety Professionals, 2019). For example, workers may become more vulnerable to accidents as they lose situational awareness and fail to react appropriately to hazards when working alongside robots, a risk illustrated by fatal crashes involving highly automated vehicles (National Transportation Safety Board, 2020). Furthermore, since robots dictate the pace of work, employees may experience heightened stress due to changes in the nature and speed of their tasks.
Many studies that occur under this generic IC will respond to CDC NIOSH’s FOW objectives within Goal 5 (NIOSH, 2021); specifically, studies may seek to address any of the following research objectives in lab, virtual, and simulation-based studies:
Evaluate the benefits and risks of robotics, to include human–machine interaction, human action recognition, and intent prediction of robots in highly impacted jobs, occupations, and industries (such as transportation, manufacturing, foundries, mining, and welding).
Investigate potential consequences of drones, autonomous vehicles, other remotely controlled mobile equipment, exoskeletons, and exosuits on worker safety, health, and well-being.
Examine the human–machine interface to determine what and how much is needed, challenges when suboptimized, and the psychosocial impact on those working with new robotics technologies (including stress in using technologies not fully understood and concerns about subservience to the technology).
Assess and improve design standards for robots to address safety concerns and increase worker trust.
Explore and evaluate robotics technology and relevant education and training to improve worker safety and health equity for disadvantaged groups.
Conduct research to understand the impact of the emerging commercial use of unmanned aerial vehicles in relation to potential deaths and injuries from falls, toxic chemical exposures, electrical hazards, and collisions (such as in transportation, construction, agriculture, utilities, public safety, and mining).
Design interventions that improve safe adoption of advanced driver-assistance system features, including ensuring workers remain engaged while driving vehicles that are partially automated, to inform best management and design practices of use interfaces.
Study potential issues of vigilance and under- or over-trust for workers who use systems that employ automation and other forms of artificial intelligence.
Investigate and reduce the risks associated with the use of artificial intelligence and computer vision to detect and prevent incidents involving contact between a human worker and a collaborative robot, autonomous mobile robot, or unmanned vehicle.
Goal 6: Evaluate the impact of innovative and emerging technologies on worker well-being. The CDC NIOSH FOW agenda (2021) also discusses the new and developing technologies that characterize Industry 4.0, such as direct reading methodologies and sensors, which have reorganized the workplace and how work is being done. For example, sensors and controls that connect to the Internet-of-Things can collect, integrate, and analyze data from a distributed industrial network to not only improve assessment of different workplace safety and health hazards and productivity, but also remotely monitor and control large numbers of devices at different locations (Chui et al. 2010; Falkenthal et al., 2016).
These capabilities and flexibilities have enhanced both productivity and safety by alerting workers to hazards, maintaining processes within acceptable risk parameters, and assisting in risk management decisions (Bloem et al., 2014). However, these advancements have not come without new challenges to worker well-being. Employees under close surveillance may take risks to uphold productivity or attempt to circumvent specific data collection efforts (Tomczak et al., 2020). Meanwhile, individuals tasked with managing multiple devices simultaneously, particularly during emergencies, may experience cognitive overload and subsequent psychosocial issues (Schulte et al., 2020).
Additionally, despite offering greater functionality than traditional sensors (Falkenthal et al., 2016), advanced or “smart” sensors, which can be worn or embedded in safety clothing, or attached to a workplace object (Nag et al., 2017, Metz, 2018) may elicit privacy concerns associated with the monitoring and tracking of certain aspects of worker performance. To this end, studies submitted under this generic IC may seek to address objectives (NIOSH, 2021) in lab or virtual/simulation-based studies such as:
Compile, evaluate, improve, and disseminate data sources that inform policies, programs, and practices evaluating the impact of new technologies on exposures and hazards.
Track positive and negative consequences and changes on worker safety, health, and well-being from using sensors, manufacturing processes, and the Internet-of-Things rather than traditional technologies.
Evaluate the effectiveness of workers using engineering controls and smart PPE to reduce exposures for those who develop and use technologies.
Create best practices to ensure ethical monitoring and surveillance (including informed consent) among workers whose data are collected by sensors.
Collect open-ended data to inform design of risk assessment guidance, control methods, and health and safety management systems protocols to inform, train, safeguard, and empower workers to develop and use technologies safely and ethically.
Anticipate and minimize potential adverse worker effects (such as lack of autonomy and job control) on worker well-being, early in the development and implementation of new technologies.
Develop methods to minimize bias in the scientific assumptions and programming that underlie technology-based designs for PPE and other protective equipment such as exoskeletons (for example, use research and computer modeling to include more diverse anthropometric data).
These new technologies, designs, construction approaches, and use cases must be tested in an efficient manner to understand the direct impact on workers and necessary revisions to performance standards or test methods to accommodate the changing technological workplace. All the above examples are applicable to most if not all occupations and industries. Data collected will be used to improve worker safety, health, and well-being outcomes through improved policies, standards, programs, and practices that surround direct reading methodologies, sensors, and robotics in the workplace.
The information collected for a project will be maintained or stored locally under strict access controls limited to the local project leader/manager or their designate. In some cases, personally identifiable information (PII) will need to be collected primarily for the purpose of facilitating payment. If it is, PII will be kept in a separate location and accessible only to the project-specific research staff. This information will be destroyed when the participant’s contribution to the project has ended. Under no circumstances will an individual be identified using a combination of variables such as gender, race, birth date, and/or other descriptors.
3. Use of Improved Information Technology and Burden Reduction
Section 2 highlighted common research objectives and complementary activities that may be proposed in this package that cover various direct reading methodologies, sensors, and robotics. Many of these studies ask the same questions of human subjects about different technologies. In almost all cases, data collected will require human subjects to complete in-person testing sessions where they will discuss, test, wear, or interact with various direct reading methodologies, sensors, and robotics while being monitored for physiological, biological, or biomechanical changes to their body or answer questions about their experiences and perceptions. Additionally, anthropometric measurements of their body and of how the technology in question fits their body may be included in the in-person testing sessions.
To reduce burden to the human subjects and to comply with the Government Paperwork Elimination Act, Public Law 105-277, title XVII, signed into law on October 21, 1998, data collection will occur in the most time efficient and technologically advanced fashion possible. Technologically advanced equipment, regular maintenance, adequate planning, sufficient staff resources, and appropriate physical environments will support every project to reduce measurement errors, human subject down time, and equipment-related delays during data collection. Measurements will only be taken when they directly relate to the advancement of the technological performance or protection for the relevant worker population as per each study’s individualized aims. Lastly, all human subjects will be informed and voluntarily consent to the specific study procedures and associated time commitment prior to the initiation of any study procedures.
Additionally, human subjects may be asked to answer surveys or open-ended questions related to their demographic background, their health, occupation, and perceptions of technologies in an effort to ensure participant safety during testing as well as characterize the human subject information relative to the collected research variables. Again, to reduce the human subject burden as per the Government Paperwork Elimination Act, these types of questionnaires will be distributed electronically whenever possible. This approach ensures data quality but decreases respondent burden with built-in skip logic. Most often, electronic platforms such as CDC’s Research Electronic Data Capture (REDCap), an approved IT platform, will be used.
Though electronic technologies will be used by many of the individual projects in this data collection, the nature of some proposed activities requires direct interaction between respondents and project staff, especially in the case of in-depth focus groups or interviews and psychological observation and monitoring.
4. Efforts to Identify Duplication and Use of Similar Information
NIOSH collaborates with other federal agencies, academic institutions, standards development organizations, and contracting mechanisms to advance their mission. NIOSH has established alliances and partnerships with other federal agencies and external partners to collaborate and share technical knowledge to improve awareness around workplace hazards and appropriate safeguards as it relates to technology. Consequently, NIOSH created two Centers charged with leading and coordinating these FOW efforts, with a focus on technology assessment and integration in the workplace that revolves around emerging recommendations and standards in advancing automation.
First, in 2014, the NIOSH Center for Direct Reading and Sensor Technologies (CDRST) was established to research and develop recommendations on the use of 21st century technologies in occupational safety and health. Both direct-reading methodologies and sensors are used to detect and monitor hazardous conditions, to assess and document intervention strategies, and especially to immediately trigger alarms in the event of unsafe conditions. Examples of direct reading and sensor technologies include real-time personal monitoring, wearable monitors, and exoskeletons including wearable robots.
Second, in 2017, NIOSH established the Center for Occupational Robotics Research (CORR) to study the nature of robots in the workplace, evaluate workplace interventions to prevent robot-related worker injuries, and develop guidance for safe interactions between humans and robots. There are several common types of robots used in occupational environments – traditional industrial robots; professional or service robots; collaborative robots; and mobile robots (e.g., drones and powered exoskeletons).
These Centers are responsible for establishing Memorandums of Understanding, Research Collaboration Agreements, and Data Use Agreements and hold regular meetings with federal and non-federal partners to ensure research and development efforts around direct reading methodologies, sensors, and robotics technologies are not only warranted but coordinated. Alliances and meetings with OSHA also occur around these topics. Lastly, NIOSH has representatives on all major standards committees allowing us to liaison with any other federal partners participating in private sector initiatives related to the project proposed in this generic submission. This generic package will allow such recommendations to be coordinated and addressed holistically and systematically across industry sectors and respiratory hazards.
5. Impact on Small Businesses or Other Small Entities
The data collection efforts reflected in this request will occur from individuals volunteering for participation in studies in their own free time. As such, the data collection will not negatively impact small businesses or other small entities with additional paperwork or task burdens. With that said, the outcomes of the research (advancement of safe development and use of direct reading methodologies, sensors, and robotics in the workplace) will support the availability of up-to-date technological information and designs that reflect evolving manufacturer, organizational, and worker needs. This will in turn decrease the burden on small employers and workers to decide how, when, why, and by whom new technologies should be used to maintain adequate protection and avoid unintended consequences. If, in the case that an individual study involves information or assessments directly related to small businesses or other small entities, the methods used to minimize burden will be explained when being submitted under this generic.
6. Consequences of Collecting the Information Less Frequently
The information collection described in this package will be completed on a reoccurring basis across the lifespan of this generic. The timeline for data collection is largely driven by regulatory agendas, timeframes established by standards development organizations, updated designs and uses of various direct reading methodologies, sensors, and robots, or by urgent needs. This data collection request and associated timeline allows for collection of information in a timely and efficient way without significant lag from need identification to solution generation or intervention. If this research were not conducted at all or in this manner, the contemporary needs and challenges of new organizations and workers each day that are being expected to integrate and use direct reading methodologies, sensors, and robotics as a part of their job may not be able to be considered efficiently enough to have large scale impact on the evolving designs, safety standards, safe integration, or manufacturing system. Thus, the workers may not be supported or protected adequately. There are no legal obstacles to reducing the burden.
7. Special Circumstances Relating to the Guidelines of 5 CFR 1320.5
This request fully complies with the regulation 5 CFR 1320.5.
8. Comments in Response to the Federal Register Notice and Efforts to Consult Outside Agencies
The Federal Register notice was published for this collection on April 23, 2024, Vol. 89, No. 79, pg. 30372-30374 (Attachment C).
No public contacts and opportunities for public comments were received.
Representatives across CDC NIOSH divisions, offices, and laboratories (DLOs) and centers were engaged to develop this request. DLO representatives and titles who provided feedback are listed in Table 1. Names of these representatives are available upon request.
NIOSH DLO or Center |
Representatives |
Division of Safety Research |
Deputy Director |
Pittsburgh Mining Research Division |
Associate Director for Science |
Spokane Mining Research Division |
Associate Director for Science |
Health Effects Laboratory Division |
Associate Director for Science |
Division of Field Studies and Engineering |
Associate Director for Science |
Western States Division |
Associate Director for Science |
National Personal Protective Technology Laboratory |
Associate Director for Science |
Center for Direct Reading and Sensor Technologies |
Center Coordinator |
Center for Occupational Robotics Research |
Center Coordinator |
9. Explanation of Any Payment or Gift to Respondents
Per OMB guidance, incentives are generally not appropriate for contractors, cooperators, grantees, or program participants because they already have a pre-existing relationship with the agency. Incentives are most appropriate where participants are being asked to travel to a site to participate in a research activity using technology and providing feedback. Incentives are generally not appropriate for questionnaires/surveys.
If an incentive is proposed, a detailed justification based on the type of collection, population of respondents, and other circumstances will be provided in the individual information collection request. Per the Office of Information and Regulatory Affairs, Office of Management and Budget guidance document Questions and Answers when Designing Surveys for Information Collections (Updated Oct. 2016), justifications will focus on data quality, burden on the respondent, past experience, improved coverage of specialized respondents, rare groups, or minority populations; reduced survey costs; and/or equity.
Each justification will cite the research literature that demonstrates significant improvements in response rates and non-response bias when applied to similar participants, data collection methods, and data collection contexts. OMB does not consider it appropriate to use private sector market rates as a justification for incentives in government information collections. The following includes expected ceiling amounts for different types of collections:
Focus groups where participates are expected to travel to a central site: Up to $40 total
Cognitive interviews or similar exercises (intensive one-on-one probing of basis for thoughts) in which participants are expected to travel to a central site: Up to $40 total
Engagement with various types of direct reading methodologies, sensors, or robotics (physical or simulated) at a central site location: Up to $40 per hour
Questionnaires/surveys/interviews: TBD, under special circumstances
For any collection over 90 minutes, participants may be offered an additional incentive to account for incidental expenses (transportation, childcare, lost wages, etc.). This will be included in all justification documents if applicable.
10. Protection of the Privacy and Confidentiality of Information Provided by Respondents
Depending on the specifics of the individual data collection project, the Privacy Act may or may not apply to an information collection. For each individual investigation, the appropriate CDC NIOSH contacts will be consulted for an official Privacy Act determination. Further, if NIOSH or its representative is receiving and/or storing personal identifiable information as a part of a specific project, then the Privacy Act may apply and the specific actions required to ensure the security of that information will be discussed in the documentation for each project submission.
Although personally identifiable information (PII) may be collected, in some instances NIOSH will not receive any identifiable information from any of the individual projects. In such cases, when the individual data collection activities require respondents to provide identifying or potentially identifying information to local project staff and/or answer sensitive questions, the information will be removed from any data sent to NIOSH, and NIOSH will, at no time, have access to any local data that contains identifiers. Local project staff will verify that any individually identifiable information that has been collected during their activities has been removed from information transmitted to or shared with NIOSH.
Certificates of confidentiality may be sought for individual data collection activities that involve sensitive and potentially identifiable information at the local project level. Also, depending on the specifics of the project, the assurance of confidentiality afforded in accordance with Section 308(d) of the Public Health Service Act (42USC242m) and the Confidential Information Protection and Statistical Efficiency Act (PL-107-347) may apply.
As methods and materials may differ between individual projects, appropriate human subjects review procedures will be conducted for each project as they are developed. Projects will acquire IRB approval when appropriate and submit documentation. Participation in all research activities is strictly voluntary. Respondents will be provided with an informed consent form prior to the start of information collection and will be allowed to ask questions about the project before deciding whether to participate. These forms will be included in each individual collection request. The consent form describes the purpose of the study, specifies specific procedures that will be conducted, and describes protections for the respondent’s privacy.
On occasion, collecting information about sensitive topics requires that we do not collect personal identifiers at any point. Collection of these identifiers may place the respondent at risk of potential harm resulting from breach of privacy. In these cases, a waiver of documentation of informed consent is requested (i.e., no respondent signatures on a consent form), but the same consent and privacy protection information is still imparted to the respondent.
Persons participating in all projects conducted or sponsored by NIOSH will be informed that their data will be maintained in a secure manner, and that the data will only be used for purposes stated in the consent form. Generally, all individually identifiable information collected by local partners would be unlinked or stripped from the data base that is submitted to CDC. Although the identities of respondents may be known to local project personnel who conduct interviews and interact with respondents, data collected regarding such sensitive topics will not be stored or accessed in a Privacy Act system of records, and the respondents’ identifying information will not be submitted to CDC. Only authorized project staff will be allowed to have access to study information (whether identifiable or not) and all information will be kept in a locked cabinet and/or locked office with limited access.
Information might be collected electronically or on paper (depending on the individual information collection request). Electronic means include handheld devices, computer-assisted self-interview (CASI), audio computer-assisted self-interview (ACASI), computer-assisted telephone interview (CATI), web-based surveys, or other point of service collection devices. Paper copies are the common mode for focus groups or interviews that may request more information around perceptions and experiences with technologies of interest. Web-based methods for survey or intervention information collection may be used. There will be no internet content directed at children under the age of 13. Individual collection requests submitted under this generic approval will describe any web-based material involved.
Electronic data collection and data management systems used for these activities will comply with the current encryption security standards from National Institute of Standards and Technology (NIST) Federal Information Processing Standards (FIPS), which meet or exceed Advanced Encryption Standards (AES). Each individual request under this generic clearance will provide adequate descriptions of information systems that will be used in their study.
11. Institutional Review Board (IRB) and Justification for Sensitive Questions
IRB requirements are investigation specific. Some investigations require IRB approval while others fall within the IRB exemption criteria (45 CFR Part 46.104) or are considered a non-research, public health surveillance activity (45 CFR Part 46.102(l)(2). For individual investigations, the appropriate CDC NIOSH contacts are consulted for an official research determination.
Each individual project ICR will address human subject participation and IRB approval. Because methods and materials may differ between individual projects, appropriate human subjects review procedures will be conducted for each project as they are developed. Projects that need IRB approval will be submitted with a copy of the approval document. If the study has been determined to be exempt from IRB, a copy of the exemption determination will be attached. If the appropriate CDC official has determined that the data/ information collection is not research involving human subjects, the information collection submitted under this generic clearance will state that IRB approval is not required.
Sensitive Questions
At times, the information collected related to human subject medical history may involve practices or matters that are commonly considered private. Race and ethnicity data, as well as diagnoses of medical conditions that may affect employability or insurability may also be viewed as sensitive or even threatening by a portion of respondents. The reasons for collection of sensitive information and their application for the improvement of CDC’s prevention efforts for the specific study sample will be addressed in the specific requests. The procedures used to obtain consent and the content of the consent form will also be explained and justified.
Sensitive personally identifying information (PII) such as social security numbers may be collected during the individual project data collections as per the individual project’s proposed methods. These methods will be outlined clearly in each individual project submission associated with this generic information collection. See section 10 Protection of the Privacy and Confidentiality of Information Provided by Respondents for more details about how this data will be handled.
12. Estimates of Annualized Burden Hours and Costs
We estimate that up to 4,000 individuals could be burdened per year with an estimated annualized burden of 68,334 hours over a three-year period (total three-year burden = 205,002 hours) from different industries. It is estimated that it will take about 5 minutes to consent individuals and then anywhere between 15 and 90 minutes to complete additional data collection instruments, depending on the study. No single data collection activity is expected to take longer than 4 hours to complete from inception of information collection to completion of all instruments or activities within the single study. We anticipate approximately 12 information collections per year which may include examination of human subject physical and psychological responses to wearing, testing, using, comparing, and/or providing feedback on various direct reading methodologies, sensors, or robotics, or measuring the fit of a technology or other protective device (physical or simulated) on the subjects’ bodies. The following table provides an estimate of the annualized burden hours over a three-year period. There is no cost to respondents other than their time.
Exhibit A.12.A - Annualized Burden Hours
A.12.B Estimated Annualized Costs
Data collections by CDC/NIOSH are generally funded through internal or external research funding and these will be noted in the specific collection requests. The annualized cost to the respondent is segmented accordingly in Exhibit A.12.B.
The United States Department of Labor, Bureau of Labor Statistics, May, 2023 (http://www.bls.gov/oes/current/oes_nat.html) data were used to estimate the hourly wage rate for the general public for the purpose of this generic request. Each project will have cost specific to the category of the respondents. Because it is not known what the wage rate category will be appropriate for the specific projects (or even whether they will be employed at all), the figure of $32.00 per hour was used as an estimate of average hourly wage across the country.
Exhibit A.12.B - Annualized Cost to Respondents
Activity |
Total Burden Hours |
Hourly Wage Rate |
Total Respondent Cost |
Data collection |
68,334 |
$30.00 |
$2,050,020 |
13. Estimates of Other Total Annual Cost Burden to Respondents and Record Keepers
NIOSH does not anticipate providing start up or other related costs to private entities.
14. Annualized Costs to the Government
Actual annualized costs to the government will vary depending on the specific needs of the individual information collection activity. Generally, each development activity will involve participation of at least one NIOSH project officer (GS-12, 13, 14, or 15 levels) who will be responsible for the project design, obtaining IRB approvals, providing project oversight, and analysis and dissemination of the results. The NIOSH project officer will provide onsite technical assistance during the data collection. In most cases, a NIOSH data manager or technical assistant’s (typically equivalent to GS-9, 11 or 12) time will also be required by one or two individuals. An estimated average cost per individual activity is listed below, but detailed costs will be submitted with each individual collection request. While many of the proposed data collection efforts will be completed at on-site laboratories thus requiring no travel, a mobile laboratory that includes the instruments outlined in the burden table may be used to travel to off-site locations. Thus, investigator travel costs are included in the annualized cost estimates to account for associated travel with mobile laboratory data collections.
Exhibit A.14.A - Annualized Cost to the Government
Expense Type |
Expense Explanation |
Annual Costs (dollars) |
Direct Costs to the Federal Government |
|
|
|
NIOSH Project Officer (GS-13/14, 0.5 FTE) |
$40,641 |
|
NIOSH data manager or technical assistant (GS-9/11, 0.5 FTE) |
$13,450 |
|
CDC NIOSH IT Security Compliance |
$100,000 |
|
NIOSH Travel (10 trips) |
$20,000 |
|
Subtotal, Direct costs |
$174,091 |
Cooperative Agreement or Contract |
Data collection equipment, participant compensation, and contractual agreements. |
$400,000 |
|
TOTAL COST TO THE GOVERNMENT |
$574,091 |
15. Explanation for Program Changes or Adjustments
This is a new data/information collection.
16. Plans for Tabulation and Publication and Project Time Schedule
Individual data collections under this generic approval will be time-limited and generally conducted only once, except in the cases of individual studies where subjects may be asked to attend several separate data collection sessions. No single data collection activity is expected to take longer than 3 years to complete from inception of information collection to the first report of findings. Proposed timelines will be submitted for each individual data collection activity. Only in rare cases would data that is collected not be published and made publicly available in aggregate form. At the time of this submission, NIOSH has not identified any such cases. It is expected that each data collection would result in at least one journal article publication. In addition, during the preliminary phase of data analysis and interpretation, each individual data collection may also be published in a proceedings for a conference administered by a professional society where experts in the domain of interest would be permitted to engage and provide feedback about the interpretation of analyses prior to final publication. Finally, findings from these information collections may be used to develop NIOSH-numbered publications such as fact sheets or infographics to ensure members of the public such as workers benefit from these information collections. In general, publication of findings is expected to occur anywhere from 6-18 months after the completion of information collection.
17. Reason(s) Display of OMB Expiration Date is Inappropriate
The display of the OMB expiration date is not inappropriate.
18. Exceptions to Certification for Paperwork Reduction Act Submissions
There are no exceptions to the certification.
References
ASSP [2019]. ASSP TR-Z15.3-2019 Technical report: management practices for the safe operation of partially and fully automated motor vehicles. American Society of Safety Professionals, https://www.assp.org/standards/standards-topics/fleet motor-vehicles-z15-1.
Bloem J, Doorn M, Duivestein S, Excoffier D, Maasl R, Ommeren E [2014]. The fourth industrial revolution. Things to tighten the link between IT and OT. VINT Report 3, https://labs.sogeti.com/research1/fourth-industrial-revolution-internet-things tighten-link-ot/.
BLS, 2020. EMPLOYER-REPORTED WORKPLACE INJURIES AND ILLNESSES – 2020. In: Survey of Occupational Injuries and Illnesses Data, 2019. Washington, DC: U.S. Department of Labor, Bureau of Labor Statistics.
Chui M, Loeffler M, Roberts R [2010]. The internet of things. McKinsey Quarterly, https://www.mckinsey.com/industries/high-tech/our-insights/the-internet-of-things.
Falkenthal M, Breitenbücher U, Christ M, Endres C, Kempa-Liehr A, Leymann F, Zimmermann M [2016]. Towards function and data shipping in manufacturing environments: how cloud technologies leverage the 4th industrial revolution. Research Gate, https://www.researchgate.net/publication/309476649_Towards_ Function_and_Data_Shipping_in_Manufacturing_Environments_How_Cloud_ Technologies_leverage_the_4th_Industrial_Revolution 16–25.
Howard J, Murashov V, Branche C [2018]. Unmanned aerial vehicles in construction and worker safety. Am J Ind Med 61(1):3–10.
Howard J, Murashov V, Lowe B, Lu M [2020]. Industrial exoskeletons: need for intervention effectiveness research. Am J Ind Med 63(3):201–208.
Metz R [2018]. The company embeds microchips in its employees, and they love it. Technology Review, https://www.technologyreview.com/s/611884/this-company embeds-microchips-in-its-employees-and-they-love-it/.
Murashov V, Hearl F, Howard J [2016]. Working safely with robot workers: recommendations for the new workplace. J Occup Environ Hyg 13(3):D61–D71.
Nag A, Mukhopadbyay S [2017]. Wearable flexible sensors: a review. IEEE Sens J 17:3949–3960.
Next Move Strategy Consulting (April 2022). Autonomous Mobile Robot Market by Type, by Application, by End-User - Global Opportunity Analysis and Industry Forecast 2022-2030. https://www.researchandmarkets.com/reports/5529480/autonomous-mobile-robot-market-by-type-by
NIOSH [2021]. The NIOSH future of work initiative research agenda. By Tamers S, Pana-Cryan R, Ruff T, Streit J, Flynn M, Childress A, Chang CC, Novicki E, Ray T, Fosbroke D, Geraci C. Cincinnati, OH: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health. DHHS (NIOSH) Publication No. 2022-105, https://doi.org/10.26616/NIOSHPUB2022105
NTSB (National Transportation Safety Board) [2020]. Tesla crash investigation yields 9 NTSB safety recommendations. National Transportation Safety Board News Release, 2/25/2020.
Schulte P, Streit J, Sheriff F, Delclos G, Felknor S, Tamers SL, Fendinger S, Grosch J, Sala R [2020]. Potential scenarios and hazards in the work of the future: a systematic review of the peer-reviewed and gray literatures. Ann Work Expos Health 64(8):786–816.
Tamers, S. L., Streit, J., Pana‐Cryan, R., Ray, T., Syron, L., Flynn, M. A., ... & Howard, J. (2020). Envisioning the future of work to safeguard the safety, health, and well‐being of the workforce: A perspective from the CDC's National Institute for Occupational Safety and Health. American journal of industrial medicine, 63(12), 1065-1084.
Tomczak D, Behrend T, Willford J, Jimenez W [2020]. I didn’t agree to these terms: electronic performance monitoring violates the psychological contract. PsyArXiv, https://psyarxiv.com/qax9u/
Appendices
Appendix A: Example Informed Consent Forms
Below, we offer three examples of informed consent forms that describe projects that we anticipate would fit in this generic information collection.
Example Project 1 occurs in the virtual reality space to simulate experiences operating a mobile demolition robot to understand how hazards in the space impact behaviors while operating the equipment.
Example Project 2 occurs in a NIOSH robots laboratory, assessing workers’ perceived safety, trust, and comfort when interacting with one or two industrial mobile robots in a set up workspace.
Example Project 3 occurs via virtual interviews to discuss frameworks being used to develop and update practices around direct reading methodologies and sensors to reduce exposure to respirable crystalline silica dust.
Example 1
Include OMB header if needed: Public reporting burden of this collection of information is estimated to average XX minutes per response, including the time for reviewing instructions, searching existing data sources, gathering and maintaining the data needed, and completing and reviewing the collection of information. An agency may not conduct or sponsor, and a person is not required to respond to a collection of information unless it displays a currently valid OMB control number. Send comments regarding this burden estimate or any other aspect of this collection of information, including suggestions for reducing this burden to CDC/ATSDR Information Collection Review Office, 1600 Clifton Road NE, MS D-74, Atlanta, Georgia 30333; ATTN: PRA (XXX). |
Form Approved OMB No. XXX Exp. Date XX/XX/XXXX |

