STD Surveillance Network (SSuN) Project Protocol * Implementation Guide
Att 4_Protocol_Clean.docx
[NCHHSTP] The STD Surveillance Network (SSuN)
STD Surveillance Network (SSuN) Project Protocol * Implementation Guide
OMB: 0920-1072
STI Surveillance Network (SSuN)
Project Protocol
&
Implementation Guide

SSuN Cycle 5
(2024 – 2029)
OMB Control# 0920-1072
Table of Contents
A. Overarching Responsibilities/Activities of Collaborators 7
i. Fidelity to Data Collection Protocols and Methods 7
ii. Adherence to Data Security and Confidentiality Requirements 7
iii. Non-Research Determination 9
iv. Participation in SSuN Meetings, Conference Calls and Collaborations 9
v. Participation in Project Evaluation and Data QA Processes 9
vi. Provision of Technical Assistance to State and Local STI Programs 10
B. CDC Responsibilities/Activities 10
i. Project Coordination and Performance Monitoring 11
i. SSuN Data Uses at the Individual Site Level 12
ii. Analysis of Aggregate SSuN Data 13
iii. SSuN Data Stratified by Site 14
D. SSuN Memorandum of Agreement 16
E. Strategy A – STI Clinic-Based Sentinel Surveillance Activities 17
F. Strategy B – Enhanced Case-Based Population Surveillance Activities 19
i. HIV Registry Matching Requirements for Strategy A 29
ii. HIV Registry Matching Requirements for Strategy B 29
H. Strategy C: Supplemental Projects 30
i. Strategy C Activities for Strategy A Recipients 30
ii. Strategy C Activities for Strategy B Recipients 32
iii. Strategy C Activities for Strategy A and B Recipients 33
CDC Collaborators
All collaborators at the Centers for Disease Control and Prevention (CDC) are staff of the National Center for HIV, Viral Hepatitis, TB, and STD Prevention (NCHHSTP), Division of STD Prevention (DSTDP), Surveillance and Data Science Branch (SDSB).
Marvin Fleming
CDC SSuN Project Officer
Eloisa Llata, MD, MPH
CDC SSuN Strategy A Medical Officer
Kristen Eberly, MPH
CDC SSuN Strategy B Epidemiologist
LaZetta Grier
CDC SSuN Data Manager
Emily Rowlinson, PhD
Lead, Enhanced Surveillance and Special Studies Team
Rebekah Frankson, MS, MPH
CDC SSuN Epidemiologist/Analyst
Introduction
The Sexually Transmitted Infection (STI) Surveillance Network (SSuN) is a collaboration of competitively selected state, county, and city health departments (HDs), funded to provide a better understanding of national, state, and local STI epidemiology. Established in 2005, the purpose of SSuN is to 1) provide enhanced behavioral, demographic, and clinical information on gonorrhea (GC) and adult syphilis cases reported to state and local HDs, 2) provide information on patients presenting for care in specialty STI clinical settings, and 3) explore innovative strategies to improve STI surveillance nationwide. There have been four prior funding cycles of SSuN, with Cycle 5 being implemented during 2024-2029. Information on prior funding cycles of SSuN can be found in Appendix 1.
Cycle 5 (2024-2029) builds upon and expands from prior cycles to create a geographically diverse network of clinical and public health partners implementing sentinel and enhanced surveillance for STIs. See below for a list of the competitively selected sites for SSuN Cycle 5.
This protocol document describes methods that funded jurisdictions will use in implementing enhanced and sentinel STI surveillance strategies. Additional information on SSuN may be obtained by contacting CDC SSuN Project staff.
SSuN Cycle 5 Jurisdictions
The following state, county and/or city HDs successfully competed for funding under CDC-RFA-PS-24-0082.
Strategy A Jurisdictions
Baltimore City Health Department
California Department of Public Health
Colorado Department of Public Health and Environment
City of Columbus Public Health
District of Columbia Health
Florida Department of Health
Indiana State Department of Health
Massachusetts Department of Public Health
New York City Department of Health and Mental Hygiene
Philadelphia Department of Public Health
San Francisco Department of Public Health
Washington State Department of Health
Strategy B Jurisdictions
Baltimore City Health Department
California Department of Public Health
Chicago Department of Public Health
City of Columbus Public Health
Florida Department of Health
Massachusetts Department of Public Health
Michigan Department of Health and Human Services
New York City Department of Health and Mental Hygiene
Philadelphia Department of Public Health
Tennessee Department of Health
Washington State Department of Health
SSuN Cycle 5 Protocol
Jurisdictions receiving funding under CDC-RFA-PS-24-0082 are required to participate in the implementation, maintenance and evaluation of sentinel and enhanced surveillance activities as requirements of their cooperative agreement; continued funding is contingent on maintaining levels of performance across all funded strategies and activities.
All SSuN collaborators are required to complete the project Memorandum of Agreement (MOA; see section D and Appendix 2) governing shared expectations for recipient conduct, collaboration and participation in analyses and dissemination of SSuN findings.
SSuN collaborators should adhere to all protocols for data collection with regards to collecting required data elements, data cleaning and quality assurance (QA), formatting and routine, secure transport of data to CDC in a timely fashion according to agreed schedules.
CDC staff will work with funded collaborators to provide for reasonable local flexibility in implementing specific activities, where necessary, to reflect local public health contexts and conditions – while assuring comparability of data across all funded areas. SSuN Epidemiologist/Medical Officers, Project Officers, and Subject Matter Experts (SMEs) will also work with collaborators to identify and address relevant training and/or technical assistance (TA) needs to ensure success of local activities. All SSuN collaborators contribute de-identified visit and case-level clinical, disease surveillance, behavioral, laboratory and other public health-related observations to aggregate national project datasets; the accuracy, validity and reliability of these data depend critically on the comparability of methods across funded sites as well as a commitment on the part of SSuN collaborators to due diligence in data collection, data cleaning and QA.
To protect the confidentiality of all SSuN data, state and local surveillance program staff agree to abide by standards embodied in, and documented by, the “Data Security and Confidentiality Guidelines for HIV, Viral Hepatitis, Sexually Transmitted Disease, and Tuberculosis Programs: Standards to Facilitate Sharing and Use of Surveillance Data for Public Health Action.” Funded jurisdictions are required to obtain a statement from their jurisdiction’s Overall Responsible Party (ORP) for HIV surveillance to document compliance as part of the award process. All personally identifiable information (PII) maintained at the local level must be securely stored and appropriately redacted before transmitting records to CDC. Data transmitted to CDC will contain only required geographic information (county, state, and census tract) as well as other demographic, clinical, and behavioral data elements specified in SSuN protocols.
SSuN values the principles embodied in patient-level protections and strives to establish and maintain the highest level of performance in protecting the confidentiality and security of all information. Patient-level data transmitted to CDC must not contain PII such as name, social security number, or other information that is unique to an individual. Unique, non-personally identified patient and event IDs are critical for the success of SSuN and specifically permit longitudinal monitoring of unique persons over time. To do this reliably, the unique identifiers (IDs) associated with individual patients and related health events must be maintained over the full period of the cooperative agreement. Moreover, because IDs assigned to uniquely identify persons are transmitted to CDC, these IDs must not contain elements of PII. All unique IDs for patients and events must be developed and maintained locally and may (or may not, depending on state/local practice or guidance from other federal surveillance programs) be the same IDs used in local surveillance data management systems or electronic health records, as locally determined.
All record-level data transmitted to CDC by collaborators must be transported using the Secure Access Management Services (SAMS) portal, which allows for encrypted, secure transport of data to CDC. Data stored at CDC are maintained on secure servers with multi-layered access restrictions. SSuN data archived at CDC are available only to DSTDP staff on an “as needed” basis. SSuN surveillance data, as with all national STI surveillance records, are governed by strict data re-release policies; disclosure of any information that could be used to identify any individual directly or indirectly on whom a record is maintained is strictly prohibited.
CDC’s Human Research Protection Office reviewed the SSuN protocol and deemed it to be a surveillance and disease control activity and not an activity of human subject research. Analysis of deidentified SSuN data does not constitute research involving human subjects; therefore, CDC’s Institutional Review Board (IRB) review was not required. All collaborating HDs must assess their local requirements for similar determinations. Where necessary, local IRB non-research exemptions or waivers should be procured, with the understanding that any additional local requirements for patient consent must be carefully balanced against public health surveillance needs and should not be burdensome to the extent of precluding the jurisdiction’s full participation in SSuN activities or significantly compromising compliance with CDC-approved protocols. Of note, no incentives are provided directly to patients for participating in SSuN activities, nor should any actual or perceived consequences devolve to patients for non-participation or refusal.
SSuN collaborators are expected to take an active role with CDC and with their SSuN colleagues from other sites, and to fully participate in individual and/or group discussions and scheduled gatherings. The purpose of these meetings, conference calls, and individual site consultations is to ensure that SSuN activities are implemented according to protocols, provide a regular forum to discuss emergent issues in STI surveillance, address site-specific issues, and collaborate on analyses and dissemination of SSuN findings. SSuN collaborators may also occasionally be called upon to serve as surveillance consultants to DSTDP for expertise on issues involving emergent and/or long-standing problems in STI surveillance at the local and national level.
