Chap_5-6-7_SF424_RR_Guide
Chap_5-6-7_SF424_RR_Guide.doc
Research and Research Training Grant Applications and Related Forms
Chap_5-6-7_SF424_RR_Guide
OMB: 0925-0001
PHS SF424 (R&R) Adobe Forms Version A Application Guide
5. Completing PHS398 Components
5.1 Overview
In conjunction with the SF424 (R&R) components, NIH and other PHS agencies grants applicants should also complete and submit additional components titled “PHS398.” Note the PHS398 components include additional data required by the agency for a complete application. While these are not identical to the PHS398 application form pages, the PHS398 reference is used to distinguish these additional data requirements from the data collected in the SF424 (R&R) components. A complete application to NIH and other PHS agencies will include SF424 (R&R) and PHS398 components. The PHS398 components include:
PHS398 Cover Letter Component (optional, however applicants are strongly encouraged to include this component)
PHS398 Cover Page Supplement (this supplements the data requirements in the R&R Cover component)
PHS398 Modular Budget Component (use only when a modular budget is submitted instead of a detailed budget)
PHS398 Research Plan Component
PHS398 Checklist Component
Complete each component using the instructions provided below.
5.2 Cover Letter Component
Applicants are encouraged to include a cover letter with the application. The cover letter is only for internal use and will not be shared with peer reviewers. The letter should contain any of the following information that applies to the application:
1. Application title.
2. Funding Opportunity (PA or RFA) title of the NIH initiative.
3. Request of an assignment (referral) to a particular awarding component(s) or Scientific Review Group (SRG). The PHS makes the final determination.
4. List of individuals (e.g., competitors) who should not review your application and why.
5. Disciplines involved, if multidisciplinary.
6. For late applications (see Late Application policy in Section 2.14) include specific information about the timing and nature of the cause of the delay.
7. When submitting a Changed/Corrected Application after the submission date, a cover letter is required explaining the reason for the Changed/Corrected Application. If you already submitted a cover letter with a previous submission and are now submitting a Changed/Corrected Application, you must include all previous cover letter text in the revised cover letter attachment. The system does not retain any previously submitted cover letters until after an application is verified; therefore, you must repeat all information previously submitted in the cover letter as well as any additional information.
8. Explanation of any subaward budget components that are not active for all periods of the proposed grant.
9. Statement that you have attached any required agency approval documentation for the type of application submitted. This may include approval for applications $500,000 or more, approval for Conference Grant or Cooperative Agreement (R13 or U13), etc.
Two types of approval documentation are cited as examples in item 6 above: NIH IC approval for an application $500,000 or more and NIH institute approval for a Conference Grant or Cooperative Agreement application (R13 or U13). To attach the approval documents to this submission, please append those referenced documents to your Cover Letter File, and upload as one attachment.
Suggested Cover Letter Format
The Division of Receipt and Referral (DRR), Center for Scientific Review (CSR) is responsible for assigning applications to ICs and to Scientific Review Groups (SRGs). DRR will be utilizing knowledge management approaches as an adjunct to the work of referral experts as part of an overall plan to shorten the time from submission to review. Analysis has shown that requests made by investigators are a valuable source of information in this process. In order to facilitate the use of these requests in conjunction with knowledge management analysis of the content of the application, applicants are requested to use the following format when assignment requests are contained in a cover letter.
List one request per line.
Place Institute/Center (IC) and SRG review requests (if both are made) on separate lines.
Place positive and negative requests (if both are made) on separate lines.
Include name of IC or SRG, followed by a dash and the acronym. Do not use parentheses.
Provide explanations for each request in a separate paragraph.
Examples:
Please assign this application to the following:
Institutes/Centers
National Cancer Institute - NCI
National Institute for Dental and Craniofacial Research – NIDCR
Scientific Review Groups
Molecular Oncogenesis Study Section – MONC
Cancer Etiology Study Section – CE
Please do not assign this application to the following:
Scientific Review Groups
Cancer Genetics Study Section – CG
The reasons for this request are [provide a narrative explanation for the request(s)].
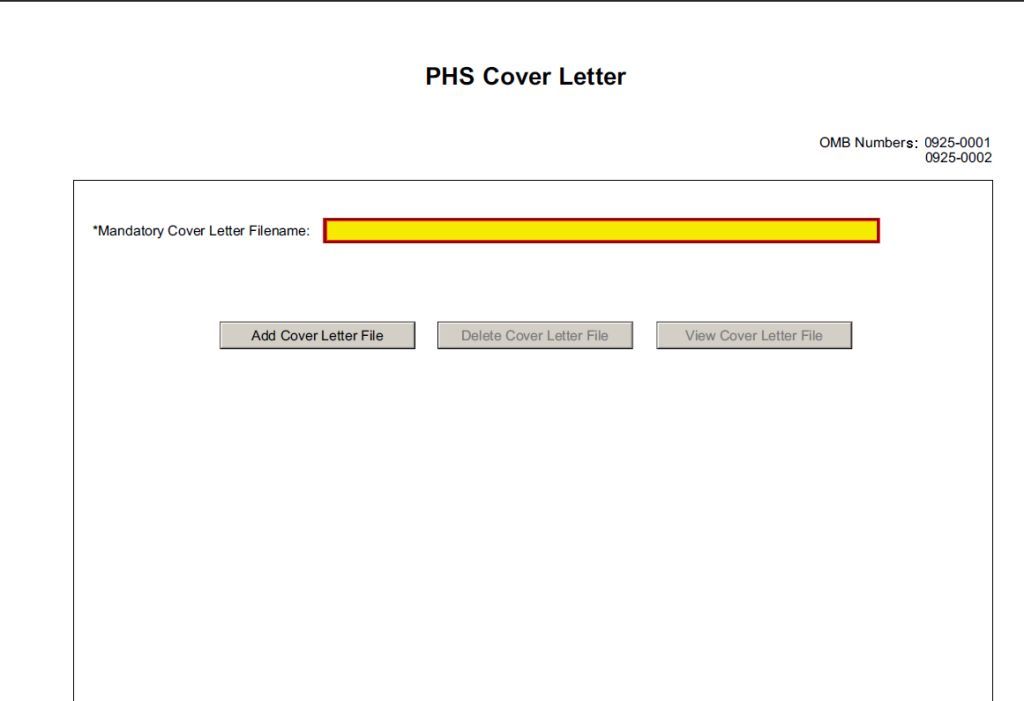
Save this information in a single file in a location you remember and convert the file to PDF. Click Add Cover Letter File, browse to where you saved the file, select the file, and then click Open. The name of the file attached will automatically appear in the “Mandatory Cover Letter Filename” field.
![]()
Once all data have been entered, click the Close Form button at the top of the form or use the scroll bar to scroll up. You will be returned to the Grant Application Package screen. To remove a document from the Submission box, click the document name to select it and then click the Move Form to Delete button. This will return the document to the Mandatory Documents Submission List or Optional Documents Submission List.