Consent to be in a Research Study Identification of Risk Factors for Demolition Robot Operators Study 1: Human, environment, and control-pad factors, and Study 2: Human behavior and perceptions of safety and trust when the robot moves unexpectedly. |
||
|
|
Key Information Summary |
Your consent is being sought for you to participate in a research study and your participation is voluntary. You will be given a copy of this form for your records. The purpose of the study is to understand what factors may lead operators of demolition robots to stand in hazardous places near the robot, and to explore the perception of operator about the safety of the demolition robot. Study 1 will take approximately 2.5 hours (of which 60 minutes you will be inside the VR simulator) and Study 2 will take approximately 2 hours (of which 40 minutes you will be inside the VR simulator). However, if you are only participating in Study 2, it will take approximately 3 hours to complete the study. Both Study 1 and Study 2 will be conducted in the NIOSH’s Virtual Reality Lab. This lab uses a CAVE-type surround screen virtual reality system, which consists of four large projected screens: front, left, right, and floor; and provides a semi-immersive virtual reality experience. You will be asked to complete a series of tasks while in a standing position; you may be required to move two or three steps in every direction. There will not be any other human subjects participating in the studies concurrently with you. During the time you complete the tasks, you will be wearing virtual reality glasses. Motion capture markers will be strapped to different parts of your body. The markers on the VR 3D glasses, as well as the markers on your waist and hand, and the markers on the Logitech remote control will serve to collect your position and motion data. Some people who use virtual reality equipment can have symptoms like those of motion sickness (general discomfort, nausea, fatigue, headache, eye strain, difficulty focusing, fullness of head, blurred vision, dizziness, and vertigo). When this happens with virtual reality equipment it can be called ‘simulator sickness’. The risks of participating in Study 1 and/or Study 2 involve developing simulator sickness symptoms. There is also a small risk that you could feel discomfort from wearing the VR goggles, and that you could develop psychological stress due to the awareness of safety failures. Throughout the study we will ask you to fill different questionnaires, including a Virtual Reality Sickness questionnaire, a Construction/Demolition Robot Experience Questionnaire, a NASA Task Load Index questionnaire, a Human-Robot Trust questionnaire, and a Post-Test questionnaire. We will make every effort to protect your privacy and you will not be individually identified in any scientific document for your participation in this study. You will be paid for your time. We will recruit up to 200 participants to complete both Study 1 and Study 2. There is a small risk you could get a respiratory infection (e.g., COVID-19, influenza) through an in-person interaction while participating in this study. To minimize your risk of exposure to viruses and maximize your protection against infection, NIOSH researchers follow COVID-19 and other relevant respiratory infectious disease guidance for CDC and NIOSH staff and workplaces. We will also clean the equipment including the VR glasses, the hand and waist markers’ straps, and the remote control, after each participant to minimize contamination or germ transmission. |
|
|
Who is conducting the study? |
The National Institute for Occupational Safety and Health (NIOSH) is a federal agency that studies worker safety and health. We are part of the Centers for Disease Control and Prevention (CDC). |
|
|
What is the purpose? |
Both Study 1 and Study 2 involve research. The purpose of Study 1 is to explore how visibility of a demolition task, environment lighting, and remote control-pad design affect an operator in operating a demolition robot. The purpose of Study 2 is to assess the perception and physical responses of the operators of demolition robots to various unexpected motions of the robot/structure. |
|
|
What will I do? |
For Study 1 you will be asked to operate a virtual demolition robot using a remote control to perform demolition work in the following scenarios:
For Study 2 you will be asked to operate the virtual demolition robot to demolish a block wall. In this study, a block in the wall will be highlighted, and you will need to move the tip of the robot’s arm to that block, and then activate the chip hammer to break off the face of the block. When this happens, a number will appear on the highlighted block for approximately one second. You will need to read the number and then press a left button in the remote control if the number is odd, or a right button if the number is even. While completing the tasks for both Study 1 and Study 2, you will be asked to walk and move around the environment and select a place where you can perform your tasks comfortably. Your movement will be recorded during the tasks by the cameras installed in the VR simulator that track motion capture markers that will be strapped to different parts of your body. Before the experiment, you will complete a questionnaire consisting of 4 questions asking about your experience in construction and your experience with demolition robots and video games. During the experiment, you will be asked to complete questionnaires to assess your task load and your trust level on the demolition robot. After completing the experiment, you will respond to a post-test questionnaire. |
|
|
When, where, for how long will I be needed? |
You will initially be screened to determine your eligibility to participate via phone. If you are eligible to participate in the study, you will be asked to visit the Virtual Reality Laboratory located at NIOSH’s facilities in Morgantown, WV. Study 1 will take approximately 2.5 hours and Study 2 will take approximately 2 hours. However, if you are only participating in Study 2, it will take approximately 3 hours to complete the study. |
6 |
Are there any risks from participating in the study? |
You will be asked to operate a virtual demolition robot similar to a video game. You may feel simulator sickness during and/or after the study due to wearing the VR glasses. Symptoms of simulator sickness include general discomfort, nausea, fatigue, headache, eye strain, difficulty focusing, fullness of head, blurred vision, dizziness, and vertigo, or fainting. If you feel the onset of any of these symptoms at any time during or after the study, please be vocal and tell the investigators immediately. The study will be stopped, and you will be guided to a comfortable chair to rest until the symptoms disappear. If you feel like you want to vomit, there will be a lined trash can that you can use; also, the researcher can walk you to the nearest restroom if you need to use it. You will not continue the study after experiencing any symptoms unless the symptoms disappear, and you tell us you desire to continue. The symptoms are likely to be temporary and expected to disappear after a rest. However, if you experience the simulator sickness symptoms in more than two occasions, the test will be terminated to guarantee your safety and well-being. To help avoid simulator sickness, before you participate in the study you will be asked some questions that will help us understand if you might be susceptible. If you have ever experienced motion sickness in the past (for example from being in a motor vehicle or plane) severe enough that you have had to stop your activity because you were sick, you could also be at increased risk of experiencing simulator sickness, and you should not participate in this study. There are no tripping hazards like cables hanging from you or laying in the floor since all the equipment uses wireless technology; also, there are no mats or rugs on the floor that could cause you to trip and fall. A researcher will monitor you throughout the study from approximately 10 feet away from you, and if you get too close to the wall, the researcher will alert you, and if necessary, will intervene to prevent you from bumping against the wall. You may also experience psychological stress due to the fact that you will be aware that there will be safety failures with the virtual demolition robot. If you feel that you are feeling stressed, then let the researcher immediately know about this situation. The researcher will immediately stop the test and reassure you that the safety failures related to the demolition robot will all be entirely simulated, and that the laboratory environment is completely safe. If you feel better and decide to continue the study, the test will continue; otherwise, the test will be terminated, and you will receive payment for your participation up to that point. You will be asked to provide your name and birth date for payment purposes only; this is considered Personal Identifiable Information. There is a very low risk related to a potential breach of confidentiality of your Personal Identifiable Information; however, we will take all the precautions necessary to protect this information. There is a very small risk you could get a respiratory infection (e.g., COVID-19, influenza) through an in-person interaction while participating in this study. To minimize your risk of exposure to viruses and maximize your protection against infection, NIOSH researchers follow COVID-19 and other relevant respiratory infectious disease guidance for CDC and NIOSH staff and workplaces. In addition, we will also clean the equipment including the VR glasses, the hand and waist markers’ straps, and the remote control, after each participant to minimize contamination or germ transmission. |
|
|
Are there other benefits? |
You will not receive any direct benefits from participating in this study. Your participation in this research will help scientists identify the best practice procedure while operating demolition robots which may advance the field of workplace safety. |
|
|
Is my participation voluntary? |
Your participation in the study is voluntary. You may choose not to answer any or all questions. You may drop out at any time, for any reason, without consequences to you. If you have completed your participation in the study but would still like to withdraw, you may do so prior to our publication of the study by contacting the researcher using the contact information on this form. If you decide to drop out of any of the studies, at any time, a partial payment will be made to you at a rate of $10 per 20 minutes, with periods less than 20 minutes rounded up. For example, if you decide to drop out after 25 minutes, you will receive $20. |
|
|
What if I am injured or harmed at a NIOSH research facility or at another location where the NIOSH research project is being conducted? |
NIOSH will summon emergency medical aid by calling 911 if needed. NIOSH will not provide payment for medical care or compensation. If you believe NIOSH has been negligent in conducting the research study and you believe you have suffered a harm as a result, you have the right to pursue a legal remedy under the Federal Tort Claims Act (28 U.S.C. §§ 2671-2680 and 28 U.S.C. § 1346(b)). To learn more about how to file a Federal Tort claim, call the General Law Division of the HHS Office of the General Counsel at (202) 619-2155 or go to https://www.hhs.gov/about/agencies/ogc/key-personnel/general-law-division/index.html. |
|
|
Will I be reimbursed or paid? |
You will be paid at a rate of $30 per hour for your time during the study. For periods of time less than one hour, you will receive partial pay of $10 per 20 minutes, with periods less than 20 minutes rounded up. For example, if it takes four and a half hours to complete the study, you will receive $140. |
|
|
What alternative procedures might benefit me? |
No alternative procedures are available for this study. |
|
|
Will my personal information be kept confidential? |
Personal information collected for the study is limited to your birth date, gender, and general employee history and type. Each participant will be assigned a study number for identification. Name will be collected in order to pay reimbursement. This information will be stored separately and not connected to any data collected during the study. The information will be destroyed after the project is completed. NIOSH is authorized to collect your personal information and will protect it to the extent allowed by law. Monitors, auditors, the IRB, and/or the regulatory authorities will be granted direct access to the subject's study records for verification of study procedures and/or data, without violating the confidentiality of the subject, to the extent permitted by the applicable laws and regulations and that, by signing a written informed consent form, the subject or the subject's legally acceptable representative is authorizing such access. Your information will not be used or distributed for future research studies even if identifiers are removed. |
|
|
Certificate of Confidentiality |
This research project has a Certificate of Confidentiality from the Centers for Disease Control and Prevention (CDC). Unless you say it is okay, researchers cannot release information that may identify you for a legal action, a lawsuit, or as evidence. This protection applies to requests from federal, state, or local civil, criminal, administrative, legislative, or other proceedings. As an example, the Certificate would protect your information from a court subpoena. There are some important things that you need to know. The Certificate does not protect your information if a federal, state or local law says it must be reported. For example, some laws require reporting of abuse, communicable diseases, and threats of harm to yourself or others. The Certificate CANNOT BE USED to stop a federal or state government agency from checking records or evaluating programs. The Certificate DOES NOT stop reporting required by the U.S. Food and Drug Administration (FDA). The Certificate also does not stop your information from being used for other research if allowed by federal regulations. Researchers may release your information when you say it is okay. For example, you may give them permission to release information to insurers, your doctors, or any other person not connected with the research. The Certificate of Confidentiality does not stop you from releasing your own information. It also does not stop you from getting copies of your own information. |
|
|
Will I or anyone else receive study results? |
Your information collected as part of the research, even if identifiers are removed, will not be used or distributed for future research studies, nor shared with any past or current employer or union. The results of the study will be documented in a journal article or a NIOSH research report. No individual results of yours will be shown.
Copies of any published work using your data can be provided to you upon publication if requested. If you would like a copy of the summary report, please contact Dr. Hugo Camargo, the project officer, at (304)285-6123, or via email at [email protected]. |
|
|
Will my personal information or samples collected from me be used in other research? |
De-identified data will be stored at the DSR research laboratory. The raw data will be entered and stored on a computer. Two levels of physical security are always maintained for the data libraries—controlled office access, and ID/password access to data storage devices. Data will be kept up to 5 years after the completion of the study. This study will comply with the CDC Data Management and Sharing Policy. Every attempt will be made to publish results in peer-reviewed journals. We will not use the information that we collect from you in future research studies or share your information with other researchers. |
|
|
Is this a Clinical Trial? |
No, this study is not a clinical trial. |
|
|
Did you receive all necessary information? |
We believe you have been given all the information that a reasonable person would want to have in order to make an informed decision about whether to participate in this study. We invite you to take this opportunity to discuss the study and have your questions answered. If you need more information, or still have questions, please ask the person who is reviewing the study with you or the study Principal Investigator (Dr. Hugo Camargo). |
|
|
Who can I talk to if I have more questions? |
For questions about the research study, contact the principal investigator, Dr. Hugo Camargo at [email protected] or (304)285-6123. For questions about your rights, your privacy, or harm to you, contact the Chair of the NIOSH Institutional Review Board (IRB) in the Human Research Protection Program at 513-533-8591. |
|
|
Your signature |
The study was explained to me. My questions were answered. I agree to be in the study. ______________________________________________________ Printed name of participant
______________________________________________________ Participant signature Date
I have accurately described this study to the participant.
______________________________________________________ NIOSH representative signature Date |
|
|
Do I wish to receive a copy of the results? |
If you wish to receive a copy of the final results of this study in the form of a journal article, please indicate below the physical address, or the email address where you want this document to be sent:
|
Example 2
Public reporting burden of this collection of information is estimated to average XX minutes per response, including the time for reviewing instructions, searching existing data sources, gathering and maintaining the data needed, and completing and reviewing the collection of information. An agency may not conduct or sponsor, and a person is not required to respond to a collection of information unless it displays a currently valid OMB control number. Send comments regarding this burden estimate or any other aspect of this collection of information, including suggestions for reducing this burden to CDC/ATSDR Information Collection Review Office, 1600 Clifton Road NE, MS D-74, Atlanta, Georgia 30333; ATTN: PRA (XXX). |
Form Approved OMB No. XXX Exp. Date XX/XX/XXXX |