SSuN staff at CDC will provide recipients with SAS data structures, format libraries, and SAS syntax for edit checking and data QA for all required datasets that must be applied prior to transmitting data to CDC. These tools should be used by funded sites to ensure that all data quality, structure, and format issues are addressed – and corrections made – before data are transmitted to CDC. Additional QA processes will be deployed at CDC before data are merged into the national datasets. SSuN collaborators are expected to actively participate in data QA processes and to collaborate with CDC staff on addressing all issues identified in datasets submitted to CDC.
Periodically, CDC may request that collaborators participate in initiatives designed to evaluate SSuN activities, provide additional information about the effectiveness or efficiency of SSuN surveillance methods, and to provide quantitative or qualitative information to CDC for future planning purposes. SSuN recipients are expected to collaborate with their CDC colleagues in these important evaluation activities.
SSuN collaborators are encouraged to provide TA on surveillance methods and best practices to the state, county, and local STI/HIV programs in their jurisdiction as part of routine SSuN activities. SSuN collaborators are a rich source of information and surveillance expertise and should proactively make themselves available to their local colleagues to improve the overall quality of STI surveillance data, especially those data routinely reported to CDC through the National Notifiable Disease Surveillance System. SSuN collaborators are asked to develop formal, written processes to share lessons-learned locally and provide for data sharing with HIV and STI surveillance programs to ensure that SSuN data are used to supplement the completeness of existing HIV and STI case data reported to CDC. Additionally, SSuN data should be used to enhance the quality of STI epidemiology reports developed and disseminated locally to guide STI prevention and control efforts.
SSuN collaborators are funded through a Cooperative Agreement rather than a grant mechanism in recognition of the substantial involvement of CDC in the development of activities, protocols, and priorities for the network – consistent with the broader goals of CDC/NCHHSTP/DSTDP. Substantial involvement by the SSuN Epidemiologist/Medical Officer, SMEs, Project Officer, and other CDC collaborators includes:
Development and dissemination of the protocol for SSuN activities
Facilitation of routine SSuN communications.
Coordination of conference calls and annual collaborator’s meeting(s).
Provision of infrastructure for secure transport of data to CDC.
Provision of TA
Provision of SAS licensure.
Monitoring of recipient progress toward achieving SSuN outcomes, including recipient implementation of data QA processes.
Management of SSuN data warehouse or other CDC central data stores to support data provisioning for collaborative analyses.
Provision of guidance and TA (where requested and/or identified by CDC) essential to implementation of activities in compliance with protocols.
Summary and aggregate reporting to CDC leadership and external stakeholders.
Ensuring that analyses and dissemination of site-specific findings from SSuN surveillance. activities are conducted collaboratively by both CDC and appropriate colleagues at participating sites.
Facilitating discussions with SSuN recipients to identify emerging trends/issues in STIs/HIV and sexual health, STI surveillance technologies and methods and other issues that merit further investigation.
Coordinating development, dissemination, and approval of proposals for multisite SSuN analytic projects.
Assisting co-authors and lead authors in the development of multisite SSuN manuscripts.
Facilitating CDC clearance for manuscripts and presentations based on multisite SSuN findings.
Working with SSuN recipients to ensure that all activities, at both the awardee and CDC level, adhere to NCHHSTP Data Security and Confidentiality Guidelines.
The SSuN Project Officer will work with each recipient to implement routine performance monitoring processes. CDC will provide periodic annotated QA Summary progress reports to collaborators that will summarize key performance metrics for the project overall and serve as the basis for comparison of these same metrics across individual sites.
Data from SSuN are expected to improve local and national STI surveillance activities, contribute to STI/HIV prevention and control programs, inform local and national STI policymaking, and increase the understanding of the epidemiology of populations being diagnosed with STIs and trends in persons seeking STI clinical services. Results and findings from SSuN are also intended to guide other national STI surveillance projects and contribute to strengthening human resources and technical infrastructure for state and local STI surveillance.
SSuN is a surveillance network that is, in part, intended to be representative of persons being diagnosed and reported with STIs in multiple participating geographic areas encompassing a significant proportion of all STIs reported nationally. An important outcome of SSuN is to disseminate findings in a timely and useful way. Many findings will be particularly useful at the local level; other results will be more meaningful after the data from all SSuN collaborators have been aggregated, cleaned, and weighted for analysis where appropriate. SSuN recipients are expected to analyze and disseminate their site-specific data and use local results to improve state and local surveillance reporting, inform STI-related health policy, and improve STI prevention and control efforts in their jurisdiction. The principles and guidelines presented in this section are intended to ensure that SSuN findings are disseminated widely and that all SSuN collaborators have opportunities to fairly participate in the process of analyzing, presenting, and participating in the development of manuscripts for submission to peer-reviewed journals.
SSuN recipients retain all rights and stewardship responsibilities regarding SSuN data collected and stored locally. Moreover, CDC data stewardship principles preclude sharing site-identified data transmitted to CDC with external parties without the explicit permission of local collaborators contributing those data. Collaborators are strongly encouraged to use their local SSuN data for routine reporting, novel descriptive or statistical analyses, presentations and/or manuscripts submitted for publication.
CDC requests that SSuN funding be acknowledged (CDC-RFA-PS-24-0082) if an analysis is presented or manuscript published that includes data collected as part of SSuN. CDC clearance is not required for site-specific data products unless a CDC collaborator is included as a co-author. Sites are asked to share local SSuN data products with CDC for inclusion in the SSuN bibliography. Moreover, it is strongly encouraged that SSuN collaborators share their ideas and plans for local analysis and publication with their SSuN colleagues at CDC and other sites through the SSUN proposal process. See the “Proposals for Analysis” section below for more information on the analytic proposal process. A SSuN Data Use/Analytic Proposal Template is also included in Appendix 3.
CDC staff will have primary responsibility for generating reports, coordinating authorship and publication of SSuN data aggregated across sites (no identified site-level stratification) and will summarize these data as requested by CDC leadership, in national surveillance reports, conference presentations, peer-reviewed journals and other internal and/or external publications. CDC staff retain the prerogative to respond rapidly to requests from DSTDP leadership for presentations of aggregate SSuN data in multiple formats and/or publications without prior notice.
Reporting of aggregate SSuN data at the national level that displays no site-specific stratifications, or only present ranges across de-identified sites, will not require co-authors or individual site-level approval or be subject to local clearance processes. However, all sites providing data used in any such analyses will be formally acknowledged if representatives from that site are not otherwise included as contributing co-authors. Reasonable effort will be made to propose such analyses to the SSuN collaborators in advance and to solicit input and co-authorship for aggregate analyses. Whenever non-CDC co-authors are included in SSuN manuscripts or presentations based on aggregate data, SSuN promotes and respects adherence to local clearance requirements when appropriate based on inclusion of a co-author from that jurisdiction.
All analyses that include data stratified by identifiable site, apart from figures presented in DSTDP’s annual STI Surveillance Report, will be disseminated as formal proposals to collaborators for discussion. Full participation as co-authors by SSuN collaborators is encouraged in these analytic projects but is not required. Co-authorship for SSuN purposes is construed to include substantive involvement in planning, methodologic decisions, data management and analysis, data visualization, manuscript drafting, implication discussions and reviews of final draft products. Contribution of data by a funded SSuN jurisdiction, in the absence of substantive involvement as described above, would not generally constitute sufficient contribution to be included as a formal co-author, consistent with the guidelines of most peer-reviewed journals.
Sites contributing data to identified, site-specific data products should identify an investigator to be included in a “SSuN Working Group” designation if there are no formal co-authors from that site; the working group will be the last formally acknowledged co-author on such manuscripts. Statements of approval for CDC clearance purposes are required of every co-author or “SSuN Working Group” member for abstracts submitted to conferences and for manuscripts submitted to journals for publication. We ask that all SSuN collaborators be conscientious in responding promptly to requests for clearance approvals.
SSuN collaborators from funded jurisdictions and DSTDP staff are all encouraged to develop proposals for analyses of SSuN data for consideration. This is a key element in disseminating the network’s findings and its important epidemiologic insights as broadly as possible and assuring that data are used to direct programmatic action. Proposals should address how the analysis will be used for public health purposes and the specific objective, data to be used (data elements, time frame), methods of analysis and briefly address the specific assumptions and how missing data may be dealt with. A SSuN Data Use/Analytic Proposal Template is included in Appendix 3.
Requests for multisite analytic data should begin with submitting the SSuN Data Use/Analytic Proposal to the SSuN Epidemiologist/Medical Officer for the applicable datasets. They in turn will review and resolve any questions directly with the requestor(s), as well as negotiate specific data elements. The SSuN Epidemiologist/Medical Officer will also serve as collaborating co-author on any abstracts/manuscripts resulting from the multisite analytic proposal. The SSuN Epidemiologist/Medical Officer processing the proposal is responsible for generating a timely e-mail to the Principal Investigator (PI) and/or Data Manager for all the sites whose data are being requested including a PDF copy of the proposal. Project Officer(s) are responsible for assisting in scheduling proposals for discussion, either as an agenda item on routinely scheduled site calls, All Sites calls, or for scheduling a specific call by request of the SSuN Epidemiologist/Medical Officer. Potential collaborators will be invited to review the proposal and to provide written comments or discuss via telephone. Collaborators should also be made aware of any deadlines for their review, and that they may elect to:
Approve the sharing of data and participate as a co-author on any resulting presentation/publications.