5.3 Cover Page Supplement Component
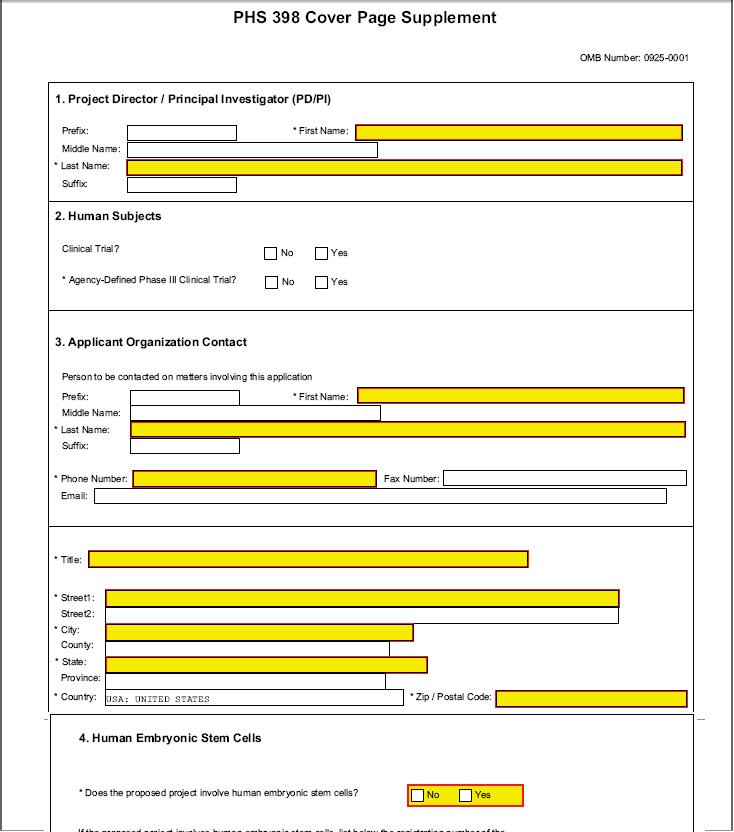
1. Program Director/Principal Investigator (PD/PI)
Field Name |
Instructions |
Prefix |
Pre-populated from the SF424 (R&R). The prefix (for example, Mr., Mrs., Rev.) for the name of the PD/PI. |
First Name |
Pre-populated from the SF424 (R&R). The first (given) name of the PD/PI. This field is required. |
Middle Name |
Pre-populated from the SF424 (R&R). The middle name of the PD/PI. |
Last Name |
Pre-populated from the SF424 (R&R). The last (family) name of the PD/PI. This field is required. |
Suffix |
Pre-populated from the SF424 (R&R). The suffix (for example, Jr., Sr., PhD) for the name of the PD/PI. |
2. Human Subjects
Field Name |
Instructions |
Clinical Trial |
Check the Yes or No box to indicate whether the project is a clinical trial. The NIH defines a clinical trial as a prospective biomedical or behavioral research study of human subjects that is designed to answer specific questions about biomedical or behavioral interventions (drugs, treatments, devices, or new ways of using known drugs, treatments, or devices). Note that Public Law 110-85, enacted 09/27/2007, mandates registration and results reporting of applicable clinical trials in ClinicalTrials.gov (see Part II and Part III). |
Agency-Defined Phase III Clinical Trial |
Check the Yes or No box to indicate whether the project is an NIH-defined Phase III clinical trial. An NIH-defined Phase III clinical trial is a broadly based prospective Phase III clinical investigation, usually involving several hundred or more human subjects, for the purpose of either evaluating an experimental intervention in comparison with a standard or control intervention or of comparing two or more existing treatments. Often the aim of such investigation is to provide evidence leading to a scientific basis for consideration of a change in health policy or standard of care. The definition includes pharmacologic, non-pharmacologic, and behavioral interventions given for disease prevention, prophylaxis, diagnosis, or therapy. Community trials and other population-based intervention trials are also included. |
3. Applicant Organization Contact
Person to be contacted on matters involving this application
Field Name |
Instructions |
Prefix |
Pre-populated from the SF424 (R&R). The prefix (e.g., Mr., Mrs., Rev.) for the person to contact on matters related to this application. |
First Name |
Pre-populated from the SF424 (R&R). The first (given) name for the person to contact on matters related to this application. This field is required. |
Middle Name |
Pre-populated from the SF424 (R&R). The middle name for the person to contact on matters related to this application. |
Last Name |
Pre-populated from the SF424 (R&R). The last (family) name for the person to contact on matters related to this application. This field is required. |
Suffix |
Pre-populated from the SF424 (R&R). The suffix (e.g., Jr., Sr., PhD) for the person to contact on matters related to this application. |
Phone Number |
Pre-populated from the SF424 (R&R). The daytime phone number for the person to contact on matters related to this application. This field is required. |
Fax Number |
Pre-populated from the SF424 (R&R). The fax number for the person to contact on matters related to this application. |
Pre-populated from the SF424 (R&R). The email address for the person to contact on matters related to this application. |
|
Title |
Enter the title for the person to contact on matters related to this application. This field is required. |
Street1 |
Enter first line of the street address for the person to contact on matters related to this application. This field is required. |
Street2 |
Enter second line of the street address for the person to contact on matters related to this application. This field is optional. |
City |
Enter the city for address for the person to contact on matters related to this application. This field is required. |
County |
Enter the county for address for the person to contact on matters related to this application. |
State |
Enter the state for address for the person to contact on matters related to this application. |
Province |
Enter the province. |
Country |
Select the country for the person to contact on matters related to this application. This field is required. |
Zip Code |
Enter the Postal Code (e.g., ZIP code) for the person to contact on matters related to this application. |

4. Human Embryonic Stem Cells
Field Name |
Instructions |
Does the proposed project involve human embryonic stem cells? |
If the proposed project does not involve human embryonic stem cells, check the No box. If the proposed project involves human embryonic stem cells, check the Yes box, and then complete the section below. |
Cell Line(s) |
List in this section the registration number of the specific cell line(s) from the NIH Human Embryonic Stem Cell Registry. |
Specific stem cell line cannot be referenced at this time. One from the registry will be used. |
If a specific line cannot be referenced at the time of application submission, check this box. |
Once all data have been entered, click the Close Form button at the top of the form or use the scroll bar to scroll up. You will be returned to the Grant Application Package screen. To remove a document from the Submission box, click the document name to select it and then click the Move Form to Delete button. This will return the document to the Mandatory Documents Submission List or Optional Documents Submission List.
5.4 Modular Budget Component
Selecting the Appropriate Budget Component
The application forms package associated with most NIH funding opportunities includes two optional budget components—(1) R&R Budget Component; and, (2) PHS398 Modular Budget Component. NIH applications will include either the R&R Budget Component or the PHS398 Modular Budget Component, but not both. (Note AHRQ does not accept modular budgets.)
To determine which budget component to use for NIH applications, consult the modular budget guidelines below. Additional guidance may also be provided in the specific funding opportunity announcement.
Modular Budget Guidelines
Modular budgets are applicable to certain research grant applications from domestic organizations requesting $250,000 or less per year for direct costs. International organizations and others that do not fall under this definition should use the detailed budget forms described in Section 4.7. Note, consortium/contractual F&A costs are not factored into the direct cost limit. They may be requested in addition to the $250,000 limit. Modular budgets are simplified; therefore, detailed categorical information is not to be submitted with the application. The modular budget is applicable only to R01, R03, R15, R21, and R34 applications.
For all modular budgets, request total direct costs (in modules of $25,000), reflecting appropriate support for the project. There will be no future year escalations. A typical modular grant application will request the same number of modules in each year. Provide an additional narrative budget justification for any variation in the number of modules requested.
NIH may request (prior to award) additional budget justification in exceptional circumstances. For further information, see http://grants.nih.gov/grants/funding/modular/modular.htm and http://grants.nih.gov/grants/funding/modular/modular_review.htm.
Using the Modular Budget Component
The Modular Budget Component provides budget fields for up to 5 years of support (e.g., budget periods 1 - 5). If requesting less than 5 years of support, complete only those years requested and leave the others blank.