Consent to be in a Research Study Investigation on safety and trust when working alongside industrial mobile robots |
||
|
|
Key Information Summary |
Consent is being sought for research, and your participation is voluntary. The purpose is to investigate human behavior and perceptions of safety and trust while interacting with multiple industrial mobile robots (IMRs) with varying characteristics (size and separation distance). The expected duration of the study is 2.5 hours. You will be asked to complete multiple tasks where you will pick-up and place boxes on a shelf while working alongside an IMR. During the study you will wear a ring to measure physiological parameters (e.g., pulse rate, heart rate, skin temperature) and motion capture markers that will be strapped/taped to various body segments and joints. The risks involve fatigue/soreness, being struck by or colliding with the mobile robot, skin irritation from elastic straps/tape, and possible breach of privacy. The study is designed to reduce each of these risks as described below. There are no direct benefits to you for participating in this research. We will recruit a total of 71 participants to complete the study. There is a small risk you could get a respiratory infection (e.g., COVID-19, influenza) through an in-person interaction while participating in this study. To minimize your risk of exposure to viruses and maximize your protection against infection, NIOSH researchers follow COVID-19 and other relevant respiratory infectious disease guidance for CDC and NIOSH staff and workplaces. |
|
|
Who is conducting the study? |
The National Institute for Occupational Safety and Health (NIOSH) is a federal agency that studies worker safety and health. We are part of the Centers for Disease Control and Prevention (CDC). |
|
|
What is the purpose? |
The purpose of this study is to investigate human behavior and perceptions of safety and trust while interacting with multiple IMRs with varying characteristics (e.g., size and separation distance) that are designed to work in close cooperation in a shared workspace. |
|
|
What will I do? |
You will be asked to complete 2 experiments that involve interacting with an IMR. Before beginning the first experiment, you will complete a demographics survey (4 questions) and a robot experience questionnaire (20 questions). In the first experiment, you will complete 12 trials where you will be approached by one or two IMRs while you are standing in the center of the laboratory. The IMR(s) will pass you on your right and/or left side. The IMR height and separation distance between you and the mobile robot(s) will vary depending on the experiment condition. After each trial you will rate your perceived comfort level (1 question). In the second experiment, you will complete 12 trials where you will complete a manual loading/unloading task while working alongside one or two IMRs. The task will involve transferring boxes between shelves located on opposite sides of the room. While you’re completing the task, one or two IMR(s) will cross the pathway between the shelves parallel to your movement direction. After each trial, you will complete a survey about your satisfaction (2 questions), perceived safety/comfort/trust in the robot (6 questions), and ratings of robot attributes (5 questions). Prior to data collection, we will equip you with a ring and motion capture markers to track and record your movements throughout the trial. |
|
|
When, where, for how long will I be needed? |
You will be asked to visit the NIOSH laboratory in Morgantown, WV for this study. Your visit will take approximately 2.5 hours, including: 30 minutes for pre-experiment preparation, 60 minutes for the first experiment, 60 minutes for the second experiment, and 5 minutes for post-study debriefing. |
6 |
Are there any risks from participating in the study? |
The probability and magnitude of harm or discomfort anticipated in this research are not greater than those in your daily life or during the performance of routine physical or psychological examinations or tests. The duration of each experiment session is expected to be 1 hour (i.e., 2 hours total). You may experience fatigue or soreness from completing tasks during each experiment. You will be given adequate rest breaks between experiment trials to recover from any fatigue and will be instructed to inform the study team if you experience any discomfort. You may be at risk of colliding with and being struck by the IMR during the experiment which may result in skin or muscle discomfort. This experience can be compared to bumping into a slow-moving object. Prior to the experiment, you will undergo training to learn how the IMRs operate and to become familiar with working alongside them. The IMRs used in this study also contain collision avoidance technology that prevents it from running into any obstacles. There is minimal risk of developing skin irritation from the elastic straps and double-sided tape used to attach the motion capture markers. We will instruct you to let us know if the markers become uncomfortable so we can readjust them immediately. There is a slight risk that the information we collect about you could be accidently disclosed to someone else, which may cause you to experience psychological or social stress due to your loss of privacy. We will minimize this risk by identifying your samples and data collection forms by code only. There is a very small risk you could get a respiratory infection (e.g., COVID-19, influenza) through an in-person interaction while participating in this study. To minimize your risk of exposure to viruses and maximize your protection against infection, NIOSH researchers follow COVID-19 and other relevant respiratory infectious disease guidance for CDC and NIOSH staff and workplaces. This includes disinfecting all equipment between participants. |
7 |
Are there other benefits? |
You will not receive any direct benefits from participating in this study. Your participation in this research will help researchers to better understand the effects of IMR design and movement characteristics on human behavior and perceived safety and trust during human-robot collaboration in the workplace. |
8 |
Is my participation voluntary? |
Your participation in the study is voluntary. You may choose to be in the study or not. You may choose to answer any or all questions. You may decline to participate or drop out at any time, for any reason, without consequences to you. If you have completed your participation in the study but would still like to withdraw, you may do so prior to publication of the study by contacting the researcher using the contact information on this form. |
9 |
What if I am injured or harmed at a NIOSH research facility or at another location where the NIOSH research project is being conducted? |
NIOSH will summon emergency medical aid by calling 911 if needed. NIOSH will not provide payment for medical care or compensation. If you believe NIOSH has been negligent in conducting the research study and you believe you have suffered a harm as a result, you have the right to pursue a legal remedy under the Federal Tort Claims Act (28 U.S.C. §§ 2671-2680 and 28 U.S.C. § 1346(b)). To learn more about how to file a Federal Tort claim, call the General Law Division of the HHS Office of the General Counsel at (202) 619-2155 or go to https://www.hhs.gov/about/agencies/ogc/key-personnel/general-law-division/index.html. |
10 |
Will I be reimbursed or paid? |
You will receive $30 per hour for your time during the study. For periods of time less than one hour, you will receive partial pay of $10 per 20 minutes, with periods less than 20 minutes rounded up. If you complete the study, you will receive a total of $80. |
11 |
What alternative procedures might benefit me? |
No alternative procedures are available to collect the information needed for this study. |
12 |
Will my personal information be kept confidential? |
NIOSH is authorized to collect your personal information and will protect it to the extent allowed by law. During the recruitment process, we will record your name, email address, and phone number. We will also record your name to reimburse you for your time during the study. This information will be stored separately in a password protected, limited access folder, and not connected to any data collected during the study. Information will be kept confidential and, to the extent permitted by applicable laws, will not be made publicly available. All identifying information and recruitment details will be destroyed upon study completion by deleting the folder used for scheduling and reimbursing participants. During the study, you will be assigned a study number for identification to protect confidentiality. Because information collected during the recruitment process is stored in a separate, password-protected folder than the coded study participant data, it will not be possible to link your study data.
|
13 |
Certificate of Confidentiality |
This research project has a Certificate of Confidentiality from the Centers for Disease Control and Prevention (CDC). Unless you say it is okay, researchers cannot release information that may identify you for a legal action, a lawsuit, or as evidence. This protection applies to requests from federal, state, or local civil, criminal, administrative, legislative, or other proceedings. As an example, the Certificate would protect your information from a court subpoena. There are some important things that you need to know. The Certificate DOES NOT protect your information if a federal, state, or local law says it must be reported. For example, some laws require reporting of abuse, communicable diseases, and threats of harm to yourself or others. The Certificate CANNOT BE USED to stop a federal or state government agency from checking records or evaluating programs. The Certificate DOES NOT stop reporting required by the U.S. Food and Drug Administration (FDA). The Certificate also DOES NOT stop your information from being used for other research if allowed by federal regulations. Researchers may release your information when you say it is okay. For example, you may give them permission to release information to insurers, your doctors, or any other person not connected with the research. The Certificate of Confidentiality does not stop you from releasing your own information. It also does not stop you from getting copies of your own information. |
14 |
Will I or anyone else receive study results? |
The results of the study will be documented in a journal article or a NIOSH research report. No individual results will be shared or published and no pictures of you will be shown. Copies can be provided to you upon publication if requested. If you would like a copy of the summary report, please contact Dr. Justin Haney, the project officer at [email protected] or 304-285-6179.
|
15 |
Will my personal information or samples collected from me be used in other research? |
We may remove your name and other identifiers from the information that we collect during the study and then use the information for future research studies without asking you for additional consent. We also may remove identifiers from the information that we collect and then share it with other researchers without asking you for additional consent. |
16 |
Is this a Clinical Trial? |
No |
17 |
Did you receive all necessary information? |
You should have been given all the information that a reasonable person would want to have in order to make an informed decision about whether to participate in this study. You should have been given the opportunity to discuss the study and have your questions answered. If you need more information, or still have questions, please ask the person who is reviewing the study with you or the study Principal Investigator (Dr. Justin Haney). |
18 |
Who can I talk to if I have more questions? |
For questions about the research study, contact the principal investigator, Dr. Justin Haney at [email protected] or 304-285-6179. For questions about your rights, your privacy, or harm to you, contact the Chair of the NIOSH Institutional Review Board (IRB) in the Human Research Protection Program at 513-533-8591. |
19 |
Your signature |
The study was explained to me. My questions were answered. I agree to be in the study. ______________________________________________________ Printed name of participant __________________________________________________ Participant signature Date I have accurately described this study to the participant. ______________________________________________________ NIOSH representative signature Date |
Example 3
Public reporting burden of this collection of information is estimated to average 60 minutes per response, including the time for reviewing instructions, searching existing data sources, gathering and maintaining the data needed, and completing and reviewing the collection of information. An agency may not conduct or sponsor, and a person is not required to respond to a collection of information unless it displays a currently valid OMB control number. Send comments regarding this burden estimate or any other aspect of this collection of information, including suggestions for reducing this burden to CDC/ATSDR Information Collection Review Office, 1600 Clifton Road NE, MS D-74, Atlanta, Georgia 30333; ATTN: PRA (XXXX-XXXX). |
Form Approved OMB Control Number: XXXX-XXXX Exp. Date XX.XX.XXX |
|