Approve the sharing of data, declining co-authorship (but with assurance of acknowledgement).
Decline permission for any sharing of site-level records.
The SSuN Epidemiologist/Medical Officer is responsible for preparing the respective datasets for multisite analyses and securing site-level permission.
All sites contributing data must identify one co-author for participation, either as a formal co-author or by inclusion in the “SSuN Working Group” designation. Sites who decline to permit the use of their data for multisite analyses must provide rationale for such decisions. Concurrent plans for a substantively similar site-level analysis by SSuN collaborators will generally not be considered a robust rationale for failing to permit use of their site-level data for inclusion in or for declining to participate in an approved SSuN multisite analysis.
Occasionally, analyses may be proposed using longitudinal data from sites that are not currently participating in SSuN, or from currently funded sites regarding data from previous cycles. Efforts will be made to contact previously participating PIs for co-authorship and approval; current collaborators may also participate as co-authors for any such analyses with consensus of the sites contributing data.
Proposals for multisite analyses from investigators that are not SSuN-funded collaborators (local interns, academic partners, etc.) must be sponsored by the PI of the site from which the proposal is submitted and must have the sponsorship of at least one SSuN Epidemiologist/Medical Officer or CDC SME. If approved through normal processes by the SSuN collaborators, the sponsoring SSuN PI will take responsibility for assuring full adherence to all appropriate SSuN processes described herein.
In many cases, preliminary data may be needed to assess the merits or feasibility of a given analysis. CDC SSuN project staff will work with investigators to conduct preliminary data exploration on the national SSuN data repository resulting in simple frequency tables and/or crosstabs to help inform the proposal; these data/tables may accompany the proposal distributed for review. All submitted proposals and any included preliminary data visualizations should be considered internal, privileged, and confidential documents.
Full access to analytic data will be provided contingent on approval of proposals; only data elements pertinent to the proposed analysis will be shared and only records for the time periods proposed in the analysis. Any SSuN data shared with external partners as part of an approved analysis proposal will be securely transported to the site sponsoring the analysis, with agreement that SSuN data will be afforded the highest level of protection at the receiving site, with limited access only for the purposes approved in the initial proposal and only by persons identified in the proposal. Sites agree to securely destroy (wipe) SSuN datasets after all analytic needs are fulfilled and further, agree that no secondary release of record-level data is permitted. Any publication requiring inclusion of full datasets as part of the publication process must be referred to the SSuN Epidemiologist/Medical Officer prior to the transfer of any SSuN datasets to third parties. In general, such uses will not be permitted.
HD collaborators funded for SSuN Cycle 5 activities will be required to complete a MOA with CDC governing the jurisdiction’s intention to provide required data, adhere to SSuN protocols for data collection, and to fully participate in SSuN collaborations as described in the cooperative agreement and this protocol document. A duly executed copy of this MOA should be completed and forwarded to CDC within one month post award. A template MOA is provided in Appendix 2.
The primary objectives of Strategy A are 1) monitoring trends in people seeking care in STI clinics, and 2) monitoring STI-related HIV prevention opportunities among persons seeking care in STI clinics. Collection of data from these settings should produce high-quality, timely clinic-based surveillance and epidemiologic data to direct public health STI prevention and control efforts and improve understanding of STI & HIV preventive services and intervention opportunities in STI-specific clinical settings. Because SSuN data are critically dependent on the quality of data, state and local STI surveillance programs are encouraged to optimize strategies to ensure high data completeness.
Population of Inference
The population of inference for the clinic-based component of SSuN includes all patients presenting for care and/or STI-related services in participating STI clinics.
Definition of STI Clinics
STI clinics are operationally defined as any clinical facility providing timely comprehensive, confidential, and culturally sensitive STI care as the facility’s primary advertised and usual function. Clinics need not be stand alone and may be integrated into broader practice settings. However, the selected facility must have a specifically identifiable STI/sexual health clinic with an annual patient census >2,000 unique patients per clinic (or >4,000 unique patients if included in a network of clinics) and can identify and extract records from their electronic health records (EHR) system for patients specifically seeking STI clinical services separately from any broader patient population.
Data Collection
Recipients will collaborate with selected STI clinical site(s) to obtain visit-level information, including the required data elements specified in the SSuN Data Dictionary, for patients who receive STI and/or sexual health services in participating STI clinics. Data abstracted will include patient demographic, behavioral, and clinical information collected during all visit encounters. The clinical information collected will be primarily related to the symptoms, prior HIV testing and results, HIV and other STI prevention opportunities, diagnosis of STI/HIV related conditions, provision of preventive care, treatment and vaccines prescribed, and STI-related laboratory tests and results. See section H below for more information on data management processes.
Patient Surveys
In addition to implementing visit-level data abstraction in STI clinics, recipients are expected to implement periodic, brief patient surveys in STI clinics participating in SSuN Strategy A. These surveys, conducted in collaboration with CDC staff, are designed to gain a better understanding of the access and utilization patterns of people who seek health services in STI clinics. Additional topics of interest may be considered but likely will be subject to approval from the Office of Management and Budget (OMB). SSuN recipients may choose when in the project period to implement their survey(s), based on local considerations, but must allow sufficient time to complete the surveys. The targeted number of surveys will be decided upon in collaboration with CDC project staff.
Although recipients may propose paper-based or technology-assisted data collection methods, the design must allow capture of voluntary, self-reported responses from patients seen consecutively during the survey administration interval. Patients should respond to the survey prior to receipt of their clinical services (e.g., in the registration area/waiting room). Conducting these periodic patient surveys can also provide the opportunity, if needed, for jurisdictions to include supplemental questions related to issues of specific interest at the discretion of the individual jurisdiction. However, this supplemental information would not be transmitted to CDC.
Patient duplication during the survey period is allowed, but only a single survey should be administered/collected per clinic visit. It is preferred, though not required, that survey data be linked to the associated SSuN patient visit record through appropriate IDs where feasible (unique visit ID, medical record number, patient name, etc.). Recipients with multiple STI specialty clinic sites contributing data to SSuN Strategy A should consider survey administration in higher-volume clinics. Data entry of survey data (if needed based on local methods proposed) will be accomplished at the STI clinic site or may be aggregated at the recipient’s HD for central data entry.
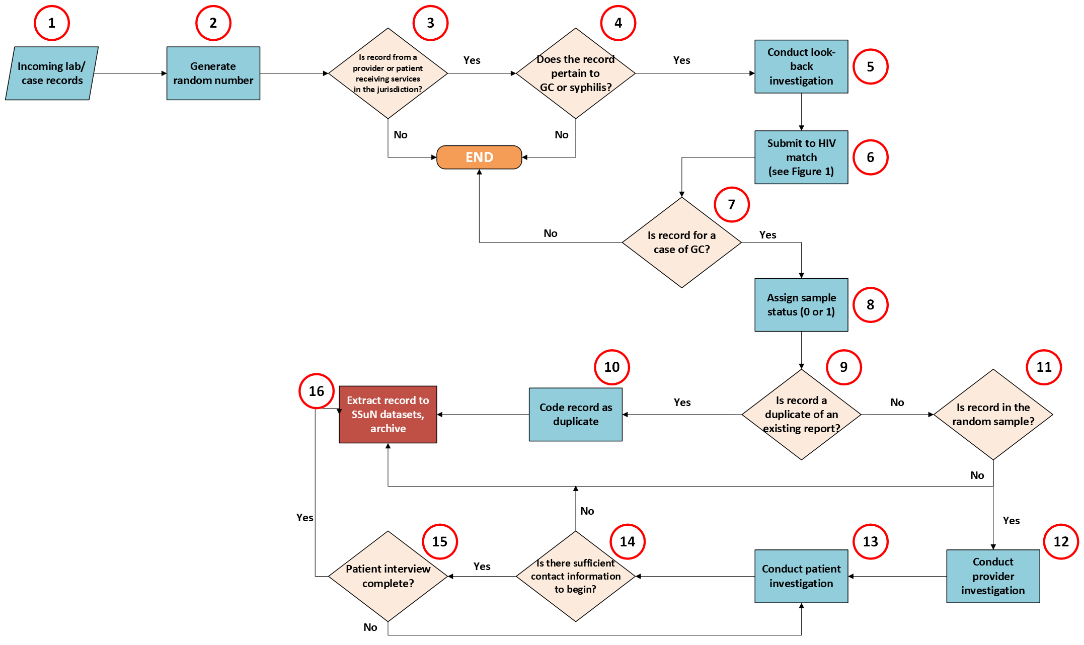
Enhanced data collection on all reported cases of GC and adult syphilis provides a valuable supplement to national case notifications, allowing assessment of key surveillance data quality measures. HIV registry matching of these cases provides information valuable for assessing progress toward HIV prevention goals and gaps in HIV preventive services among persons diagnosed and reported with GC and adult syphilis. Additional patient and provider data obtained on a representative sample of GC cases allows for valid estimation of case characteristics often missing or not present in routine case reporting.
The primary activities of Strategy B are to:
Extract, clean, and recode a full census of reported GC and adult syphilis cases to a case dataset with enhanced data elements including case and patient deduplication indicators.