5.4.1 Periods 1 through 4
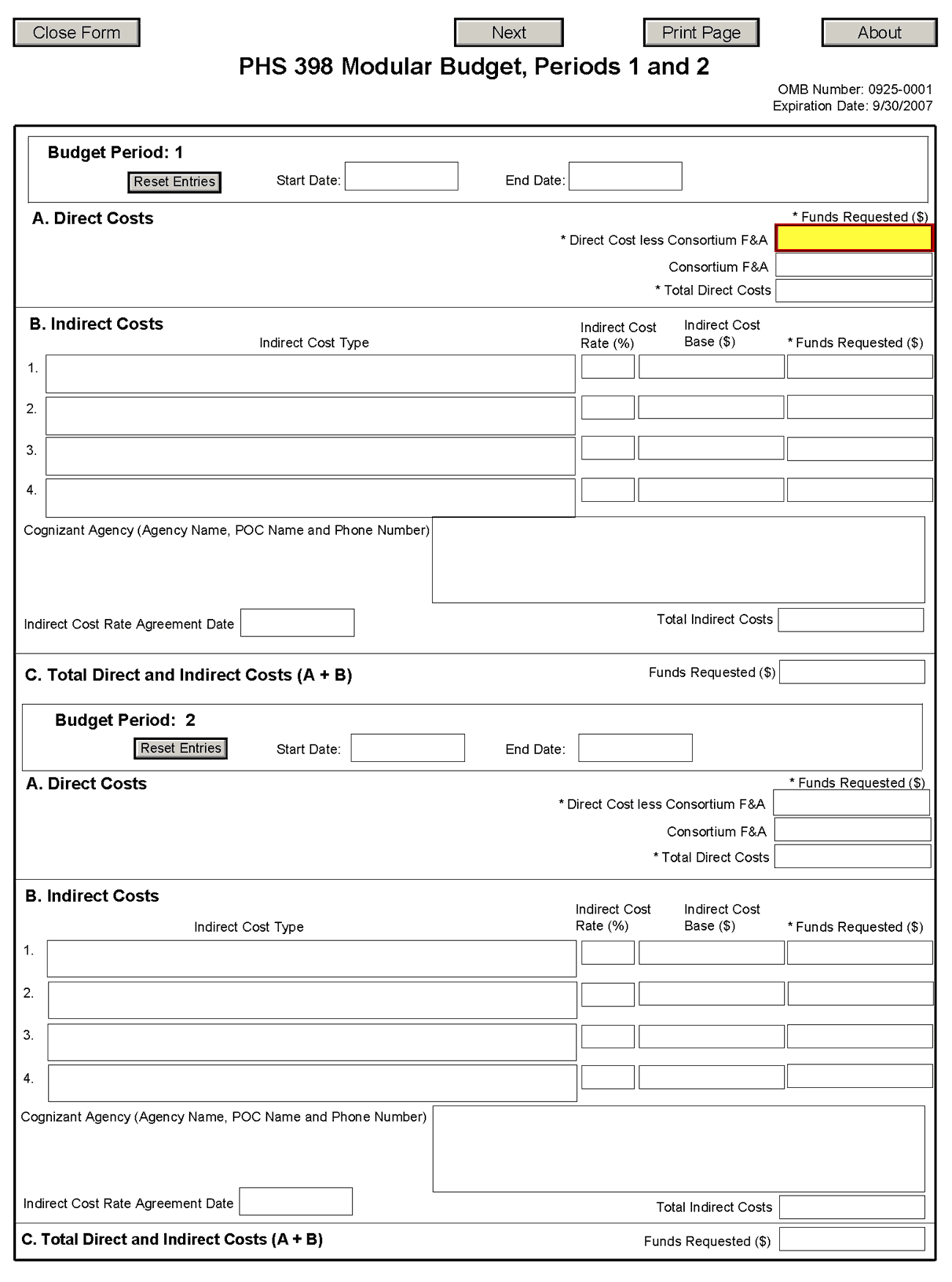
NOTE: The fields are the same for budget periods 1 through 5, the following instructions can be used for each.
Budget Period
Field Name |
Instructions |
Start Date |
Enter the requested/proposed start date of the budget period. Use the following format: MM/DD/YYY. |
End Date |
Enter the requested/proposed end date of the budget period. Use the following format: MM/DD/YYY. |
A. Direct Costs
Field Name |
Instructions |
Direct Cost less Consortium F&A |
Enter the amount of direct costs, less actual consortium F&A costs for this budget period. This figure must be in $25,000 increments, and it may not exceed $250,000. Actual consortium F&A costs are excluded from this figure. |
Consortium F&A |
If this project involves a consortium, enter the actual consortium F&A costs for this budget period. If this project does not involve a consortium, leave blank. |
Total Direct Costs |
The total direct costs. This field auto-calculates. |
B. Indirect Costs
Field Name |
Instructions |
Indirect Cost Type |
Indicate the type of base (for example, Salary & Wages, Modified Total Direct Costs, Other [explain]), and indicate if Off-site. If more than one rate/base is involved, use separate lines for each. If you do not have a current indirect rate(s) approved by a Federal agency, indicate, “None—will negotiate” and include information for a proposed rate. Use the budget justification if additional space is needed. |
Indirect Cost Rate (%) |
Indicate the most recent Indirect Cost rate(s) (also known as Facilities & Administrative Costs [F&A]) established with the cognizant Federal office, or in the case of for-profit organizations, the rate(s) established with the appropriate agency. If you have a cognizant/oversight agency and are selected for an award, you must submit your indirect rate proposal to that office for approval. If you do not have a cognizant/oversight agency, contact the awarding agency. Currently this field will not allow a figure greater than 100% to be entered. If the Indirect Cost Rate exceeds 100%, use 2 lines to show the entire calculation. |
Indirect Cost Base ($) |
Enter the amount of the base for each indirect cost type. |
Funds Requested ($) |
Enter funds requested for each indirect cost type. |
Cognizant Agency (Agency Name, POC Name and Phone Number) |
Enter the name of the cognizant Federal Agency, name, and phone number of the individual responsible for negotiating your rate. If no cognizant agency is known, enter “None.” |
Indirect Cost Rate Agreement Date |
If you have a negotiated rate agreement, enter the agreement date. |
Total Indirect Costs |
The total funds requested for indirect costs. This field auto-calculates. |
C. Total Direct and Indirect Costs (A+B) Funds Requested ($)
The total funds requested for direct and indirect costs. This field auto-calculates.
![]()
Once you have entered all required information for budget periods 1 and 2, press the Next button or scroll down to enter information for subsequent budget periods.
5.4.2 Period 5 and Cumulative
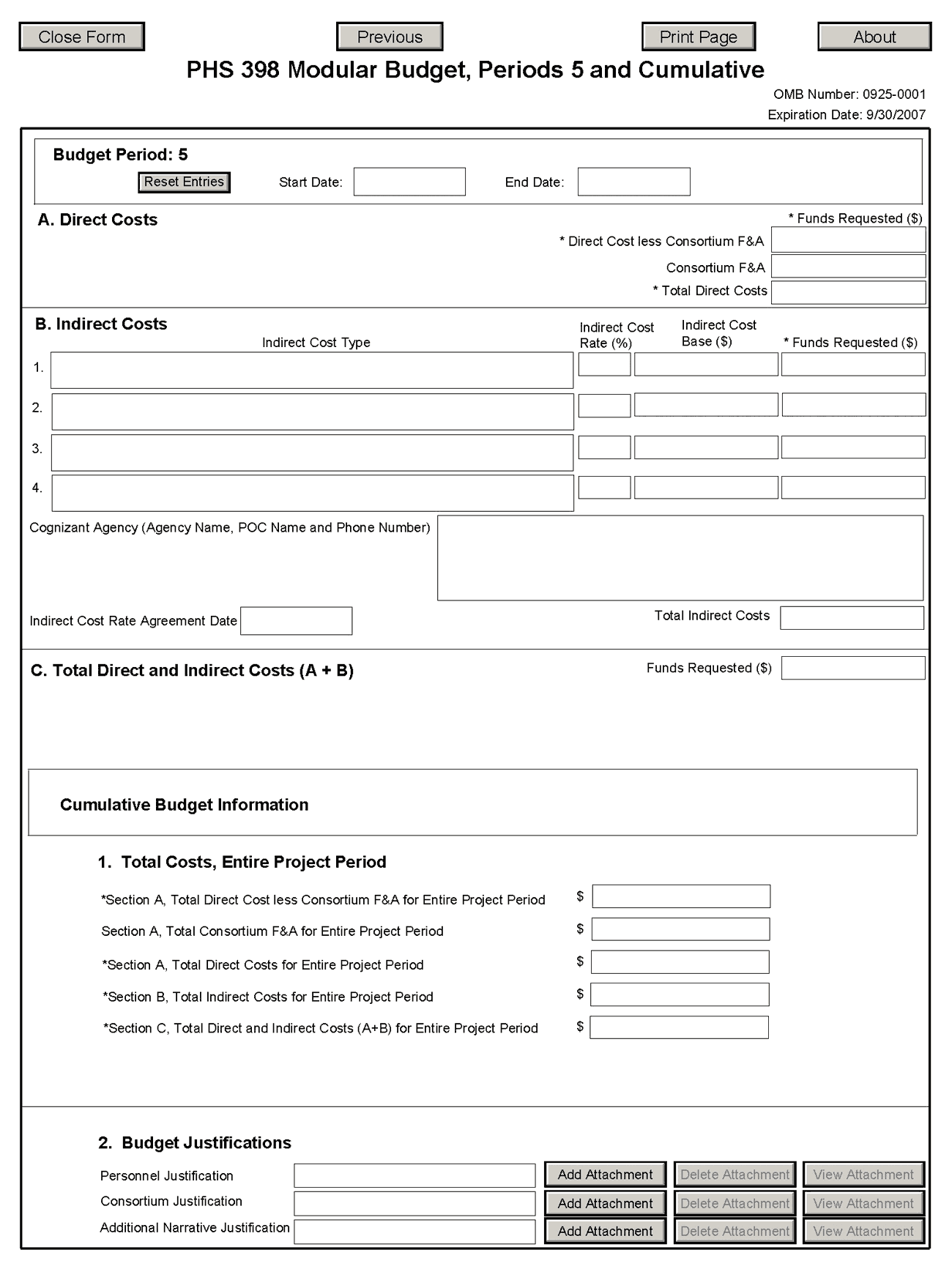
Cumulative Budget Information
All values for the Cumulative Budget Information are calculated automatically. They equal the summations of the amounts that you have entered previously for each of the individual budget periods. Therefore, no data entry is allowed or required, in order to complete this “Cumulative Budget” section.