Consent to be in a Research Study Assessing Industrial Hygiene Practices Across Hazardous Work Environments |
||
|
|
Key Information Summary |
The National Institute for Occupational Safety and Health (NIOSH) is a federal agency that studies worker safety and health. NIOSH is part of the Centers for Disease Control and Prevention (CDC). NIOSH is leading research to document public health supplies and risk management practices that might apply direct reading methods (DRM) to minimize exposure to respirable dust with crystalline silica (RD/CS). This project is engaging representatives in the mining, construction, and oil and gas extraction (OGE) industries to compare methods and practices among these sectors.
We will conduct interviews and focus groups with employees who are responsible for industrial hygiene practices at various levels of the organization. Each data collection will last no more than one hour. Before starting, we will ask you to verbally indicate whether you agree to participate. Questions will primarily focus on current work practices to mitigate exposure to respirable dust that contains crystalline silica (RD/CS), use of that involve DRM and other technologies, challenges to those activities, and what can be done to help operationalize their use in the field. We will document answers in writing, but we will not audio record conversations. Participation is voluntary. You may refuse to answer any questions and stop your participation at any time without any consequences. All data collection will occur during work hours.
Participation in this research involves minimal risks. There is a small risk that collected information in focus groups, specifically, will be accidentally released. We will minimize the risk by identifying each focus group with a unique code that cannot be linked back to a workplace or to you. You can self-assign how you want to be addressed during the focus group. Due to a group setting, we are not able to guarantee actions of peers who participate in the focus groups once finished. However, the focus group ground rules will stress the importance of not sharing any of the discussion outside of the focus group. We will further minimize risk by releasing only summaries of information in reports, presentations, and publications. Only NIOSH researchers in this project will have access to focus group data. If these accommodations bring discomfort, anyone is welcome to request a one-on-one interview in lieu of participating in a focus group. |
|
|
Who is conducting the study? |
The National Institute for Occupational Safety and Health (NIOSH) is a federal agency that studies worker safety and health. We are part of the Centers for Disease Control and Prevention (CDC). |
|
|
What is the purpose? |
This field is still relatively new with little operationalization for how DRM can be used in the workplace. The purpose of this research is to: 1) understand the pubic health supplies, technologies, and practices used to manage exposure and risk to RD/CS.2) understand if and how your company has integrated DRM into its industrial hygiene practices to reduce exposure to RD/CS; 2) better understand challenges in implementing these new supplies and technologies in the field; and 3) use this information to draft a playbook that entails needs, priorities, and guidelines for integrating and using data from new DRM. |
|
|
What will I do? |
You will be asked to voluntarily participate in an interview or focus group – either in-person or virtual. A NIOSH researcher will ask about the use of DRM and other technologies specific to the job roles and responsibilities of an industrial hygienist. We will take notes to capture all answers. |
|
|
When, where, for how long will I be needed? |
Virtual data collection will occur via a virtual platform (i.e., Microsoft Teams). In-person data collection will meet at your place of employment or a pre-arranged place (e.g., conference room). You will participate during your usual work hours. Participation will take no more than one hour, including time for consent. |
6a |
Are there any risks from participating in the study? |
If you participate in a focus group discussion, there is a potential risk of a loss of confidentiality because you will be sharing your opinions among the group. Although we are asking all participants in the group to keep information shared confidential, NIOSH cannot ensure this aspect of your confidentiality, causing a slight risk that the information we collect could be accidently disclosed to someone else. This may cause you to experience psychological or social stress due to your loss of privacy. We will minimize this risk by identifying all data by code and by only releasing summaries of all data. Your name, email, and phone number were only collected for the purpose of scheduling the focus group. All of this information will be destroyed once the focus group is conducted. If these accommodations bring discomfort, anyone is welcome to request a one-on-one interview in lieu of participating in a focus group. For data collection being conducted virtually, there is no risk you could get COVID-19 or other respiratory infectious disease through an in-person interaction while participating in this study. However, there is a [very small] risk you could get a respiratory infection (e.g., COVID-19, influenza) through an in-person interaction while participating in this study. To minimize your risk of exposure to viruses and maximize your protection against infection, NIOSH researchers will follow COVID-19 and other relevant respiratory infectious disease guidance for CDC and NIOSH staff and workplaces. |
7 |
Are there benefits? |
No one will be reimbursed or paid for participation. However, you may indirectly benefit; specifically, this topic can influence company programs to support a reduction in serious incidents and exposure risks – benefiting the worker population across various industries. Also, to help enhance the benefits for this study, we will ensure that you are aware of all publicly available NIOSH-approved materials and resources about DRM that has been used to minimize exposure. |
8 |
Is my participation voluntary? |
Your participation in the study is voluntary. You may choose to answer any or all questions. You may decline to participate or drop out at any time, for any reason, with no penalty or loss of benefits to which you are otherwise entitled. |
9 |
What if I am injured or harmed at a NIOSH research facility or at another location where the NIOSH research project is being conducted? |
NIOSH will summon emergency medical aid by calling 911 if needed and if the work is conducted on a NIOSH facility. If the work is conducted at a field site, it is the responsibility of the company to call 911. NIOSH will not provide payment for medical care or compensation. If you believe NIOSH has been negligent in conducting the research study and you believe you have suffered a harm as a result, you have the right to pursue a legal remedy under the Federal Tort Claims Act (28 U.S.C. §§ 2671-2680 and 28 U.S.C. § 1346(b)). To learn more about how to file a Federal Tort claim, call the General Law Division of the HHS Office of the General Counsel at (202) 619-2155 or go to https://www.hhs.gov/about/agencies/ogc/key-personnel/general-law-division/index.html. |
10 |
Will I be reimbursed or paid? |
You will not be paid or reimbursed for participating. |
11 |
What alternative procedures might benefit me? |
No alternative procedures are available for this study.
|
12 |
Will my personal information be kept confidential? |
NIOSH will protect your information to the extent allowed by law. In this study, results are anonymous as we are not collecting or recording personal identifiers. You will be assigned a code throughout the study and in no records or notes will you be referenced by name. Your name, email, and phone number were only collected for the purpose of scheduling. All of this information will be destroyed once the discussion is conducted. |
13 |
Certificate of Confidentiality |
This research project has a Certificate of Confidentiality from CDC. Unless you say it is okay, researchers cannot release information that may identify you for a legal action, a lawsuit, or as evidence. This protection applies to requests from federal, state, or local civil, criminal, administrative, legislative, or other proceedings. As an example, the Certificate would protect your information from a court subpoena. There are some important things that you need to know. The Certificate DOES NOT protect your information if a federal, state, or local law says it must be reported. For example, some laws require reporting of abuse, communicable diseases, and threats of harm to yourself or others. The Certificate CANNOT BE USED to stop a federal or state government agency from checking records or evaluating programs. The Certificate DOES NOT stop reporting required by the U.S. Food and Drug Administration (FDA). The Certificate also DOES NOT stop your information from being used for other research if allowed by federal regulations. Researchers may release your information when you say it is okay. For example, you may give them permission to release information to insurers, your doctors, or any other person not connected with the research. The Certificate of Confidentiality does not stop you from releasing your own information. It also does not stop you from getting copies of your own information. |
14 |
Will I or anyone else receive study results? |
If you wish, we will provide an aggregated report of results by your industry and combined, within 6 months of study ending. We will not share individual results with the organization, union, or individual employees. |
15 |
Will my personal information or samples collected from me be used in other research? |
We may remove other identifiers from the information that we collect and then use the information for future research studies without asking you for additional consent. We also may remove identifiers from the information that we collect and then share it with other researchers without asking you for additional consent. |
18 |
Who can I talk to if I have more questions? |
If you have questions about the project, you can contact the Principal Investigator, Dr. Emanuele Cauda ([email protected]). For questions about your rights, your privacy, or harm to you, contact the Chair of the NIOSH Institutional Review Board (IRB) in the Human Research Protection Program at (513) 533-8591 or [email protected]. |
19 |
Waiver of signature |
The study was explained to me. My questions were answered. I agree to be in the study. |
Appendix B: Example Data Collection Instruments
DEMOGRAPHICS
Subject ID #: ________________