Conduct look-back investigations on all reported cases of GC and adult syphilis. This includes the search of existing HD records, matching and merging with electronic or other laboratory data, and matching to HIV and other disease registries.
Select a random sample from the universe of reported GC cases.
Initiate provider investigations on all cases selected in the random sample to obtain relevant clinical and GC treatment information.
Initiate enhanced patient investigations of GC cases selected in the random sample.
Methods
Extraction of Case, Laboratory, Treatment, and Provider Records
GC and adult syphilis ‘records’ are defined as provider case reports, laboratory records, or any other original source documents as appropriate given the specific surveillance infrastructure in funded jurisdictions. Each record must contain a unique event ID and a unique patient ID. The patient ID should represent a single individual and must be static (i.e., must remain the same throughout the project period for any given individual patient to allow for longitudinal tracking over time). All case, laboratory, and treatment data associated with reported GC and adult syphilis cases are to be extracted, reformatted, and assembled into a separate dataset related to the case data through unique IDs. Unique provider records associated with reported cases of GC and adult syphilis will be extracted and assembled into a separate, related dataset, updated annually. Records will be linked to case records with a unique provider number. Required data elements for all datasets and the appropriate response options are included in the SSuN Data Dictionary.
Case-Level Look-Back Investigations
At a minimum, case records for reported GC and adult syphilis cases will be compared with existing disease and laboratory registries to determine if the patient of record has ever been reported to the HD as a case of GC, chlamydia, syphilis, viral hepatitis, or tuberculosis occurring within 365 days of the specimen collection/diagnosis date of current GC or syphilis case report. This should be documented and included in the appropriate data elements the SSuN record. If multiple diagnoses are found, only the most recent diagnosis should be retained. It should also be determined whether the record represents a ‘duplicate case record’, which is defined as a reported GC diagnosis within the previous 30 days or for syphilis cases, a syphilis diagnosis consistent with the current stage; if record is a duplicate of an existing report, this should be documented and included in the SSuN record as P1_CaseDup=1. For duplicate cases/records, the earliest specimen collection date (used to determine duplicate status) and report date should be documented.
Generating a Random Sample of Cases
Collaborating HDs will develop the capacity to generate a random sample of all reported cases of GC within the first three months of funding. The most effective way to achieve this result is to modify local STI surveillance information systems to incorporate this functionality by creating a system variable associated with individual records of confirmed cases/events. This variable should be populated with the results of a random number generator (generally a system function that randomly generates number between 0 and 1) which runs only once at the time the case is entered into their system, regardless of whether the record is created automatically based on incoming laboratory data or manually based on review of internal or external case or laboratory reports. Random number functions are also available in SQL, Oracle, and most other software platforms.
A useful analogy is that as each case is entered into the system, dice are rolled and the result frozen for that unique case; the ‘dice’ should not be rolled again once the initial result is recorded. The variable or data element containing this ‘frozen’ random number must be permanently stored in the underlying case/event record and available for export for use in constructing SSuN datasets and for directing subsequent SSuN enhanced case investigations. Because this random number is generated uniquely for each individual record, it is irrelevant whether subsequent investigations determine that the case/event is a duplicate or is out-of-jurisdiction.
Each system will have unique characteristics from the programming/development perspective, but the result must be a random number permanently associated with each unique case or event record that can be used to select a sample for enhanced investigation regardless of any subsequent disposition of that specific record.
Using Random Numbers to Assign Sample Status
Once a random number is associated with individual cases/events, this number will be used to assign a sample indicator based on the desired sample fraction for cases of interest to SSuN. This can be done either internally in the local STI surveillance data management system or externally using SAS, SQL, or other software.
For example, if a sample fraction of 30% is desired, and the random number generator returns a value between 0 and 1, all records with a value between 0 and 0.3 should be selected, and the sample indicator (P1_RandSamp) assigned a value of 1. For records with values > 0.3, the sample indicator should be assigned a value of 0. All records with a sample indicator equal to 1 constitute approximately a 30% random sample.
Consider that it may be desirable to have different sample fractions in different counties (or other geographic units) based on available resources for follow-up or to balance workloads based on morbidity levels. The same random number can be used, but the geographic unit would be incorporated into the assignment of the sample indicator. For example, given three counties with local IDs of 01, 02 & 03, and desired sample fractions of 10%, 20% and 50%, the sample indicator would be assigned this way using SAS code:
P1_RandSamp=0;
If county=01 and (0 LE RandomNumber LE 0.1) then P1_RandSamp=1;
If county=02 and (0 LE RandomNumber LE 0.2) then P1_RandSamp =1;
If county=03 and (0 LE RandomNumber LE 0.5) then P1_RandSamp =1;
Note that this schema can be deployed using a macro that could get the fraction and county data from an external source such as an excel workbook or other source to provide greater flexibility in changing the sample fraction over time, by geographic area or by disease. SSuN recipients are encouraged to develop and deploy flexible means of sampling cases wherever possible.
The ‘universe’ for assigning a random number will include ALL cases of laboratory confirmed GC cases diagnosed and reported from ALL public and private sources within the geographic boundaries of the collaborating jurisdiction.
Records should be individually assigned a random number at the time they are received into the jurisdiction’s STI surveillance information system (or batched in a timely manner daily, or at a minimum, weekly if randomization is performed external to the STI surveillance information system) such that all records meeting the sampling criteria based on information contained in the report (provider located in jurisdiction, laboratory confirmed diagnosis of GC) are assigned a random number.
The assignment of the 'sample' is determined by comparing the random number to a predetermined fraction based on the disease and geography, as determined locally (as mentioned above). To identify the record's inclusion in the sample, the variable (P1_RandSamp) will have values of 0 and 1.
A sufficient volume of records should be included in the resulting random samples to fulfil stated project objectives:
For jurisdictions with >50,000 GC cases reported in 2023, the minimum acceptable target for completed patient interviews is 2.5% of all reported cases.
For jurisdictions with 30,000–50,000 GC cases reported in 2023, the minimum acceptable target for completed patient interviews is 3.0% of all reported cases.
For jurisdictions with 10,000–30,000 GC cases reported in 2023, the minimum acceptable target for completed patient interviews is 3.5% of all reported cases.
For jurisdictions with <10,000 cases reported in 2023, the minimum acceptable target for completed patient interviews is 4% of all reported cases.
The patient interview completion rate will be calculated as the ratio of completed interviews to the number of cases in the sample; the interview completion rate target for SSuN investigations is 65%.
“Completed” patient interviews refer to fully complete or substantially complete (partial), patient interview. An interview will be considered as ‘partial’ if complete demographic and sex/number of sex partner information is obtained but other information is refused, or the interview was terminated prematurely. However, every effort must be made to complete all data elements on the patient interview; periodic recipient performance reviews will include assessments of missing or incomplete patient-reported data elements.
Jurisdictions will conduct routine and frequent (e.g., quarterly) QA activities to assess the representativeness of their samples, with particular attention to equal probability of sampling by patient characteristics (at a minimum by sex, age, and geographic region within jurisdictions and source of report).
Jurisdictions will ensure that appropriate data are available on the universe of all reported cases to calculate valid stratification and non-response weights for their sampled cases.
Provider Investigations
Case records that meet the following criteria should be referred for brief provider investigations:
Case falls within the random sample.
Record is a confirmed GC case and is not a duplicate of a previously reported case.
Diagnosing provider/facility is ascertained and is within funded jurisdiction.
Patient determined to reside within jurisdiction at the time of diagnosis.
For these investigations, the diagnosing provider is contacted to provide additional information about clinical characteristics, treatment(s) prescribed, the specific care setting and demographics of the patient. These investigations can be conducted and completed either by direct contact with providers (over the phone) or through other methods such as secure fax, mail, or other means if the confidentiality of patient information is strictly maintained. Provider investigations also provide an opportunity to obtain more recent contact information necessary for completing the enhanced patient investigations, especially if this information is missing from laboratory or case reports. SSuN recipients must institute QA and follow-up procedures to ensure the highest possible completion rate for provider investigations, including tracking the status of investigations and periodic re-contact to ensure completion.
Patient/Case Investigations
Criteria for referral to enhanced patient/case investigations include:
Case falls within the random sample.
Record is a confirmed GC case and is not a duplicate of a previously reported case.
Patient determined to reside within jurisdiction at the time of diagnosis.
Initial case report was received by HD ≤60 days of the diagnosis (or specimen collection) date.
Enhanced patient/case investigations may be conducted either by phone or in-person with a minimum of four documented contact attempts at various times (evenings/weekends, etc.) and using a range of contact methods (SMS, phone calls, mail, etc.). Sites are required to develop local protocols and data collection instruments (paper and/or electronic) for investigators, provide training for conducting direct patient contact and to address local human subject protection requirements, as necessary.
All reasonable attempts must be made to obtain reliable contact information for cases eligible for patient interviews. Methods for obtaining contact information for patients may include vital record searches, registry searches, direct provider contact, social media (following local HD policies), and/or driver’s license and/or vehicle registration registries, if available.
Jurisdictions should integrate SSuN data collection into local partner services and treatment assurance protocols to prevent duplicate patient and/or provider contacts; SSuN-related data elements may be collected during routine partner services if these data are collected in a manner consistent with SSuN protocols.