If any of the amounts displayed on this form appears to be incorrect, you may correct it by adjusting one or more of the values that contribute to that total. To make any such adjustments, you will need to revisit the appropriate budget period form(s), to enter corrected values.
Modular Budget Justifications
Field Name |
Instructions |
Personnel Justification |
List all personnel, including names, number of person months devoted to the project (indicate academic, calendar, and/or summer) and roles on the project. Do not provide individual salary information. Since the modules should be a reasonable estimate of costs allowable, allocable, and appropriate for the proposed project, you must use the current legislatively imposed salary limitation when estimating the number of modules. For guidance on current salary limitations contact your office of sponsored programs. NIH grants also limit the compensation for graduate students. Compensation includes salary or wages, fringe benefits, and tuition remission. This limit should also be used when estimating the number of modules. See: http://grants.nih.gov/grants/guide/notice-files/NOT-OD-02-017.html. Save this information in a single file in a location you remember. Click Add Attachment, browse to where you saved the file, select the file, and then click Open. |
Consortium Justification |
Provide an estimate of total costs (direct plus facilities and administrative) for each year, rounded to the nearest $1,000. List the individuals/organizations with whom consortium or contractual arrangements have been made, along with all personnel, including percent of effort (in person months) and roles on the project. Do not provide individual salary information. Indicate whether the collaborating institution is foreign or domestic. While only the direct cost for a consortium/contractual arrangement is factored into eligibility for using the modular budget format, the total consortium/contractual costs must be included in the overall requested modular direct cost amount. Save this information in a single file in a location you remember. Click Add Attachment, browse to where you saved the file, select the file, and then click Open. |
Additional Narrative Justification |
If the requested budget requires any additional justification, such as variations in the number of modules requested, save this information in a single file in a location you remember. Click Add Attachment, browse to where you saved the file, select the file, and then click Open. |
Once all data have been entered, click the Close Form button at the top of the form or use the scroll bar to scroll up. You will be returned to the Grant Application Package screen. To remove a document from the Submission box, click the document name to select it and then click the Move Form to Delete button. This will return the document to the Mandatory Documents Submission List or Optional Documents Submission List.
5.5 Research Plan Component

The Research Plan should include sufficient information needed for evaluation of the project, independent of any other document (e.g., previous application). Be specific and informative, and avoid redundancies.
1. Application Type
This field is pre-populated from the SF424 (R&R) Cover Component. Corrections to this field must be made in that component.
2. Research Plan Attachments (See also Section 2.3.2 Creating PDFs for Text Attachments)
Although many of the sections of this application are separate PDF attachments, page limits referenced in the instructions and/or funding opportunity announcement must still be followed. Agency validations will include checks for page limits (and use of appropriate font). Some accommodation will be made for sections that, when combined, must fit within a specified limitation.
Text attachments should be generated using word processing software and then converted to PDF using PDF generating software. Avoid scanning text attachments to convert to PDF since that causes problems for the agency handling the application. In addition, be sure to save files with descriptive file names.
Do not include any information in a header or footer of the attachments. A header will be system-generated that references the name of the PD/PI. Page numbers for the footer will be system-generated in the complete application, with all pages sequentially numbered.
Since a number of reviewers will be reviewing applications as an electronic document and not a paper version, applicants are strongly encouraged to use only a standard, single-column format for the text. Avoid using a two-column format since it can cause difficulties when reviewing the document electronically.
Full-sized glossy photographs of material such as electron micrographs or gels must only be included within the page limits of the Research Strategy. The maximum size of images to be included should be approximately 1200 x 1500 pixels using 256 colors. Figures must be readable as printed on an 8.5 x 11 inch page at normal (100%) scale.
Investigators must use image compression such as JPEG or PMG. Do not include figures or photographs as separate attachments either in the Appendix or elsewhere in the application.
Separate Attachments
Separate attachments have been designed for the Research Plan sections to maximize automatic validations conducted by the eRA system. When the application is received by the agency, all of the Research Plan sections will be concatenated in the appropriate order so that reviewers and agency staff will see a single cohesive Research Plan.
While each section of the Research Plan needs to eventually be uploaded separately, applicants are encouraged to construct the Research Plan as a single document, separating sections into distinct PDF attachments just before uploading the files. In this way the applicant can better monitor formatting requirements such as page limits. When validating for page limits, the eRA Commons will not count the white space created by breaking the text into separate files for uploading.
When attaching a PDF document to the actual forms, please note you are attaching an actual document, not just pointing to the location of an externally stored document. Therefore, if you revise the document after it has been attached, you must delete the previous attachment and then reattach the revised document to the application form. Use the “View Attachment” button to determine if the correct version has been attached.
Page Limits
R01 applicants must follow the page limits described in the table in 2.6-1 unless the FOA specifies otherwise. All tables, graphs, figures, diagrams, and charts must be included within the Research Strategy page limit. If PAs or RFAs contain specific page limits, those instructions always supersede these instructions.
All applications and proposals for NIH funding must be self-contained within specified page limits. Agency validations will include checks for page limits. Some accommodation will be made for sections that when combined must fit within a specified limitation. Note that while these computer validations will help minimize incomplete and/or non-compliant applications, they do not replace the validations conducted by NIH staff. Applications found not to comply with the requirements may be delayed in the review process. Unless otherwise specified in an NIH solicitation, Internet website addresses (URLs) may not be used to provide information necessary to the review because reviewers are not obligated to view the Internet sites. Moreover, reviewers are cautioned that they should not directly access an internet site (except to review publications cited in the Biographical Sketch or Progress Report publication list) as it could compromise their anonymity.
Notice of Proprietary Information
Applicants are discouraged from submitting information considered proprietary unless it is deemed essential for proper evaluation of the application. However, when the application contains information that constitutes trade secrets, or information that is commercial or financial, or information that is confidential or privileged, make sure you have checked the “Yes” box of question #3 in the “Other Project Information” component. Identify the pages in the application that contain this information by marking those paragraphs or lines with an asterisk (*) in the left-hand margin. Include at the beginning of the Research Plan which pages contain asterisks and a note stating “The following sections marked with an asterisk contain proprietary/privileged information that (name of Applicant) requests not be released to persons outside the Government, except for purposes of review and evaluation.”