Demographic Survey
What is your age? ________________________
What is your gender? (check one)
Male
Female
Nonbinary
Is your vision normal or corrected to normal?
Yes
No (please inform researcher)
Is your hearing normal or corrected to normal?
Yes
No (please inform researcher)
Which racial/ethnic category best describes you? (Select all that apply.)
American Indian or Alaska Native
Asian
Black or African American
Hispanic, Latino or Spanish origin
Native Hawaiian or Other Pacific Islander
White
Other racial group not listed here
Prefer not to answer
Job experience
Current manufacturing, warehouse, or stockroom employee: Yes | No
Years of working in the manufacturing industry, warehousing industry, or in a stockroom: _______ years
Job and Experience Surveys
Robot Experience Questionnaire
Prior experience with robots
Have you had any prior experience with robots? YES | NO
If you answered ‘No’ to the previous question, skip the following 3 questions and go to Q2.
Which of the following types of robots do/did you use or interact with? Check all that apply.
Assistive or service robots, such as those in domestic or public settings
Traditional industrial robots, such as those that work in robotic cells and cages away from humans
Collaborative robots, that physically interact with humans in industrial settings
Mobile robots or autonomous ground vehicles
Aerial robots or drones
Wearable robots or exoskeletons
Other: ________________
How long have you used or worked with the technology that you selected above?
______________ hours/weeks/months/years
How often have/had you worked in direct interaction or within a shared work area with the selected robot(s)?
Never
Hardly ever
Occasionally
Quite often
Frequently
Nearly all the time
The following questions will ask about your attitudes towards robots in general. Please indicate to what extent you agree or disagree with each of the following statements on a 5-point scale:
I would feel uneasy if robots really had emotions.
(I strongly disagree) 1---2---3---4---5 (I strongly agree)
Something bad might happen if robots developed into living beings.
(I strongly disagree) 1---2---3---4---5 (I strongly agree)
I would feel relaxed talking with robots.
(I strongly disagree) 1---2---3---4---5 (I strongly agree)
I would feel uneasy if I was given a job where I had to use robots.
(I strongly disagree) 1---2---3---4---5 (I strongly agree)
If robots had emotions, I would be able to make friends with them.
(I strongly disagree) 1---2---3---4---5 (I strongly agree)
I feel comforted being with robots that have emotions.
(I strongly disagree) 1---2---3---4---5 (I strongly agree)
The word “robot” means nothing to me.
(I strongly disagree) 1---2---3---4---5 (I strongly agree)
I would feel nervous operating a robot in front of other people.
(I strongly disagree) 1---2---3---4---5 (I strongly agree)
I would hate the idea that robots or artificial intelligences were making judgments about things.
(I strongly disagree) 1---2---3---4---5 (I strongly agree)
I would feel very nervous just standing in front of a robot.
(I strongly disagree) 1---2---3---4---5 (I strongly agree)
I feel that if I depend on robots too much, something bad might happen.
(I strongly disagree) 1---2---3---4---5 (I strongly agree)
I would feel paranoid talking with a robot.
(I strongly disagree) 1---2---3---4---5 (I strongly agree)
I am concerned that robots would be a bad influence on children.
(I strongly disagree) 1---2---3---4---5 (I strongly agree)
I feel that in the future society will be dominated by robots.
(I strongly disagree) 1---2---3---4---5 (I strongly agree)
VIRTUAL REALITY EXPERIENCE QUESTIONNAIRE
1. Prior experience with virtual reality.
Have you had any prior experience using any virtual reality devices? YES | NO
If you answered ‘No’ to the previous question, skip the following 3 questions.
What type of virtual reality device do you use or have previously used.
Head mounted display or virtual reality headset (e.g., Oculus Rift, Meta Quest, HTC VIVE, etc.)
Virtual reality CAVE
Mixed reality glasses (e.g., Microsoft HoloLens, Google Glasses)
Other: ________________
How long had you used the technology that you selected above?
_______________ hours/weeks/months/years
How often do you use the technology that you selected above?
Never
Hardly ever
Occasionally
Quite often
Frequently
Nearly all the time
CONSTRUCTION/DEMOLITION Robot Experience Questionnaire
Prior experience in the construction industry
Have you worked previously in the construction industry?
YES | NO
How many years?
What tasks did you perform? (Please check all that apply)
☐ Site preparation |
☐ Installation of windows |
☐ Insulation |
☐ Site Work |
☐ Concrete slabs |
☐ Roofing |
☐ Foundation work |
☐ Plumbing |
☐ Equipment Operator |
☐ Framing |
☐ Electric |
Other_______________________ |
2. Prior experience operating an excavating machine
Do you have experience operating an excavating machine?
YES | NO
How long have you used or worked with excavating machines?
______________ years
Prior experience with demolition robots
Do you have experience operating a demolition robot?
YES | NO
How long have you used or worked with demolition robots?
______________ years
What type of structures did you demolish using these robots? (Please check all that apply)
☐ Residential buildings |
☐ Commercial buildings |
☐ Highway/exterior structures |
Characteristic of the demolition robot remote control: (Please check all that apply)
☐ Cabled remote control |
☐ Wireless remote control |
Pre- and Post-Assessments
Baseline, In Progress, Post Questionnaires Wearable Robot Lab Study
Date (MM/DD/YEAR): _____ / _____ / __________
Name: ______________(First)_________________(Last) (Assigned Study ID:_______)
Email address for follow-up contact: __________________________________
Workstation/Production line name: __________________
Birth Sex: ________________ Male/Female/Not Specified
Date of Birth: _________
Height: ____ ft. ____ in.
Weight: _______ lbs
Job Title: ______________________________________
What is the name of the passive shoulder exoskeleton (PSE) you used in the past year? _______________ I don’t know the name
I don’t use shoulder exoskeletons (If you choose this option, go to question No. 23)
Please answer each question based on how you have typically felt on the days you worked. Please mark on the line the point that you feel represents your perception.
11A. When I work, I really exert myself to the fullest.
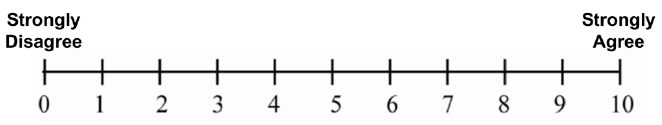
11B. I feel exhausted at the end of shift.