Figure 1 demonstrates a sample flow for conducting Strategy B activities.
Figure 1. Strategy B Jurisdiction-Level Process Flow for Collection of Case and Enhanced Provider/Patient Investigation Data.
All funded jurisdictions are required to match the STI clinic patients (Strategy A) and/or reported cases of gonorrhea and adult syphilis (Strategy B) to the jurisdiction’s HIV registry, depending on which strategies they were funded for. For the purposes of SSuN, the jurisdiction’s “HIV registry” is a term expressly defined to mean eHARS, the CDC-provided surveillance data management system for HIV case surveillance that constitutes the universe of HIV case data officially reported to CDC. However, some jurisdictions may maintain supplemental registry databases in synchrony with the jurisdiction’s official eHARS repository; these may provide similar functionality and validity for fulfilling SSuN’s HIV matching requirements if the data are comprehensive, reflect the full geographic extent of SSuN activities within the funded jurisdiction, and allow for extraction of required SSuN data elements, including HIV-related laboratory information. Case data extracted for SSuN should be matched with the HIV registry periodically, however a minimum requirement is that matches should be performed at least twice a year, with one matching event coinciding with submission of annual, cleaned SSuN datasets (due annually in March).
Data matching or linking records between data sources can be an important means of strengthening STI and HIV surveillance data, including identifying co-infections, improving the completeness of existing databases, and guiding public health program activities. The choice of IDs used in matching records is up to the recipient but in general, variables with the greatest specificity should be used. The matching process will strictly be performed at the recipient level; CDC will not conduct these matches nor receive patient IDs. Although jurisdictions will propose methods specific to their jurisdiction (e.g., software, matching methodology), the expectation is that matching will be automated and fine-tuned for maximum efficiency. Grantees will be able to assess their local burden of HIV co-infection among patients presenting for STI care in STI clinics or among reported cases of GC and/or adult syphilis. Matches will also enable CDC to do the following:
Evaluate HIV testing/status among STI clinic patients or among persons diagnosed with or at risk for GC or other STIs, including the ability to stratify by demographics, geography, behavioral risk, and diagnosing provider characteristics.
Understand the proportion of STI clinic patients or persons diagnosed with or at risk for GC or other STIs who are HIV-negative and eligible for and/or receiving HIV pre- or post-exposure prophylaxis (PrEP/PEP, at time of STI diagnosis) and to stratify these outcomes by multiple demographic, behavioral, and healthcare factors.
Understand the proportion of STI clinic patients or persons diagnosed with or at risk for GC or other STIs who are HIV-positive and in HIV-primary care, on antiretroviral therapy (ART), and virally suppressed, and to stratify these outcomes by multiple demographic, behavioral, and healthcare factors.
Provide relevant patient-level and aggregate information at the recipient level to assist their jurisdiction’s CDC-funded HIV Surveillance and Prevention programs to resolve cases with no risk reported/no identified risk (NRR/NIR), better monitor HIV care status of HIV-positive individuals, monitor local prevalence patterns, track current residence, and to better understand gaps in and opportunities for promotion and uptake of high impact STI-related HIV prevention interventions.
It is highly recommended that recipients collaborate with their CDC-funded HIV Surveillance units to address reciprocal information sharing to ensure that desired patient demographics, sexual orientation, HIV testing, and or treatment data are available to CDC-funded HIV Surveillance staff for related evaluations and to enhance the completeness of HIV case surveillance data. There is no requirement for data abstracted from the HIV registry to be shared back to clinical facilities for the purpose of patient-level interventions or public health actions. However, if jurisdictions choose to develop processes by which to share data back with clinical partners, SSuN would have no objections otherwise.
All SSuN patient records should have a disposition for result of HIV registry match. In cases where there is uncertainty or matching discrepancies are noted, a manual review of the matching variables is strongly suggested. For patients that are matched to a record in the HIV registry, recipients are asked to abstract patient-level data on the date of the earliest indication of HIV infection and documented mode of transmission. In addition, recipients are required to obtain HIV diagnostic and laboratory data that are available in the HIV registry for matched patients, including the earliest recorded initial HIV–positive diagnostic test date and HIV viral load (VL) and CD4+ test date(s)/result(s) after October 1st, 2023, with specimen collection dates within 12 months of the patient visit or patient disease report (12-month look-back).
Figure 2 provides an example of the flow of a jurisdiction’s HIV matching process.
Figure 2. Example of Jurisdiction-Level HIV Match Process Flow.
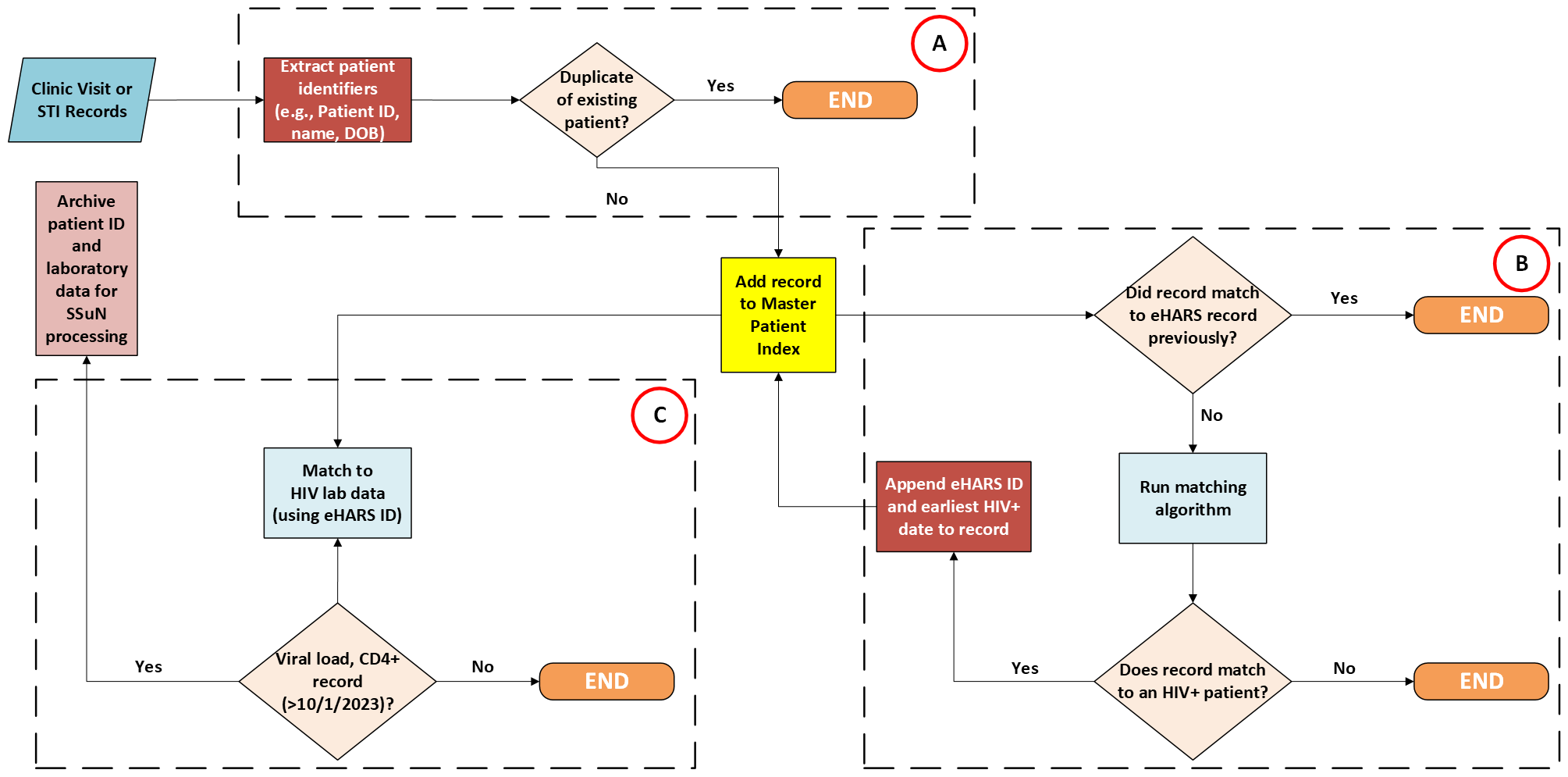
The process of HIV record matching is summarized into three broad steps: A) deduplication of clinic patient records by patient identifiers, B) identifying matching entries, and C) extraction of HIV data among matched entries. It is suggested, though not required, that jurisdictions develop and maintain a master patient index in which unique, deduplicated records of patients who have previously matched an HIV registry entry are kept centrally to obviate the need to rerun the patient through a matching algorithm. However, if a previously matched patient presents to a participating STI clinic for subsequent visits, the patient’s HIV laboratory (viral load/CD4+) data will need to be updated based on the date of each additional clinic visit.
Recipients are required to collaborate with selected STI clinical facilities to conduct matching of all clinic patients (regardless of diagnosis) seeking care at participating STI/sexual health clinics with the jurisdiction’s eHARS, or similar comprehensive HIV case registry. If collaborating STI clinics are independent entities, they are expected to provide patient IDs, as requested by the state/local HIV surveillance authority for routine matching.