When information in the application constitutes trade secrets or information that is commercial or financial, or information that is confidential or privileged, it is furnished to the Government in confidence with the understanding that the information shall be used or disclosed only for evaluation of this application. If a grant is awarded as a result of or in connection with the submission of this application, the Government shall have the right to use or disclose the information to the extent authorized by law. This restriction does not limit the Government’s right to use the information if it is obtained without restriction from another source.
Begin each text section of the Research Plan with a section header (e.g., Introduction, Specific Aims, Research Strategy, etc).
Field Name |
Instructions |
1. Introduction to Application (for Resubmission or Revision only) |
See specific instructions in 2.7 Resubmission Applications and 2.8 Revision Applications on the content of the Introduction. First time (new) applications should not include an Introduction unless specified in the FOA. The Introduction is limited to one page unless specified otherwise in the FOA. Save this information in a single file in a location you remember. Click Add Attachment, browse to where you saved the file, select the file, and then click Open. |
2. Specific Aims |
State concisely the goals of the proposed research and summarize the expected outcome(s). List succinctly the specific objectives of the research proposed, e.g., to test a stated hypothesis, create a novel design, solve a specific problem, challenge an existing paradigm or clinical practice, address a critical barrier to progress in the field, or develop new technology. Specific Aims are limited to one page. Save this information in a single file in a location you remember. Click Add Attachment, browse to where you saved the file, select the file, and then click Open. |
3. Research Strategy |
Organize the Research Strategy in the specified order and using the instructions provided below. Start each section with the appropriate section heading—Significance, Innovation, Approach. Experimental details should be cited using the Bibliography and References Cited section (see Instruction section 4.4.8) and need not be detailed in the Research Strategy. Follow the page limits for the Research Strategy in the table of page limits at 2.6-1, unless specified otherwise in the FOA. (a) Significance
(b) Innovation
(c) Approach
Save this information in a single file in a location you remember. Click Add Attachment, browse to where you saved the file, select the file, and then click Open. |
4. Inclusion Enrollment Report |
If the renewal or revision application involves clinical research, then you must report on the enrollment of research subjects and their distribution by ethnicity/race and sex/gender. See Part II, Section 4.3 for more detailed instructions on which Target and Enrollment Report or Table to use. |
5. Progress Report Publication List (Renewal Applications Only) |
List the titles and complete references to all appropriate publications, manuscripts accepted for publication, patents, and other printed materials that have resulted from the project since it was last reviewed competitively. When citing articles that fall under the Public Access Policy, were authored or co-authored by the applicant and arose from NIH support, provide the NIH Manuscript Submission reference number (e.g., NIHMS97531) or the Pubmed Central (PMC) reference number (e.g., PMCID234567) for each article. If the PMCID is not yet available because the Journal submits articles directly to PMC on behalf of their authors, indicate “PMC Journal – In Process.” A list of these journals is posted at: http://publicaccess.nih.gov/submit_process_journals.htm. Citations that are not covered by the Public Access Policy, but are publicly available in a free, online format may include URLs or PMCID numbers along with the full reference (note that copies of these publications are not accepted as appendix material, see 5.5.15). |
Field Name |
Instructions |
6. Protection of Human Subjects |
Refer to Part II, Supplemental Instructions for Preparing the Human Subjects Section of the Research Plan. See separate sections below for other human subjects related sections that may apply. Do not use the protection of human subjects section to circumvent the page limits of the Research Strategy. Save this information in a single file in a location you remember. Click Add Attachment, browse to where you saved the file, select the file, and then click Open. Unless an explanation is necessary, if Human Subjects research is not involved, and you have checked the box marked “No” on the Other Project Information Component, you need not include any additional information in this section. |
7. Inclusion of Women and Minorities |
To determine if Inclusion of Women and Minorities applies to the application, see Part II, Supplemental Instructions for Preparing the Human Subjects Section of the Research Plan, Sections 4.2 and 5.6. Save this information in a single file in a location you remember. Click Add Attachment, browse to where you saved the file, select the file, and then click Open. |
8. Targeted/Planned Enrollment |
If this application involves the Inclusion of Women and Minorities, complete the Targeted/Planned Enrollment Table for each protocol; see Part II Supplemental Instructions for Preparing the Protection of Human Subjects Section of the Research Plan, Section 4.3. Save this information in a single file in a location you remember. Click Add Attachment, browse to where you saved the file, select the file, and then click Open. |
9. Inclusion of Children |
To determine if Inclusion of Children applies to the application, see Part II Supplemental Instructions for Preparing the Human Subjects Section of the Research Plan, Sections 4.4 and 5.7. Save this information in a single file in a location you remember. Click Add Attachment, browse to where you saved the file, select the file, and then click Open. |
Other Sections
Field Name |
Instructions |
10. Vertebrate Animals |
If Vertebrate Animals are involved in the project, address each of the five points below. If all or part of the proposed research involving vertebrate animals will take place at alternate sites (such as project/performance or collaborating site(s)) identify those sites and describe the activities at those locations. Although no specific page limitation applies to this section of the application, be succinct. Failure to address the following five points will result in the application being designated as incomplete and will be grounds for the PHS to defer the application from the peer review round. Alternatively, the application's impact/priority score may be negatively affected. The five points are as follows: 1. Provide a detailed description of the proposed use of the animals in the work outlined in the Research Strategy section. Identify the species, strains, ages, sex, and numbers of animals to be used in the proposed work. 2. Justify the use of animals, the choice of species, and the numbers to be used. If animals are in short supply, costly, or to be used in large numbers, provide an additional rationale for their selection and numbers. 3. Provide information on the veterinary care of the animals involved. 4. Describe the procedures for ensuring that discomfort, distress, pain, and injury will be limited to that which is unavoidable in the conduct of scientifically sound research. Describe the use of analgesic, anesthetic, and tranquilizing drugs and/or comfortable restraining devices, where appropriate, to minimize discomfort, distress, pain, and injury. 5. Describe any method of euthanasia to be used and the reasons for its selection. State whether this method is consistent with the recommendations of the American Veterinary Medical Association (AVMA) Guidelines on Euthanasia. If not, include a scientific justification for not following the recommendations. If the involvement of animals is indefinite, provide an explanation and indicate when it is anticipated that animals will be used. If an award is made, prior to the involvement of animals the grantee must submit to the NIH awarding office detailed information as required in 1-5 above and verification of IACUC approval. If the grantee does not have an Animal Welfare Assurance then an appropriate Assurance will be required (see Part III, 2.2). Do not use the vertebrate animal section to circumvent the page limits of the Research Strategy. Save this information in a single file in a location you remember. Click Add Attachment, browse to where you saved the file, select the file, and then click Open. |
11. Select Agent Research |
Select Agents are hazardous biological agents and toxins that have been identified by DHHS or USDA as having the potential to pose a severe threat to public health and safety, to animal and plant health, or to animal and plant products. CDC maintains a list of these agents. See http://www.cdc.gov/od/sap/docs/salist.pdf. If the activities proposed in the application involve only the use of a strain(s) of Select Agents which has been excluded from the list of select agents and toxins as per 42 CFR 73.3, the Select Agent requirements do not apply. Use this section to identify the strain(s) of the Select Agent that will be used and note that it has been excluded from this list. The CDC maintains a list of exclusions at http://www.cdc.gov/od/sap/sap/exclusion.htm. If the strain(s) is not currently excluded from the list of select agents and toxins but you have applied or intend to apply to DHHS for an exclusion from the list, use this section to indicate the status of your request or your intent to apply for an exclusion and provide a brief justification for the exclusion. If any of the activities proposed in your application involve the use of Select Agents at any time during the proposed project period, either at the applicant organization or at any other performance site, address the following three points for each site at which Select Agent research will take place. Although no specific page limitation applies to this section, be succinct. 1. Identify the Select Agent(s) to be used in the proposed research. 2. Provide the registration status of all entities* where Select Agent(s) will be used.