12. Please estimate the average time for using the PSE during a typical work day in the past year.
Less than one hour per day
About 1-2 hours per day
About 2-4 hours per day
More than 4 hours per day
None of the above, please specify: _______________________
13. Please estimate the average number of days using the PSE during a typical week in the past year.
Less than or equal to one day per week
About 2-3 days per week
About 4-5 days per week
None of the above, please specify: _______________________
14. What was your perception of the thermal comfort (and/or feelings of sweatiness) when using the PSE?
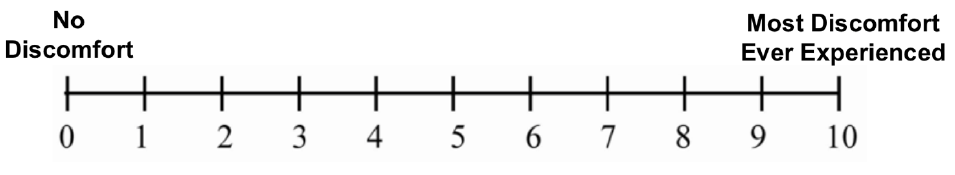
15. What was your perception of balance (or any sense of imbalance) while using the PSE?
16. Did you feel that your range of motion was at all limited while wearing the PSE?
17. What was your perception of overall comfort and the fit of the PSE when performing your job?
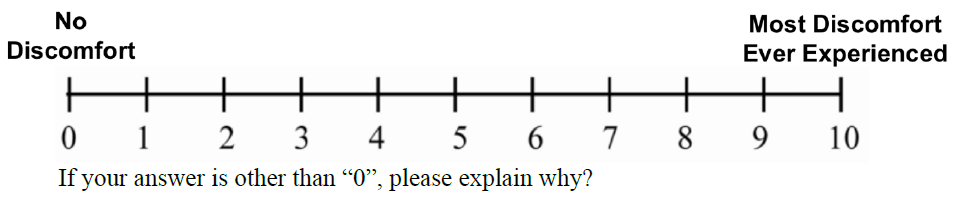
__________________________________________________
18. What was your perception of overall safety when performing your job with the PSE, compared to when not wearing the vest?
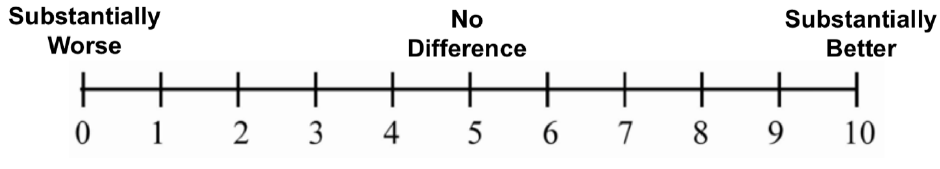
19. Overall, did the PSE positively or negatively affect your task performance during a typical work week?

20. What do you most like about using the PSE?
____________________________________________________
21. What do you least like about using the PSE?
____________________________________________________
Post Questionnaire to Assess Preferred Layout of a Police Cruiser Design (Human factors assessment)
Participant Number: ______________
1. How many years have you been driving a police cruiser? _________________
2. On average, how many hours per work shift do you spend in a police cruiser? _______________
3. On a scale 0 to 9 (0 means no experience, 9 means high experience), please rate the amount of your experience in using the MDT while driving. Please put a check in the box below the number you have chosen:
0 |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
|
|
|
|
|
|
|
|
|
|
4. For the touchscreen task you have just completed, do you prefer the MDT installed on top of the center console, or the same MDT installed on the pole to the right of the center console? Circle one below.
A. MDT on the center console
B. MDT to the right of the center console
C. No preference.
5. For the license plate number entry task you have just completed, do you prefer the use the MDT installed on top of the center console, or the same MDT installed on the pole to the right of the center console? Circle one below.
A. MDT on the center console
B. MDT to the right of the center console
C. No preference
6. In the space below, please comment on your positive or negative experience using the center-console-installed MDT while driving. Feel free to skip to the question if you do not have comments/suggestions.
Positive:_________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________
Negative: ________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________
Other comments/Suggestions:
7. In the space below, please comment on your positive or negative experience using the post-installed MDT to the right of center console while driving. Feel free to skip to the question if you do not have comments/suggestions.
Positive:_________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________
Negative: ________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________
Other comments/Suggestions: ________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________
8. For the radio zoning task you have just completed, do you prefer the radio installed on
A.
Flat center console: 
B.
Angled center console:

C. No preference
9. For the light/siren control task you have just completed, do you prefer the radio installed on
A.
Flat center console: 
B.
Angled center console:

C. No preference
10. In the space below, please comment on your positive or negative experience using the radio installed on the flat center console. Feel free to skip to the question if you do not have comments/suggestions.
Positive:_________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________
Negative: ________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________
Other comments/suggestions:
________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________
11. In the space below, please comment on your positive or negative experience using the light/siren control unit installed on the flat center console. Feel free to skip to the question if you do not have comments/suggestions.
Positive:_________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________
Negative: ________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________
Other comments/suggestions:
________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________
12. In the space below, please comment on your positive or negative experience using the radio installed on the angled center console. Feel free to skip to the question if you do not have comments/suggestions.
Positive:_________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________
Negative: ________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________
Other comments/suggestions:
________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________
13. In the space below, please comment on your positive or negative experience using the light/siren control unit installed on the angled center console. Feel free to skip to the question if you do not have comments/suggestions.
Positive:_________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________
Negative: ________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________
Other comments/suggestions:
________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________
ANTHROPOMETIC MEASUREMENTS
Calculated PCA Panel Head Size (circle one):
Small Short/Wide Medium
Calculated PCA Panel Cell Number: __________
Weight (kg) |
|
(kg) |
Stature (cm) |
|
(cm) |
Head circumference |
|
(mm) |
Interpupillary Distance |
|
(mm) |
Menton-Sellion Length (Face Length) |
|
(mm) |
Subnasale-Sellion Length |
|
(mm) |
Nasal Root Breadth |
|
(mm) |
Nose Breadth |
|
(mm) |
Nose Protrusion |
|
(mm) |
Minimum Frontal Breadth |
|
(mm) |
Bizygomatic Breadth (Face Breadth) |
|
(mm) |
Bigonial Breadth |
|
(mm) |
Head Breadth |
|
(mm) |
Using an automated application, measure 19 facial/head dimensions adapted from Zhuang and Bradtmiller (2005) (below). These dimensions were identified in the 2005 study as relevant to fit.
Measure and Record 19 Facial/Head Dimensions
1 |
Bigonial breadth |
11 |
Lip length |
2 |
Bitragion chin arc |
12 |
Maximum frontal breadth |
3 |
Bitragion coronal arc |
13 |
Menton-Sellion length |
4 |
Bitragion frontal arc |
14 |
Minimum frontal breadth |
5 |
Bitragion subnasale arc |
15 |
Nasal root breadth |
6 |
Bizygomatic breadth |
16 |
Neck circumference |
7 |
Head breadth |
17 |
Nose breadth |
8 |
Head circumference |
18 |
Nose protrusion |
9 |
Head length |
19 |
Subnasale-Sellion Length |
10 |
Interpupillary distance |
|
|
* Zhuang and Bradtmiller (2005). Head-and-Face Anthropometric Survey of U.S. Respirator Users, Journal of Occupational and Environmental Hygiene. 2:11, 567-576.
Physiological Measurements
STRESS TEST RESULTS
Name: Subject’s name
Your
Value Comments
Age: 42
Height (inches): 69
Weight (pounds): 230
Rest Heart Rate
(beats/minute): 74
Rest Blood Pressure
(mm Hg): 138/90
Max. Hear Rate
(beats/minute): 183
Max. oxygen
Consumption
(ml/kg/min): 30.4
Perceived Rate of Exertion
Borg-RPE
Rate your level of exertion while performing the task on the following scale. (Borg RPE (Borg 1982)) (Descriptions are provided as a guide. You can pick any number, even where there is no description provided)
-
6
No exertion at all
No muscle fatigue, breathlessness, or difficulty breathing
7
Extremely light
Very, very light
8
9
Very Light
Like walking slowly, for a short while, very easy to talk
10
11
Light
Like a like exercise at your own pace
12
Moderate
13
Somewhat hard
Fairly strenuous and breathless. Not so easy to talk
14
Heavy and strenuous. An upper limit for fitness training, as when running or walking fast
15
Hard
16
17
Very hard
Very strenuous, you are tired and breathless. Very difficult to talk.
18
19
Extremity hard
The most strenuous effort you have ever experienced
20
Maximal exertion
Maximal heaviness
Borg-CR10
Rate your level of exertion while performing the task on the following scale. (Borg CR-10 (Borg 1982)) (Descriptions are provided as a guide. You can pick any number, even where there is no description provided)
-
0
Nothing at all
0.3
0.5
Extremely weak
Just noticeable
0.7
1
Very weak
1.5
2
Weak
Light
2.5
3
Moderate
4
5
Strong
Heavy
6
7
Very strong
8
9
10
Extremely strong
“Maximal”
|
*
Absolute maximum
Highest Possible
Body Function Assessments
Procedure for conducting shoulder function assessments
PART 1. Strength Assessment-20 min
Equipment: Tension dynamometer, strapping/belt fixed at anchor point (Katoh, 2015).
Exertion task |
Posture |
Limb positions |
Dynanometer position |
Belt fixation |
Picture |
Shoulder flexion |
Supine |
0° shoulder flexion, 0° abduction, elbow slightly bent, forearm in pronation |
Humerus lateral supracondylar ridge |
Bed leg below arm |
|
Shoulder extension |
Seated (a) |
0° shoulder flexion, 0° abduction, 0° elbow flexion, forearm in pronation |
Olecranon |
Stairs baluster parallel to arm |
|
External rotation
|
Seated (b) |
45° shoulder flexion, 135° elbow flexion |
Styloid process of the ulna |
Stairs baluster parallel to forearm |
|
Internal rotation
|
Seated (b) |
45° shoulder flexion, 135° elbow flexion |
Styloid process of the ulna |
Stairs baluster parallel to forearm |
|
Steps:
(a) The examiner holds the subject’s shoulder on the measured side.
(b) To prevent abduction-adduction, a 5 kg bag of sand is fixed between the elbow and the baluster.
(c) Three exertions to be averaged. Values must be within 15%.
Data Recording Sheet:
Exertion task |
Measurement #1 |
Measurement #2 |
Measurement #3 |
Note |
Shoulder flexion |
|
|
|
|
Shoulder extension |
|
|
|
|
External rotation |
|
|
|
|
Internal rotation |
|
|
|
|
Procedure for conducting shoulder function assessments
PART 2. Range of Motion/Mobility – 10 min
Equipment Needs: Manual goniometer, PT treatment table.
Pictures below obtained from: https://otassessments.wordpress.com/events-list/shoulder/ AND https://shouldercomplexgocatsnmu.weebly.com/range-of-motion.html.
Steps: Use a manual goniometer to measure the following maximal angles. Ask participants to sit upright on a stool. Note that three measurement values must be within 15%.
Angle |
Picture |
Measurement #1 |
Measurement #2 |
Measurement #3 |
Note |
Shoulder flexion |
|
|
|
|
|
Shoudler extension |
|
|
|
|
|
Shoulder abduction |
|
|
|
|
|
Lateral rotation |
|
|
|
|
|
Medial rotation |
|
|
|
|
|
Procedure for conducting shoulder function assessments
PART 3. Functional Movement: Endurance/Overhead work tolerance Fit-HANSA (Overhead work) – 10 mins (Kumta et al., 2012)
Equipment will be made by NIOSH and delivered to study sites prior to data collection.

Steps:
A shelf is placed at the subject’s eye level with an attachable plate, perpendicular to the shelf, projecting out toward the subject.
One bolt is placed in the top notch of the attachable plate and a second bolt is placed in the third notch down the same column so that there is an empty notch between them.
Ask the participant to stand with feet apart at shoulder width, flat on the ground. When their hands are held up, the elbows should be bent in the starting position, as shown in the above picture.
Ask the participant to use both arms above the shoulder to perform the test (picture above). The participant will unscrew the bolt in notch 1 (top) and move down the bolt to notch 2 (middle); move the bolt in notch 3 (bottom) to notch 1 (top), then move the bolt in notch 2 down to notch 3 into the plate. The subject repeatedly screws and unscrews bolts in the top 3 holes in the plate, simulating sustained overhead work.
Pattern of movement:
The bolt in notch 1 (top) moves down to notch 2.
The bolt in notch 3 (bottom) moves up to notch 1.
The bolt in notch 2 moves down to notch 3.
This pattern is repeated until 5 minutes have elapsed or the subject feels unable to continue (see test-stopping criteria below).
Standardized verbal instruction for step 4:
“Screw and unscrew the bolts by staying in the top 3 holes. We want you to hold the nut and turn the standoff. Do NOT twirl the screw. If you drop a bolt, keep your arms up in the air and a tester will give you another one so that you don't bring your arms down.”
The tester always has one or two extra bolts ready to go.
Test Stopping Criteria:
Each task can be continued for up to 5 minutes, but is terminated based on the following stopping rules:
The subject stops or states it is too uncomfortable to continue.
The subject is severely off pacing to the extent that they are unable to complete one repetition of the movement within 2 beats of the metronome.
The subject substitutes using trunk/whole body movement and cannot correct with feedback for 5 successive repetitions of the task.
The examiner believes the subject is at risk of injury or adverse complication if tests were to continue.
At completion, participants provide perceived exertion rating using the Borg CR-10 scale below. The tester circles one of the numbers below for documenting the perceived exertion effort.

Motion Measurement Cameras
Perceived Usability Assessments
NASA Task Load Index
Please place a mark [X , | , +] on the line to indicate your response. (Hart & Staveland 1988)
-
Mental Demand
How mentally demanding was the task?

Low High
Physical Demand
How physically demanding was the task?

Low High
Temporal Demand
How hurried or rushed was the pace of the task?

Low High
Performance
How successful were you in accomplishing what you were asked to do?

Low High
Effort
How hard did you have to work to accomplish your level of performance?

Low High
Frustration
How insecure, discouraged, irritated, stresses, and annoyed were you?