All reported cases of GC and adult syphilis (all stages) are to be matched with the jurisdiction’s HIV registry. Jurisdictions should coordinate with their jurisdiction’s CDC-funded HIV Surveillance program to conduct periodic person-based matching, mindful that both STI and HIV case registries are dynamic; new patients are added continuously as new diagnoses are reported. Previously unmatched STI patients should be re-submitted to all subsequent matches to identify subsequent HIV diagnoses/reports and to ensure that complete information is available for all GC and adult syphilis cases reported throughout the full period of the SSuN cooperative agreement.
Strategy C Supplemental Projects are intended to be shorter-duration, limited-scope activities designed to improve the quality and utility of STI surveillance data and to pilot methods for monitoring the burden of disease, cost, and adverse health outcomes across a range of STIs, and laboratory and provider settings. Examples of anticipated/potential outcome and outputs include but are not limited to an improved ability to 1) ascertain and respond to emergent issues of concern in STI surveillance, 2) ascertain co-factors affecting access to (and use of) preventive services, and 3) ability to integrate, manage, de-duplicate, visualize, interpret and rapidly report on trends in STI-related laboratory and case data using local surveillance data management systems.
SSuN recipients are required to apply for at least one but no more than two Strategy C activities. These activities may change, be revised, or be retired, reflecting Divisional priorities and emergent STI issues. Protocols and methods may be recipient based or collaboratively developed post-award depending on the number of jurisdictions participating.
C.1: Improvements to Medical Record Systems in STI Clinics Supporting Collection of SSuN Data for Strategy A
Applicants will propose innovative approaches to automate, increase efficiency, and lower burden data collection from clinic patients and to initiate design/requirements collection or to implement ‘off-the-shelf’ solutions achievable with available resources to enhance and facilitate Strategy A visit-level data management, data cleaning, automated reporting, or enhanced analysis and visualization to support SSuN activities and to monitor patient care.
Expected outputs:
Documented system requirements and implementation plan.
Documented system modifications, datasets, data entry screens, and/or reports.
C.2: HIV-PrEP Patient Monitoring or Doxycycline Post-Exposure Prophylaxis Monitoring
Develop clinic-based panel of users of HIV PrEP or doxycycline post-exposure prophylaxis (doxyPEP) and provide for quarterly monitoring of STI screening, STI behavioral risks, incident diagnoses of STIs and PrEP (or doxyPEP) use, and maintenance as documented through prescriptions or dispensing. Expected outputs:
Quarterly reporting/monitoring of the proportion of:
Eligible patients referred to, initiating, and maintaining HIV PrEP or doxyPEP.
HIV PrEP/doxyPEP users screened for STIs in compliance with guidelines.
HIV PrEP/doxyPEP users diagnosed with STI(s).
HIV PrEP/doxyPEP users maintaining HIV PrEP/doxyPEP at quarterly intervals.
HIV PrEP/doxyPEP users lost to follow-up at quarterly intervals.
C.3: Technology to Enhance HIV PrEP Monitoring
Following SSuN protocols, recipients will implement clinic-based technologies for HIV PrEP adherence support, recall/scheduling management and monitoring (SeraAlert, SMS, etc.)
Expected outputs:
Documented system requirements and implementation plan.
Proportion of HIV PrEP patients enrolling and actively using platform.
Evaluation of the platform’s user (patient) acceptability and effectiveness.
C.4: Evaluate HIV PrEP Uptake and Retention
Recipients will conduct comprehensive analysis of co-factors of HIV PrEP initiation and maintenance, with focus on women of color. May include records-based analyses supplemented with qualitative methods such as surveys, focus groups and/or interviews. Compliance with local IRB requirements will be required.
Expected outputs:
Comprehensive analysis of co-factors facilitating HIV PrEP uptake and maintenance.
Comprehensive analysis of co-factors inhibiting HIV PrEP uptake and maintenance.
Programmatic recommendations for enhancing HIV PrEP uptake among women of color.
C.5: Enhancements to Local Surveillance Data Systems Supporting Collection of SSuN Data for Strategy B
Recipients will propose innovative approaches to automated, efficient, and lower burden data collection for patient interview data and to initiate design/requirements collection or to implement ‘off-the-shelf’ solutions achievable with available resources. These can include SMS, REDCap or other secure web or mobile device-enabled data collection tools or enhancements to data systems used to manage and facilitate Strategy B data management, data cleaning, automated reporting, or enhanced analysis and visualization of SSuN data.
Expected outputs:
Documentation of system requirements.
Evaluation of system enhancements with respect to:
Patient response rates.
Completeness of key data elements.
Recommendations for future modifications/enhancements.
C.6: Implementing Syphilis Automated Record Search and Review Algorithm
Recipients funded for this activity will develop automated processes to eliminate ore greatly reduce the need for manual record searches when reactive syphilis serologies are routinely reported to HDs among syphilis cases. Automation of this process will free-up valuable human resources to conduct prioritized investigations to identify syphilis cases, offer partner services, and break the chain of transmission within sexual networks.
Expected outputs:
Implementation of CDC-provided syphilis automated record search and review algorithm.
Evaluation of algorithm performance:
Quantify time from report to prioritization of case follow-up.
Estimated saving versus manual review effort using traditional reactor grids.
Additional evaluation in collaboration with CDC SME.
Recommendations for future modifications/enhancements.
C.7: Neonatal HSV (nHSV) Case Investigations and Medical Record Abstraction
Recipients funded for this activity will collaborate with CDC subject-matter experts to identify key clinical, laboratory and maternal characteristics useful for developing useful, reliable, specific, and sensitive case criteria for surveillance purposes.
Expected outputs:
In collaboration with CDC, deploy a medical record abstraction tool to collect vital infant and maternal data elements.
Identify cases of nHSV infection and abstract required clinical, demographic, laboratory, and treatment data.
Recommendations for future surveillance of nHSV.
Evaluate potential, candidate case definitions against formal criteria:
Timeliness for case reporting purposes.
Specificity of definition to rule out non-cases.
Sensitivity to detect neonatal infections.
C.8: Innovative Analyses of Multisite Data from SSuN Cycles 3 & 4
Recipients funded for this activity will propose to support innovative analysis of multisite data from Strategy A or Strategy B surveillance activities leading to dissemination of findings to relevant stakeholders through peer-reviewed publications or abstract-driven conferences. They must describe the specific analytic question, proposed analytic methods and potential impact of findings. All multisite analyses and manuscript preparation will be proposed, approved, and completed in collaboration with SSuN Science Officers at CDC and with the consent and participation of PIs from former or current SSuN sites providing data. Funds may be used to support existing SSuN staff, or to support fellows, interns or collaborating academic partners through appropriate local contractual mechanisms (however, tuition reimbursement is NOT an allowable budget item).
Expected outputs:
One or more peer-reviewed publication(s) or conference presentation(s) based substantially on multisite SSuN data. Other data sources may be incorporated as needed (e.g., American Community Survey or other US Census data for ecologic analyses).
C.9: Surveillance Focus Activity of Local Interest
Recipients may propose to collaborate with CDC to implement a locally relevant focus activity designed to implement activities that address issues pertinent to improving/enhancing local STI surveillance capacity, efficiency, timeliness, completeness, representativeness of surveillance data, and/or to analyze, interpret and disseminate local STI surveillance data to relevant stakeholders in innovative and meaningful ways. In collaboration with CDC, recipients will evaluate, document, and disseminate findings of the activity.
Expected outputs:
One (or more) analytic products or project reports comprehensively describing goals, objectives, methods, findings, and recommendations.
Strategy A data elements will be abstracted from the participating clinic’s electronic medical record system and will include patient demographic, behavioral, and clinical information collected during all visit encounters (Table 1).
Table 1. Required Strategy A Datasets.
Strategy A Datasets |
Content |
Frequency of Submission |
Clinic Visit Records |
SAS file containing visit-level records of demographic, behavior, and clinical information, cumulative from beginning of calendar year |
Every 2 months |
Clinic Diagnosis Records |
SAS file containing all STI-related diagnosis associated with each visit (separate record for each diagnosis), cumulative from beginning of calendar year |
Every 2 months |
Clinic Laboratory Records |
SAS file containing all STI-related laboratory tests associated with each visit (separate record for each test), cumulative from beginning of calendar year |
Every 2 months |
Clinic Treatment Records |
SAS file containing all STI-related treatment tests associated with each visit (separate record for each medication), cumulative from beginning of calendar year |
Every 2 months |
Clinic Metadata File |
Descriptive/services/policy information for each clinic for that calendar year in a separate record |
Initial submission, updated annually |
Year-End Datasets |
Final, cleaned and validated annual cumulative datasets containing all records for the previous calendar year. These serve as the primary analytic data. |
Due March 15th each year of the cooperative agreement |
Records from the visit, diagnosis, laboratory, and treatment files must include a unique, non-personally identifiable 1) patient ID, 2) event ID (unique for each visit), and 3) site ID to ensure multiple visits by the same patient are captured and longitudinal monitoring can be performed. The unique patient and event IDs should be assigned by either the state or local HD or the sentinel clinic and are created solely for the purposes of surveillance; neither should itself be a medical record number. It is imperative that the unique IDs associated with individual patients and related health events be maintained, static, and immutable over the full course of the cooperative agreement. If applicable, it is strongly encouraged to use the same unique ID for an individual patient if there exists multiple participating STI clinics within a network in a single jurisdiction. If the recipient is a past SSuN recipient, it is highly preferred that the static patient IDs be maintained in Cycle 5. Each jurisdiction will also be identified by a unique site ID and every clinical facility will have its own unique facility ID, both of which will be prepopulated by CDC.