*An “entity” is defined in 42 CFR 73.1 as “any government agency (Federal, State, or local), academic institution, corporation, company, partnership, society, association, firm, sole proprietorship, or other legal entity.” 3. Provide a description of all facilities where the Select Agent(s) will be used.
If you are responding to a specific funding opportunity announcement (e.g., PA or RFA), address any requirements specified by the FOA. Reviewers will assess the information provided in this Section, and any questions associated with Select Agent research will need to be addressed prior to award. Save this file in a location you remember. Click Add Attachment, browse to where you saved the file, select the file, and then click Open. |
12. Multiple PD/PI Leadership Plan |
For applications designating multiple PDs/PIs, a leadership plan must be included. A rationale for choosing a multiple PD/PI approach should be described. The governance and organizational structure of the leadership team and the research project should be described, including communication plans, process for making decisions on scientific direction, and procedures for resolving conflicts. The roles and administrative, technical, and scientific responsibilities for the project or program should be delineated for the PDs/PIs and other collaborators. If budget allocation is planned, the distribution of resources to specific components of the project or the individual PDs/PIs should be delineated in the Leadership Plan. In the event of an award, the requested allocations may be reflected in a footnote on the Notice of Grant Award. Save this file in a location you remember. Click Add Attachment, browse to where you saved the file, select the file, and then click Open. |
13. Consortium/Contractual Arrangements |
Explain the programmatic, fiscal, and administrative arrangements to be made between the applicant organization and the consortium organization(s). If consortium/contractual activities represent a significant portion of the overall project, explain why the applicant organization, rather than the ultimate performer of the activities, should be the grantee. The signature of the authorized organizational official on the SF424 (R&R) cover component (Item 18) signifies that the applicant and all proposed consortium participants understand and agree to the following statement: The appropriate programmatic and administrative personnel of each organization involved in this grant application are aware of the agency’s consortium agreement policy and are prepared to establish the necessary inter-organizational agreement(s) consistent with that policy. Save this information in a single file in a location you remember. Click Add Attachment, browse to where you saved the file, select the file, and then click Open. |
14. Letters of Support (e.g., Consultants) |
Attach all appropriate letters of support, including any letters necessary to demonstrate the support of consortium participants and collaborators such as Senior/Key Personnel and Other Significant Contributors included in the grant application. Letters are not required for personnel (such as research assistants) not contributing in a substantive, measurable way to the scientific development or execution of the project. For consultants, letters should include rate/charge for consulting services. Save this information in a single file in a location you remember. Click Add Attachment, browse to where you saved the file, select the file, and then click Open. |
15. Resource Sharing Plan(s) |
NIH considers the sharing of unique research resources developed through NIH-sponsored research an important means to enhance the value and further the advancement of the research. When resources have been developed with NIH funds and the associated research findings published or provided to NIH, it is important that they be made readily available for research purposes to qualified individuals within the scientific community. See Part III, 1.5 Sharing Research Resources. 1. Data Sharing Plan: Investigators seeking $500,000 or more in direct costs (exclusive of consortium F&A) in any year are expected to include a brief 1-paragraph description of how final research data will be shared, or explain why data-sharing is not possible. Specific Funding Opportunity Announcements may require that all applications include this information regardless of the dollar level. Applicants are encouraged to read the specific opportunity carefully and discuss their data-sharing plan with their program contact at the time they negotiate an agreement with the Institute/Center (IC) staff to accept assignment of their application. See Data-Sharing Policy or http://grants.nih.gov/grants/guide/notice-files/NOT-OD-03-032.html. 2. Sharing Model Organisms: Regardless of the amount requested, all applications where the development of model organisms is anticipated are expected to include a description of a specific plan for sharing and distributing unique model organisms or state why such sharing is restricted or not possible. See Sharing Model Organisms Policy, and NIH Guide NOT-OD-04-042. 3. Genome Wide Association Studies (GWAS): Applicants seeking funding for a genome-wide association study are expected to provide a plan for submission of GWAS data to the NIH-designated GWAS data repository, or an appropriate explanation why submission to the repository is not possible. GWAS is defined as any study of genetic variation across the entire genome that is designed to identify genetic associations with observable traits (such as blood pressure or weight) or the presence or absence of a disease or condition. For further information see Policy for Sharing of Data Obtained in NIH Supported or Conducted Genome-Wide Association Studies, NIH Guide NOT-OD-07-088, and http://grants.nih.gov/grants/gwas/. 4. Human Specimen and/or Data Research Resource Repositories: Applicants seeking funding for human specimen and/or data research resource repositories are expected to provide a brief description of the procedures and policies that will govern the collection, storage, and use of human specimens and/or data for research. For the purposes of this policy, a research resource repository is defined as an entity that collects, stores or distributes human biological specimens and/or data expressly for the purpose of sharing of specimens and data for current and/or future research. For further information, see NIH Guide NOT-OD-XX-XXX, and http://XXXXXXXXX. Save this information in a single file in a location you remember. Click Add Attachment, browse to where you saved the file, select the file, and then click Open. |
Only one copy of appendix material is necessary. Use the add attachments button to the right of this field to complete this entry. A maximum of 10 PDF attachments is allowed in the Appendix. If more than 10 appendix attachments are needed, combine the remaining information into attachment #10. Note that this is the total number of appendix items, not the total number of publications. When allowed there is a limit of 3 publications that are not publicly available (see below for further details and check the FOA for any specific instructions), though not all grant activity codes allow publications to be included in the appendix. Do not use the appendix to circumvent the page limits of the Research Strategy. Appendix material may not appear in the assembled application in the order attached, so it is important to use filenames for attachments that are descriptive of the content. A summary sheet listing all of the items included in the appendix is also encouraged but not required. When including a summary sheet, it should be included in the first appendix attachment. Applications that do not follow the appendix requirements may be delayed in the review process. New, resubmission, renewal, and revision applications may include the following materials in the Appendix (note, however, that some FOAs do not permit publications):
(Do not include unpublished theses, or abstracts/manuscripts submitted (but not yet accepted) for publication.)
Items that must not be included in the appendix:
|
Once all data have been entered, click the Close Form button at the top of the form or use the scroll bar to scroll up. You will be returned to the Grant Application Package screen. To remove a document from the Submission box, click the document name to select it and then click the Move Form to Delete button. This will return the document to the Mandatory Documents Submission List or Optional Documents Submission List.