Low High
For each pair of factors, which factor do you feel contributed more to your workload for the task.
-
Mental Demand
vs.
Physical Demand
Mental Demand
vs.
Temporal Demand
Mental Demand
vs.
Performance
Mental Demand
vs.
Effort
Mental Demand
vs.
Frustration
Physical Demand
vs.
Temporal Demand
Physical Demand
vs.
Performance
Physical Demand
vs.
Effort
Physical Demand
vs.
Frustration
Temporal Demand
vs.
Performance
Temporal Demand
vs.
Effort
Temporal Demand
vs.
Frustration
Performance
vs.
Effort
Performance
vs.
Frustration
System Usability Scale
Please mark your response for each of the following questions (System Usability Scale (SUS) | Usability.gov)
-
Strongly disagree
Strongly agree
1
2
3
4
4
I think that I would like to use the exoskeleton frequently.
I found the exoskeleton unnecessarily complex.
I thought the exoskeleton was easy to use.
I think that I would need the support of a technical person to be able to use this exoskeleton.
I found the various functions in this exoskeleton were well integrated.
I thought there was too much inconsistency in this exoskeleton.
I would imagine that most people would learn to use this exoskeleton very quickly.
I found the exoskeleton very cumbersome to use.
I felt very confident using the exoskeleton.
I needed to learn a lot of things before I could get going with this exoskeleton.
Self-perception data related to technology comfort and usability
Subject ID ____________________ Date _________________________________
In general, how easy was it to don and doff this technology?
(1 = easy, 2 = acceptable, 3 = not easy). Circle one number.
1 2 3
How do you rate the weight of this technology?
(1 = light, 2 = comfortable but slightly heavy, 3 = heavy). Circle one number.
1 2 3
How do you rate the comfort of this technology?
(1 = very poor, 2 = poor, 3 = acceptable, 4 = good, 5 = very good). Circle one number.
1 2 3 4 5
How do you rank the noise of this technology?
(1 = very loud, 2 = loud, 3 = acceptable, 4 = quiet). Circle one number.
1 2 3 4
How do you rank this technology for your visibility?
(1 = very poor visibility, 2 = poor visibility, 3 = acceptable, 4 = good visibility, 5 = very good visibility). Circle one number.
1 2 3 4 5
Considering all aspects of the technology, rate your experience using it as a part of your job?
(1 = very poor, 2 = poor, 3 = acceptable, 4 = good, 5 = very good). Circle one number.
1 2 3 4 5
----------------------------------------------------------------------------------------------------
COMPLETE THE FOLLOWING QUESTIONS RELATED TO COMFORT AND TOLERABILITY
----------------------------------------------------------------------------------------------------
DISCOMFORT
For each of the following, please rate your current level of discomfort described by each category (Please select one response for each item below.)
|
No discomfort at all |
|
|
|
|
Very Uncomfortable |
|
0 |
1 |
2 |
3 |
4 |
5 |
1. tightness |
□ |
□ |
□ |
□ |
□ |
□ |
2. irritation |
□ |
□ |
□ |
□ |
□ |
□ |
3. itching |
□ |
□ |
□ |
□ |
□ |
□ |
4. pinching |
□ |
□ |
□ |
□ |
□ |
□ |
5. pain |
□ |
□ |
□ |
□ |
□ |
□ |
6. bruising |
□ |
□ |
□ |
□ |
□ |
□ |
7. rash |
□ |
□ |
□ |
□ |
□ |
□ |
9. heat/warmth |
□ |
□ |
□ |
□ |
□ |
□ |
10. sweat/moisture buildup |
□ |
□ |
□ |
□ |
□ |
□ |
11. lack of fresh air |
□ |
□ |
□ |
□ |
□ |
□ |
12. nausea |
□ |
□ |
□ |
□ |
□ |
□ |
13. headache |
□ |
□ |
□ |
□ |
□ |
□ |
GENERAL WEARING EXPERIENCE
Please rate the level at which you are currently experiencing the following symptoms: (Please select one response for each item below.)
|
Not experiencing at all |
|
|
|
|
Experiencing to a very high degree |
|
0 |
1 |
2 |
3 |
4 |
5 |
1. dizziness |
□ |
□ |
□ |
□ |
□ |
□ |
2. loss of energy/tiredness/fatigue |
□ |
□ |
□ |
□ |
□ |
□ |
3. claustrophobia |
□ |
□ |
□ |
□ |
□ |
□ |
4. shortness of breath |
□ |
□ |
□ |
□ |
□ |
□ |
5. difficulty breathing |
□ |
□ |
□ |
□ |
□ |
□ |
THERMAL SENSATION
Please rate the thermal sensation that you are experiencing right now for your whole body: (Please select one response for each item below.)
|
Very Cold |
Cold |
Cool |
Slightly Cool |
Slightly Warm |
Warm |
Hot |
Very Hot |
|
-4 |
-3 |
-2 |
-1 |
1 |
2 |
3 |
4 |
1. Face |
□ |
□ |
□ |
□ |
□ |
□ |
□ |
□ |
2. Whole body |
□ |
□ |
□ |
□ |
□ |
□ |
□ |
□ |
FUNCTION
Think about your experience while wearing the equipment/technology and rate your level of agreement with each statement regarding your work today: (Please select one response for each item below.)
|
Strongly Disagree |
Disagree |
Agree |
Strongly Agree |
|
1 |
2 |
3 |
4 |
1. the technology affected my concentration while working (always adjusting) |
□ |
□ |
□ |
□ |
2. I had difficultly verbally communicating with others |
□ |
□ |
□ |
□ |
3. I had difficultly hearing others |
□ |
□ |
□ |
□ |
4. the equipment obstructed my vision |
□ |
□ |
□ |
□ |
5. the technology interfered with my job duties (quick to leave room, less interaction) |
□ |
□ |
□ |
□ |
Self-Perception Surveys and other Semi-structured Questions
Robot-HUMAN TRUST Evaluation Questionnaire
Please mark the percentage for each question.
What percentage of the time do you believe this robot will… |
% |
||||||||||
0 |
10 |
20 |
30 |
40 |
50 |
60 |
70 |
80 |
90 |
100 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Schaefer, K. (2013). The perception and measurement of human-robot trust. (Unpublished doctoral dissertation). University of Central Florida, Orlando, FL.
Semi-structured interview questions about direct reading methodologies and sensors
Introduction
Welcome and introduction of research team
Good morning/afternoon. My name is __________ and I am here with my colleague(s) ____________. We work at the National Institute for Occupational Safety and Health (NIOSH) which is an institute within Centers for Disease Control and Prevention (CDC). This project is support by the NIOSH Mining Program and we are also here on behalf of NIOSH’s Center for Direct Reading and Sensor Technologies (CDRST). We thank you for taking the time to join us today.
Overview
We invited you to come here today to talk about public health and industrial hygiene practices, direct reading methods, commonly referred to as DRM, and other supplies or technologies that are used to assess and mitigate respirable dust with crystalline silica (RD/CS) in the workplace. We are particularly interested in learning about the current industrial hygiene practices, the driving factors and uses of DRM and challenges to its implementation to better understand how to standardize and guide the use of DRMs from a systems approach in the future.
Your feedback will help us 1) understand the current approaches and practices used to manage exposure and risk to RD/CS.2) understand how your company has integrated DRM into its industrial hygiene practices to reduce exposure to RD/CS; 3) better understand challenges in implementing these new technologies in the field; and 4) use this information to draft a playbook that entails needs, priorities, and guidelines for integrating and using data from new DRM. We will share what we learn with others in the scientific community to advance work in this area. Again, the information shared will be deidentified.
Ground Rules
Over the next 60 minutes, I will ask you to consider a series of questions. Please think of this process as a conversation. Keep in mind that there are no right, or wrong answers and we want to hear what you have to say.
[IF IN FOCUS GROUP]: We ask that you do not share anything we discuss here outside of the focus group, including descriptors of individual participants. If you would like to share your experiences but feel uncomfortable relating them to you personally, please create hypothetical situations or use generic examples to illustrate your points (for example, one of my co-workers or someone I know conduct that practice for this...). This will help us maintain the confidentiality of your information.
[IF VIRTUAL]: At this point, I am going to turn my camera on so you can see me during the discussion. But there is no expectation for you to turn your camera on. This is your choice.
There are no right or wrong answers to these questions. Your participation is voluntary, and you can decline participation at any time by leaving this meeting or simply remaining quiet during questions you do not want to answer.
OPENING QUESTION/ICEBREAKER
First, let’s introduce ourselves. Please tell us your identifier but do not disclose your name or the name of your organization. After you introduce yourself, please tell me about:
The type of organization and industry in which you work.
Your job position and primary roles and responsibilities within your company.
The length of time you have been working in your current job/industry.
Experience level with various direct reading methods or other technologies specific to exposure reduction of RD/CS.
Thank you.
QUESTIONS
Introductory questions – specific to exposure management and then the integration and use of direct reading methodologies to manage exposures.
Starting with the health hazard RD/CS more specifically, how is the exposure to respirable dust and crystalline silica managed at each worksite?
How do you manage workers’ exposure to RD/CS at different levels of the operation? In other words, what are your responsibilities versus responsibilities at your regional or corporate office to ensure consistency?
Can you describe the industrial hygiene activities adopted (exposure assessment, periodic monitoring, task monitoring) for respirable dust and crystalline silica?
Has your company adopted any defined framework, such as risk management, to control workers’ exposure to RD/CS? If so, how are these implemented in the field?
We would like to understand your view of direct reading methods in general – what is your view toward these technologies for health and safety?
What about for RD/CS specifically?
Tell me about the different types of direct reading supplies and technologies you use as a part of your job specific to reducing exposure to RD/CS.
Generally, how long have you been using these instruments or technologies?
In general, how often do you employ these instruments or technologies?
Out of the DRM you just described do you feel one is more important for worker health and safety? And, least important?
What are some of advantages of the DRM you feel is best (i.e., more important)?
What are some disadvantages?
Which control strategies or work practices are adopted by the company to manage the exposure to respirable dust and crystalline silica at the worksite?
Can you share an example of how the use of one DRM method has changed a process, procedure, or practice on your site? (any example from the hierarchy of controls works).
What other ways are exposure monitoring data used in decision making for control technologies and work practices?
In what ways are frontline workers involved in this decision-making process?
Are you involved in the selection of DRM at your workplace?
Who selects any new DRM for your workplace?
In your opinion, does the DRM that is currently available meet the health and safety needs of workers specific to reducing exposure to RD/CS? Why or why not?
In general, what do you like most about using DRM methods and technologies?
Is there a type of DRM you like?
Why do you like it?
In general, what do you dislike most about using DRM methods and technologies?
What would you change if you could?
[If applicable] describe a situation where your employees complained of specific DRMs interfering with job tasks or requirements.
Why do you dislike it?
Industry-/job-/organization-specific factors affecting the effectiveness of HS&IH practices.
Now, we would like to understand industry-, job-, and/or organization-specific issues that impact your application of HS&IH practices and use of DRM to reduce RD/CS in your sector.
What are some specific job tasks in your industry make it difficult to assess and mitigate for exposure assessment?
Could changes to the practices address these challenges?
Could adoption of existing or new DRM address these challenges?
What are some features specific to [your industry – mining, construction or OGE] that make it difficult to conduct HS&IH mitigation practices for RD/RC?
Could changes to the practices address these challenges?
Could adoption of existing or new DRM address these challenges?
What guidelines, frameworks, or resources have been particularly helpful in informing your company’s current practices (e.g., AIHA, ASSP, etc.)? And specifically for DRM?
How have you used these to inform or update your own guidelines?
What NIOSH resources (if applicable) have been consulted by you or your company to inform or update your own guidelines?
What are some gaps in the current resources and guidelines available that must be updated for industry professionals?
Is this gap specific to your industry? If so, why?
Learning from the data.
How are you using the data collected from DRMs and other methods to address exposure to RD/CS?
Specifically, use of samples from personal or area monitoring?
Statistical modelling – and if so, what tools and for what purpose?
Interactions with employees on site?
Which performance metrics are used by the company to evaluate the management of the risk for the exposure of the workers to crystalline silica and respirable dust?
In which way can the advanced monitoring approaches provide support to the company in raising awareness among workers and additional training on risk hazard?
On the horizon and future needs
How is your company or site, specifically, planning to evolve their HS&IH practices and how DRMs and complementary technologies are being used?
What about the evolution of data analysis techniques with DRM output?
For each new technology adopted, there is an associated burden for the company (cost, training for new technologies, and maintaining additional equipment). How do you think the benefits can balance the additional burden?
Standardized use of DRM is important, as we have been discussing today. What are some ways that DRM could be better integrated with your company’s overall health and safety management system (HSMS) or other complementary system?
Is there anything else that you feel I should have asked, or that you would like to add?
That’s all the questions I have.
Thank you very much for sharing your thoughts with us and for a great conversation.
Biomechanics measurements and eye tracking measures
Examples of Technologies that Collect Data Outlined in Burden Table



Virtual reality glasses to detect eye movement and head sway (left). Heart rate monitor to collect changes in heart rate during participation (middle); and force plate with biomarkers to detect balance and postural sway during participation (right).

Use of a head mounted display to receive a technological interaction with different robots, using controllers to select communication modalities


Illustrations of Dikablis eye trackers being worn by the participant in a stationary vehicle while responding to virtual reality simulations on TV monitors.
Task Performance Measures
Graphical User Interface Information to Set up and Record in Virtual Reality Setting Prior to Testing
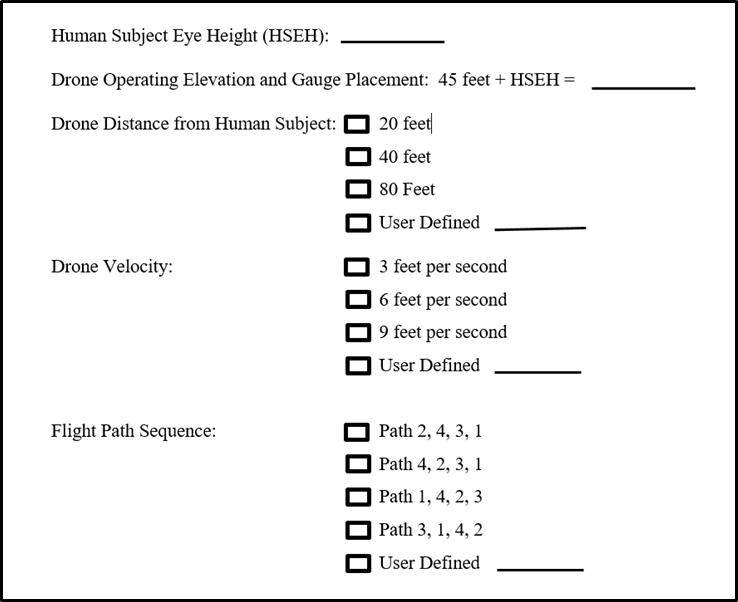

Stemma tactile push-button by DigiKey (Digikey, part number 1528-4431-ND) will be placed on the steering wheel (2 o’clock position, keyboard, and siren/light controls to record movement time of the participant’s right hand/arm as he/she performs the reaching tasks (Figure 4). These switches complete an electrical circuit when pressure is applied to the device by the participant, which then gives the participant a perceptible click in response, indicating current flow. Current flow is turned off when the switch is released.
TEST SUBJECT PAYMENT FORMS

| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| File Modified | 0000-00-00 |
| File Created | 2024-10-27 |
© 2026 OMB.report | Privacy Policy