The visit file will serve as the ‘parent’ record as it serves as the record of the actual clinical encounter. The patient ID, event ID, site ID, facility ID, and visit date variables found in every record will be the ‘key’ variables; null values will not be accepted for these variables. These key variables will be used to link records from the diagnosis, laboratory, and treatment files to the visit or parent record. Visit-level records from the diagnosis, laboratory, and treatment files that do not link to a parent record are considered ‘orphan’ records. These records should be reconciled at the local level before transmission of data to CDC. Facility-based characteristics included in the metadata file will be collaboratively defined by SSuN collaborators post-award and will be updated annually.
Strategy B data elements will be abstracted from the participating jurisdiction’s STI surveillance information system and will include case demographic, laboratory, and treatment information (Table 2).
Table 2. Required Strategy B Datasets.
Strategy B Datasets |
Content |
Frequency of Submission |
Case Records |
SAS file containing all diagnosed and reported GC cases, cumulative from beginning of calendar year |
Every 2 months |
Case Laboratory Records |
SAS file containing all STI-related laboratory tests (from all sources, including electronic laboratory reporting) associated with reported case, cumulative from beginning of calendar year |
Every 2 months |
Case Treatment Records |
SAS file containing all STI-related treatments/medications for reported cases, cumulative from beginning of calendar year |
Every 2 months |
Provider Reference File |
SAS file containing each unique reporting facility/provider documented in separate record that reported one or more GC cases in the previous calendar year |
Initial submission, updated annually |
Year-End Datasets |
Final, cleaned and validated annual cumulative datasets containing all records for previous calendar year. These serve as the primary analytic data. |
Due March 15th each year of cooperative agreement |
Data obtained for Strategy B will come from numerous sources within the HD and will need to be locally merged, recoded, and appropriately structured to facilitate merging into the national SSuN datasets. CDC will provide SAS data structures with variable names, lengths and types defined for all requested datasets. Local data should be transformed to conform to these data structures and include only the requested data elements, properly coded, and in appropriate data formats.
Funded jurisdictions are expected to maintain rigorous procedures to ensure the quality and validity of data before submitting to CDC, including but not limited to, completing data verification, recoding, and appropriately structuring the data to facilitate merging into national SSuN datasets. In collaboration with Data Managers in each jurisdiction, CDC will prepare syntax for data validation that will provide for the minimum data QA required. Jurisdictions will apply these validation checks and fix errors in records prior to transmission. In cases where errors are repeatedly introduced from underlying, primary data sources that cannot be corrected, an “exception” file should be maintained locally and applied to the dataset before transmission to fix historical errors that recur because of the cumulative nature of SSuN data processes.
Required Strategy A, B, and C (optional) datasets will be securely transmitted to CDC on a staggered schedule. On the 15th of each month, sites will transmit each of the datasets on an alternating basis. For example, on March 15, sites would send the Strategy A data, and then on April 15, sites would send Strategy B data, alternating throughout the year. Data for each transmission should be cumulative for that calendar year and complete through the last day of the month from two months prior. For example, for data due on May 15, the dataset should contain records from January 1 through March 31. This allows approximately 6 weeks for case follow-up, for data to be cleaned, properly coded and all QA processes to be completed prior to transmission. When the 15th falls on a holiday or weekend, datasets will be due the first business day following the holiday. A data transmission schedule will be distributed to SSuN collaborators post award.
Record-level data will only be transmitted to CDC following SAMS protocols. CDC will formally acknowledge all data transmissions and communicate all validation results. Datasets failing to comply with pre-determined data structures will be rejected, with notification to sites. Sites must re-format, recode or resolve issues and retransmit corrected datasets within five working days to remain in compliance with SSuN requirements.
Datasets received at CDC will be validated and merged into the national SSuN database; the national dataset will be maintained current as of the end of the previous reporting month for the purposes of reporting process measures back to funded jurisdictions. Funded sites will receive an individual data QA summary report documenting the status of all datasets received to date, identifying any datasets that were due and not received, and the on-time status of all transmissions as part of grants management and QA processes.
Appendix 1
History of the STI Surveillance Network
The Sexually Transmitted Infection (STI) Surveillance Network (SSuN) was established in 2005 to create a robust network of geographically diverse collaborating health departments (HDs) with the capacity to implement a wide variety of enhanced STI surveillance activities, the flexibility to modify activities over time as trends and emergent issues demand, and the ability to use surveillance data in a timely way to inform STI prevention policy at all levels of the public health infrastructure to guide STI programmatic action.
SSuN expanded in 2008 to include 12 collaborating HDs and further strengthen capacity in terms of public health workforce, data management and IT infrastructure. Activities funded in 2008 included monitoring the prevalence of STIs, HIV, viral hepatitis, and risk behaviors in MSM, assessing trends in the burden of genital wart disease in patients attending STI clinics, monitoring HIV testing coverage in patients attending STI clinics, and implementing population-based enhanced GC surveillance to provide estimates of demographic and behavioral characteristics of diagnosed and reported cases.
In 2013, ten sites were funded to maintain the network’s core focus on sentinel surveillance in STI clinics and expanded these sentinel surveillance activities to include patients being seen in reproductive health/family planning settings. Case-based enhanced surveillance activities were revised to include brief provider investigations to obtain important clinical and treatment information, additional look-back data from HD records and added interview questions related to care-seeking behaviors, HIV preventive services such as pre-exposure prophylaxis (PrEP) and sexual network/partnership characteristics. Revisions to data management processes with respect to data quality assurance, and collection of fully relational laboratory, provider, treatment, and diagnoses datasets enhanced the utility of data across both core surveillance components of SSuN in the 2013–2019 funding cycle. Additionally, weighting algorithms were developed to ensure timely weighted analysis of sampled cases and routine dissemination of findings.
In 2019, we funded 11 collaborating HDs in Cycle IV (2019 - 2024) to continue the network’s focus on enhanced STI surveillance through three primary surveillance strategies: Strategy A (sentinel surveillance activities in STI specialty clinics serving populations at risk for HIV and STIs), Strategy B supporting enhanced, case-based surveillance among reported cases of STI (gonorrhea [GC] and adult syphilis). We also expanded HIV surveillance registry matching beyond reported GC and syphilis cases to include all patients attending participating specialty STI clinics in Strategy A. Additionally, resources to support shorter-term, STI surveillance activities were provided to enhance local surveillance capacity and to investigate issues of specific concern to STI epidemiology. Examples of these Strategy C activities included migrating local surveillance information systems to reporting cases in an HL7-compliant format for national STI reporting and enhanced analysis of local or multisite STI surveillance data. Through this combination of collaborative surveillance activities, SSuN provides uniform, comprehensive, and enhanced information that supplements the information received via routine case reports on a representative sample of STI cases and for a census of patients attending selected STI clinics.
Past SSuN Funding Cycles
Data Collection Cycle |
No of Grantees |
Jurisdictions |
2005-2008 |
6 |
Colorado Department of Public Health and Environment Minnesota Department of Health New York City Department of Health and Mental Hygiene San Francisco Department of Health Virginia Department of Health Washington State Department of Health |
2008-2013 |
11 |
Alabama Department of Public Health Baltimore City Health Department Chicago Division of Public Health Colorado Department of Public Health and Environment Connecticut Department of Health Los Angeles County Department of Health Services Louisiana Office of Public Health New York City Department of Health and Mental Hygiene Philadelphia Health Department San Francisco Department of Health Washington State Department of Health |
2013-2019 |
10 |
Baltimore City Health Department California Department of Public Health Florida Department of Health Massachusetts Department of Public Health Minnesota Department of Health Multnomah County Health Department NYC Department of Health & Mental Hygiene Philadelphia Department of Public Health San Francisco Department of Public Health Washington State Department of Health |
2019-2024 |
11 |
Baltimore City Health Department California Department of Public Health Columbus Public Health Florida Department of Health Indiana State Department of Health Multnomah County Health Department NYC Department of Health & Mental Hygiene Philadelphia Department of Public Health San Francisco Department of Public Health Utah Department of Health and Human Services Washington State Department of Health |
Appendix 2
Memorandum of Agreement
Collection and USE of STI Surveillance Network (SSuN) Surveillance Data between
The Division of STD Prevention (DSTDP),
National Center for HIV/AIDS, Viral Hepatitis, STD and TB Prevention (NCHHSTP)
and
<Insert State Department of Health>
PURPOSE
The purpose of this agreement is to provide a mutually agreed framework between CDC and funded entities for the collection, sharing and release of surveillance data collected as part of STI Surveillance Network (SSuN) activities.