5.6 Checklist Component
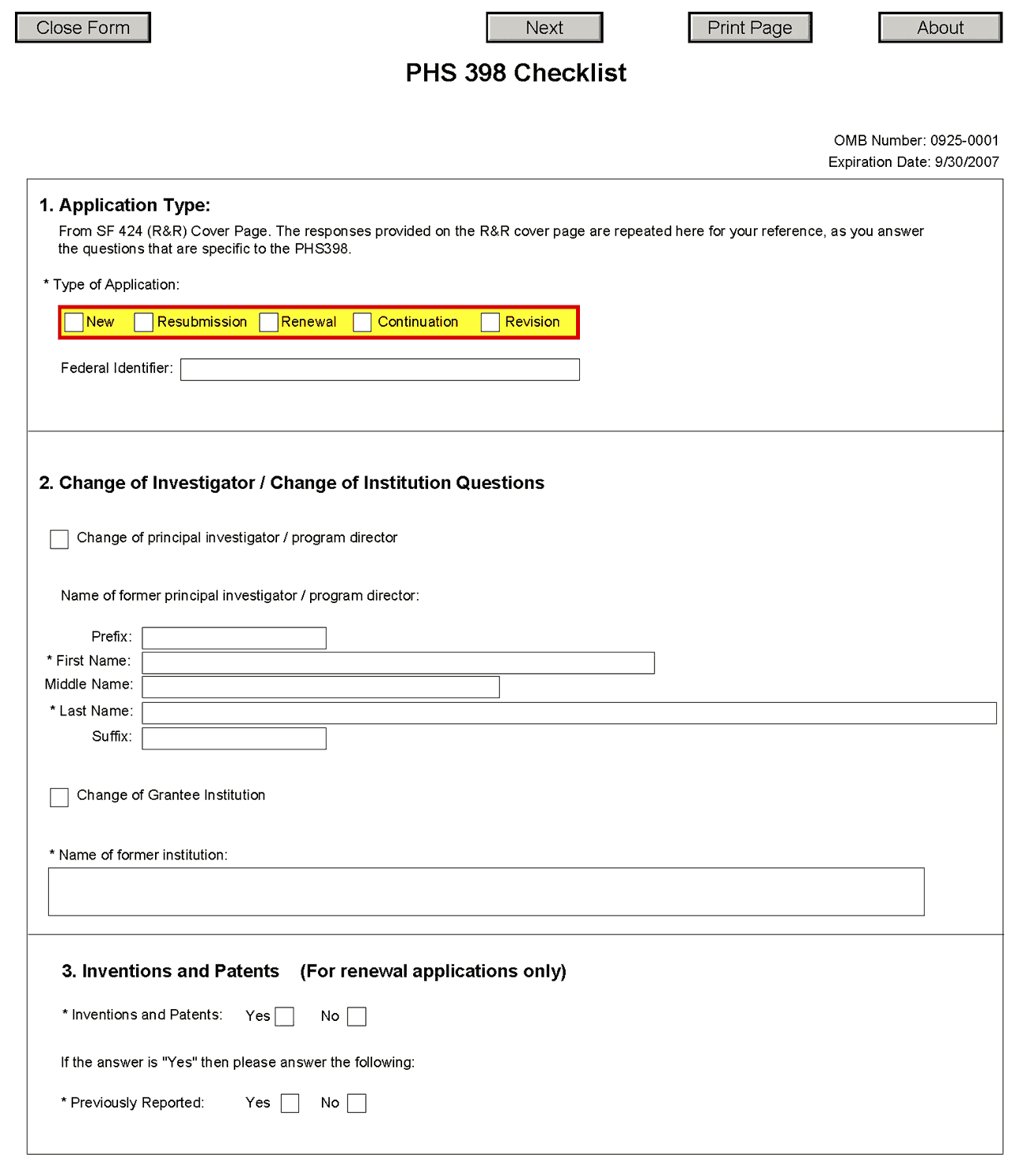
1. Application Type
Field Name |
Instructions |
Type of Application |
This field is pre-populated from the SF424 (R&R) Cover Component. Corrections to this field must be made in that component. |
Federal Identifier |
This field is pre-populated from the SF424 (R&R). Corrections to this field must be made in that component. For New applications this field will be blank. |
2. Change of Investigator/Change of Institution Questions
Field Name |
Instructions |
Change of Program Director/Principal Investigator |
Check this box if this application reflects a change in PD/PI from the one who was indicated on a previous application. This is not generally applicable to a “New” application. |
Prefix |
If this application reflects a change in PD/PI, enter the name prefix (for example, Mr., Mrs., Rev.) of the former PD/PI. |
First Name |
If this application reflects a change in PD/PI, enter the first name of the former PD/PI. |
Middle Name |
If this application reflects a change in PD/PI, enter the middle name of the former PD/PI. |
Last Name |
If this application reflects a change in PD/PI, enter the last name of the former PD/PI. |
Suffix |
If this application reflects a change in PD/PI, provide the suffix (for example, Jr., Sr., PhD) of the former PD/PI. |
Change of Grantee Institution |
Check this box if this application reflects a change in grantee institution from the one that was indicated on a previous application. This is not generally applicable to a “New” application. |
Name of Former Institution |
If this application reflects a change in grantee institution, enter the name of the former institution. |
3. Inventions and Patents (For renewal applications only)
Field Name |
Instructions |
Inventions and Patents |
This block need only be completed if submitting an R&R “Renewal” application. If no inventions were conceived or reduced to practice during the course of work under this project, check the No box. The remaining parts of the item are then not applicable. If any inventions were conceived or reduced to practice during the previous period of support, check the Yes box. |
Previously Reported |
If you checked the Yes box for Inventions and Patents, above, indicate whether this information has been reported previously to the PHS or to the applicant organization official responsible for patent matters. |
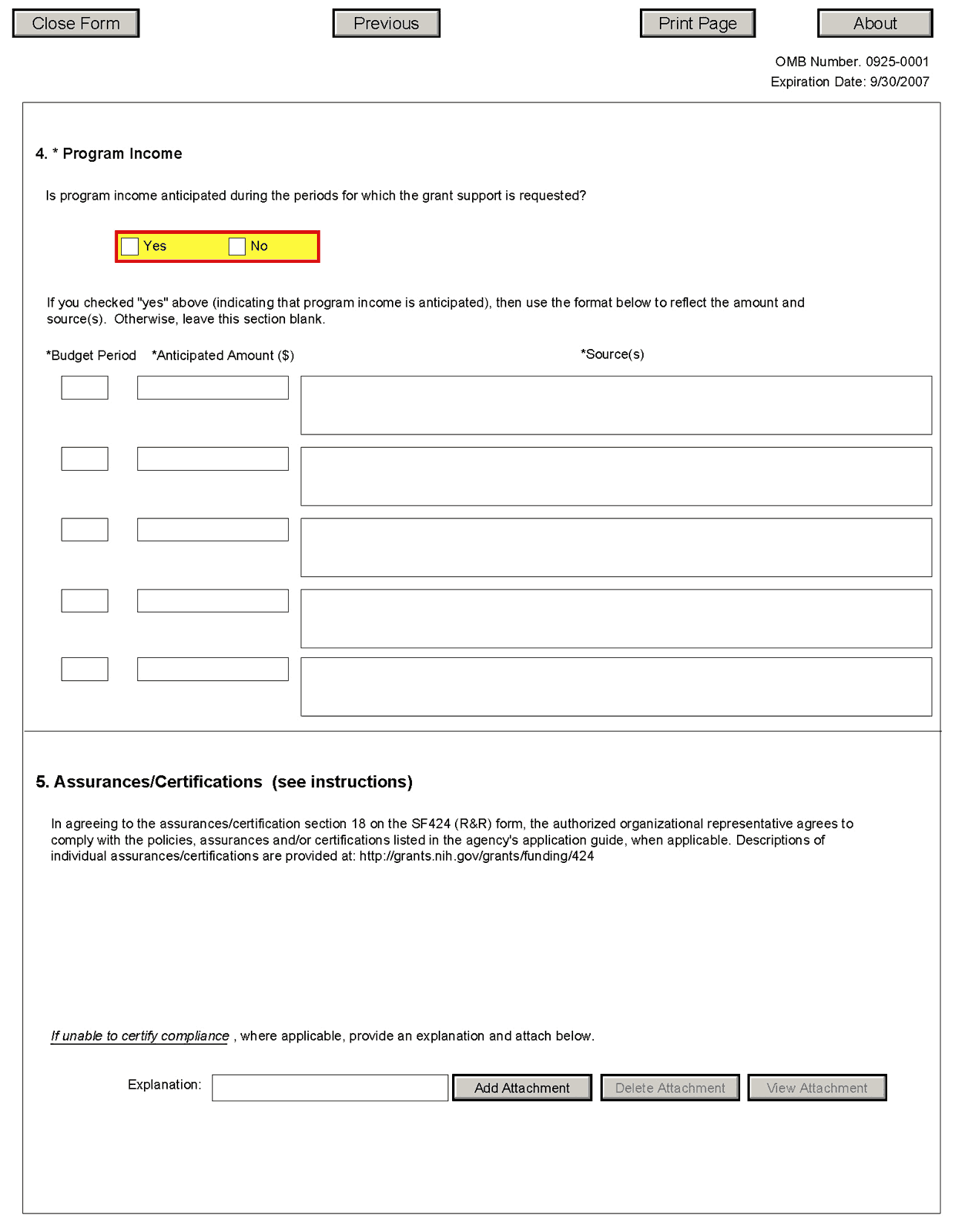
Field Name |
Instructions |
Is program income anticipated during the periods for which the grant support is requested? |
If program income is anticipated during the periods for which the grant support is requested, check the Yes box, and then complete the section below. If no program income is anticipated, check the No box and leave the following section blank. |
Budget Period |
If program income is anticipated, enter the budget periods. If the application is funded, the Notice of Award will provide specific instructions regarding the use of such income. |
Anticipated Amount ($) |
If program income is anticipated, enter the amount anticipated for each budget period listed. |
Source(s) |
If program income is anticipated, enter the source for each budget period listed. |
5. Assurances/Certifications
In agreeing to the assurances/certification section 18 of the SF424 (R&R) form, the authorized organizational representative agrees to comply with the following policies, assurances and certifications when applicable. Descriptions of individual assurances/certifications are provided in Part III: Policies, Assurances, Definitions, and Other Information.