BACKGROUND & OBJECTIVES
The STI Surveillance Network is comprised of state/local and/or city health departments funded by cooperative agreement (CDC-RFA-PS24-0082) to implement common protocols for enhanced and sentinel STI surveillance. The purpose of SSuN is to improve the capacity of national, state, and local STI programs to detect, monitor, and respond rapidly to trends in STIs through enhanced data collection, reporting, analysis, visualization and interpretation of disease information. Data are collected locally by funded jurisdictions following prescribed protocols, cleaned, formatted and transported to CDC for merging into national datasets that will be used by SSuN collaborators and CDC subject matter experts for a broad range of reporting and analysis as provided for in SSuN protocol documents. This Memorandum of Agreement is intended to explicitly document concurrence of funded sites with SSuN data collection protocols, procedures and guidelines for the protection and use of SSuN data.
STORAGE OF SSuN DATA
The health department identified above agrees to send to CDC appropriately de-identified datasets with data elements (Appendix 4) as specified in SSuN protocols on all persons visiting collaborating STD clinics, and for all persons diagnosed and reported with gonorrhoea and/or all stages of adult syphilis. Separate SAS datasets will be required for clinic patient visits, diagnoses associated with patient clinic visits, laboratory observations associated with patient clinic visits, treatments associated with patient clinic visits, reported cases of gonorrhoea and adult syphilis, laboratory data associated with reported cases of gonorrhoea and adult syphilis, treatment data associated with reported cases of gonorrhoea and adult syphilis, and information on providers reporting cases of gonorrhoea and adult syphilis.
Sites will send SSuN data through SAMS using specified encryption methods and biologic specimens (if required for Strategy C activities) through approved carriers per CDC-supplied protocols. CDC agrees to accept and securely store these data and specimens, accessible only to SSuN project or CDC laboratory staff. Data will not be integrated into other datasets maintained by CDC and will at all times be stored on secure servers with fully restricted access. Biologic specimens (if required for supplemental projects) will be received directly by DSTDP’s Laboratory Reference and Research Branch.
To protect the confidentiality of persons reported with STIs, state and local surveillance program staff and/or contractors agree to abide by the Data Security and Confidentiality Guidelines for NCHHSTP (http://www.cdc.gov/nchhstp/programintegration/docs/PCSIDataSecurityGuidelines.pdf) and will be required to document compliance as part of annual project reporting. Full names, street addresses, social security numbers, telephone numbers, or any other specific identifying information will not be sent to CDC. Databases will contain geographic information at the census tract level as well as other demographic, clinical, and behavioral data elements specified in SSuN protocols collaborative developed by SSuN collaborators. Census tract data collected in the population component will be linked with US census data (American Community Survey and decennial Census data) and all such internal datasets will also be stored on secure servers with fully restricted access.
The Surveillance and Data Science Branch in the Division of STD Prevention is charged with the responsibility of maintaining the security and confidentiality and the scientific integrity of all SSuN databases, dataset and subsequent analyses. Appropriate CDC staff will be designated custodians of the SSuN data and accept full responsibility for observance of all conditions of use and for establishment and maintenance of CDC-standard security precautions to prevent unauthorized use. Other CDC staff in the Division of STD Prevention may be granted access to dataset derived from SSuN data as needed for legitimate data management or analytic purposes.
STI Surveillance Network Principal Collaborators will be promptly notified of any CDC personnel changes that affect access to data collected and managed for this project. All CDC staff with access to SSuN data will remain current with the annual Health and Human Services Information Security Awareness Training. A record of the completion of security training for all CDC staff is maintained by the CDC Information Technology Services Office (ITSO).
CDC may retain SSuN data as long as the data are protected as described herein and local use of these data to direct STI surveillance and prevention activities is ongoing. CDC will annually review the need for the data with SSuN Principal Collaborators, and shall destroy all copies of the data if it is determined that no further analysis will be conducted.
DATA RE-RELEASE & USE
Local collaborators retain full control of and rights to analysis, research, and publication of their locally collected data, regardless of whether these data are also provided to CDC as part of SSuN activities. However, collaborators agree to acknowledge CDC funding in publications resulting from analyses of data collected through SSuN funding. Principal Collaborators may request and receive multi-site SSuN dataset for specific analytic purposes provided the SSuN Project Officer and the Principal Collaborator (or designated representative) of sites contributing data have reviewed and approved the analysis proposal. Proposals for such analyses must include all of the information required in SSuN protocols prior to consideration for approval.
AUTHORSHIP & ACKNOWLEDGEMENT
All collaborators are encouraged to participate in generating and proposing analytic ideas, conducting analysis, drafting abstracts and manuscripts as first or with colleagues as co-authors. At least one co-author from each site is strongly recommended for all analyses using site-specific or site-stratified data.
This agreement may be amended at any time in writing by mutual agreement of CDC and SSuN Principal Collaborators. Such amendments will not be binding unless and until they are signed by personnel authorized to bind each of the parties.
SIGNATURES
_______________________________________________________________________________
R. Luke Shouse, MD, MPH Date
Chief, Surveillance and Data Science Branch,
Division of STD Prevention,
National Center for HIV, Viral Hepatitis, STD and TB Prevention
Centers for Disease Control and Prevention
______________________________________________________________________________
Emily Rowlinson, PhD Date
Team Lead, Enhanced Surveillance and Special Studies Team
Surveillance and Data Science Branch,
Division of STD Prevention,
National Center for HIV, Viral Hepatitis, STD and TB Prevention
Centers for Disease Control and Prevention
______________________________________________________________________________
Marvin Fleming Date
Project Officer – STD Surveillance Network (SSuN)
Surveillance and Data Science Branch,
Division of STD Prevention,
National Center for HIV, Viral Hepatitis, STD and TB Prevention
Centers for Disease Control and Prevention
Collaborators:
I/We have read and agree to stipulations in the Memorandum of Agreement for collection, stewardship, uses and transmission of data to CDC for CDC-RFA-PS24-0082, STI Surveillance Network (SSuN).
___________________________________________________________________________________
Health Department SSuN Principal (or Co-) Collaborator(s) Date
Name:___________________________________ Name:___________________________________
Title:____________________________________ Title:____________________________________
Agency:__________________________________ Agency:__________________________________
Appendix 3
SSuN Data Use/Analytic Proposal Template
1) Date proposal initiated: _______/________/________
2) Proposal submitted by:
☐SSuN Site Collaborator ☐DSTDP Staff ☐ Other CDC Staff/Fellow ☐External/Academic Partner
3) Title of proposed analysis: ______________________________________________________________
4) First author name: ________________________________________________________________
5) Affiliation (SSuN Site, Division/Branch/Team, academic institution, etc.)
_____________________________________________________________________________________
6) If applicable, include SSuN Principal Investigator/CDC Project Officer sponsor here.
_____________________________________________________________________________________
7) Additional collaborators/investigators:
_____________________________________________________________________________________
_____________________________________________________________________________________
_____________________________________________________________________________________
(Not binding. Final product may or may not include these or may include additional co-authors.)
8) Intended audience for analysis: (e.g., abstract or manuscript, name of conference or meeting)
_____________________________________________________________________________________
9) SSuN data to be used: (e.g., single site data [specify site]; multisite data [specify sites], specific variables requested).
_____________________________________________________________________________________
_____________________________________________________________________________________
_____________________________________________________________________________________
_____________________________________________________________________________________
_____________________________________________________________________________________
10) Describe the study design, including analytic methods.
_____________________________________________________________________________________
_____________________________________________________________________________________
_____________________________________________________________________________________
11) Describe how this analysis will be translated into program action.
_____________________________________________________________________________________
_____________________________________________________________________________________
_____________________________________________________________________________________
12) Describe the proposed timeline.
_____________________________________________________________________________________
_____________________________________________________________________________________
_____________________________________________________________________________________
13) Additional description or notes (optional).
_____________________________________________________________________________________
_____________________________________________________________________________________
_____________________________________________________________________________________
_____________________________________________________________________________________
_____________________________________________________________________________________
_____________________________________________________________________________________
_____________________________________________________________________________________
_____________________________________________________________________________________
_____________________________________________________________________________________
Requestors are reminded that any multisite SSuN data they receive for analytic purposes are to be governed by full data security and confidentiality protections at the collaborating site, are to be used only for the intended analytic purpose, may not be subsequently shared with any other entity (in whole or in part) and must be securely deleted/destroyed upon completion of the proposed analytic work. For the purposes of analytic integrity, the original datasets will be preserved at CDC.
Publication in open-access or other journals requiring supplemental data be provided to the publisher is not encouraged; additional permission must be procured and documented from all sites providing data and it is the responsibility of the investigator/lead author to provide such documentation to the CDC Science Officer(s) in advance of submission.
Reviewing Site________________________________________________________________________
We Approve Use of our Data: Yes☐ No☐
We request formal Co-Authorship: Yes☐ No☐
Principal Investigator: (Print)____________________________________________________________
Signature: ____________________________________________________Date____________________
Notes: _______________________________________________________________________________
_____________________________________________________________________________________
_____________________________________________________________________________________
_____________________________________________________________________________________
_____________________________________________________________________________________
Appendix 4
SSuN Protocol Change Log/Revision History
Date |
Section |
Revision Description |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | Torrone, Elizabeth A. (CDC/OID/NCHHSTP) |
| File Modified | 0000-00-00 |
| File Created | 2025-05-31 |
© 2025 OMB.report | Privacy Policy