Human Subjects Research; Research on Transplantation of Human Fetal Tissue; Research Using Human Embryonic Stem Cells; Women and Minority Inclusion Policy; Inclusion of Children Policy; Vertebrate Animals; Debarments and Suspension; Drug Free Workplace; Lobbying; Non-Delinquency of Federal Debt; Research Misconduct; Civil Rights; Handicapped Individuals; Sex Discrimination; Age Discrimination; Recombinant DNA, including Human Gene Transfer Research; Financial Conflict of Interest; Smoke-Free Workplace; Prohibited Research; Select Agent Research; Program Director/Principal Investigator(s) Assurance; Impact of Grant Activities on the Environment and Historic Properties; and Institutions Receiving Awards for Training of Graduate Students for Doctoral Degrees.
If you are unable to certify compliance with the applicable policies, assurances, and certifications listed, please provide an explanation in a separate file. Click Add Attachment, browse to where you saved the file, select the file, and then click Open.
![]()
Once all data have been entered, click the Close Form button at the top of the form or use the scroll bar to scroll up. You will be returned to the Grant Application Package screen. To remove a document from the Submission box, click the document name to select it and then click the Move Form to Delete button. This will return the document to the Mandatory Documents Submission List or Optional Documents Submission List.
6. Peer Review Process
Overview
NIH policy is intended to ensure that applications for funding submitted to the NIH are evaluated on the basis of a process that is fair, equitable, timely, and conducted in a manner free of bias. The NIH dual peer review system is mandated by statute in accordance with section 492 of the Public Health Service Act and federal regulations governing "Scientific Peer Review of Research Grant Applications and Research and Development Contract Proposals" (42 CFR Part 52h).
The first level of review is carried out by a Scientific Review Group (SRG) composed primarily of non-federal scientists who have expertise in relevant scientific disciplines and current research areas. The second level of review is performed by Institute and Center (IC) National Advisory Councils or Boards. Councils composed of both scientific and lay members are chosen for their expertise, interest, or activity in matters related to health and disease. Only applications that are favorably recommended by both the SRG and the Advisory Council may be recommended for funding. Only the NIH Institute or Center may make actual funding decisions.
A detailed description of what happens to a research project grant application after it is received for peer review can be found at the following location: http://grants.nih.gov/grants/peer_review_process.htm. Additional information about charters and membership of SRGs, Councils, and Boards can be obtained from the appropriate agency. Information on CDC review procedures is located at http://www.cdc.gov/od/science/PHResearch/peerreview.htm.
Streamlining
The initial scientific peer review of most applications will also include a process in which only those applications deemed by the reviewers to have the highest scientific and technical merit, generally the better half of the applications under review, will be discussed at the SRG meeting, assigned an impact score, and receive a second level review. Applications in the lower half are reviewed by SRG members but they are not discussed or scored at the SRG meeting. This process allows the reviewers to focus their discussion on the most meritorious applications.
Before the review meeting, each reviewer and discussant assigned to an application will give a separate score for each of the five core review criteria and a preliminary impact score for that application (see below). The preliminary impact scores will be used to determine which applications will be discussed.
Scoring
SRG members are instructed to evaluate research applications by addressing the five core review criteria (see below) and additional review criteria as applicable for the application. However, Requests for Applications (RFAs) and other types of funding opportunities (e.g., construction grants and fellowship applications) may list different and/or additional review criteria and considerations.
For each application that is discussed, a final overall impact score will be given by each eligible committee member (without conflicts of interest) following the panel discussion. Each member’s impact score will reflect his/her evaluation of the overall impact of the project in its entirety, rather than an arithmetic formula applied to the reviewer’s scores given to each criterion. The final impact score for each discussed application will be determined by calculating the arithmetic average of all the eligible members’ impact scores, and multiplying the average by 10.
As part of the initial merit review, and regardless of whether an application is discussed or not discussed (streamlined), all applicants will receive a written critique, called a Summary Statement, unless stated otherwise in the FOA. The Summary Statement represents a combination of the reviewers' written comments and scores for individual criteria. The Summary Statement for discussed applications includes the Scientific Review Officer's summary of the members' discussion during the SRG meeting; the final impact score; the recommendations of the SRG, including budget recommendations; and administrative notes of special considerations. For applications that are not discussed by the full committee, the scores of the assigned reviewers and discussants for the five core criteria will be reported individually on the Summary Statement. Final impact scores are not given for applications that are not discussed.
Research Project Evaluation Criteria
Overall Impact. Reviewers will provide an overall impact score to reflect their assessment of the likelihood for the project to exert a sustained, powerful influence on the research field(s) involved, in consideration of the following five core review criteria, and additional review criteria (as applicable for the project proposed).
Core Review Criteria. Reviewers will consider each of the five review criteria below in the determination of scientific and technical merit, and give a separate score for each. An application does not need to be strong in all categories to be judged likely to have major scientific impact. For example, a project that by its nature is not innovative may be essential to advance a field.
Significance: Does the project address an important problem or a critical barrier to progress in the field? If the aims of the project are achieved, how will scientific knowledge, technical capability, and/or clinical practice be improved? How will successful completion of the aims change the concepts, methods, technologies, treatments, services, or preventative interventions that drive this field?
Investigator(s): Are the PD/PIs, collaborators, and other researchers well suited to the project? If Early Stage Investigators or New Investigators, do they have appropriate experience and training? If established, have they demonstrated an ongoing record of accomplishments that have advanced their field(s)? If the project is collaborative or multi-PD/PI, do the investigators have complementary and integrated expertise; are their leadership approach, governance and organizational structure appropriate for the project?
Innovation: Does the application challenge and seek to shift current research or clinical practice paradigms by utilizing novel theoretical concepts, approaches or methodologies, instrumentation, or interventions? Are the concepts, approaches or methodologies, instrumentation, or interventions novel to one field of research or novel in a broad sense? Is a refinement, improvement, or new application of theoretical concepts, approaches or methodologies, instrumentation, or interventions proposed?
Approach: Are the overall strategy, methodology, and analyses well-reasoned and appropriate to accomplish the specific aims of the project? Are potential problems, alternative strategies, and benchmarks for success presented? If the project is in the early stages of development, will the strategy establish feasibility and will particularly risky aspects be managed?
If the project involves clinical research, are the plans for 1) protection of human subjects from research risks, and 2) inclusion of minorities and members of both sexes/genders, as well as the inclusion of children, justified in terms of the scientific goals and research strategy proposed?
Environment: Will the scientific environment in which the work will be done contribute to the probability of success? Are the institutional support, equipment and other physical resources available to the investigators adequate for the project proposed? Will the project benefit from unique features of the scientific environment, subject populations, or collaborative arrangements?
Additional Review Criteria. As applicable for the project proposed, reviewers will consider the following additional items in the determination of scientific and technical merit, but will not give separate scores for these items.
Protections for Human Subjects. For research that involves human subjects but does not involve one of the six categories of research that are exempt under 45 CFR Part 46, the committee will evaluate the justification for involvement of human subjects and the proposed protections from research risk relating to their participation according to the following five review criteria: 1) risk to subjects, 2) adequacy of protection against risks, 3) potential benefits to the subjects and others, 4) importance of the knowledge to be gained, and 5) data and safety monitoring for clinical trials.
For research that involves human subjects and meets the criteria for one or more of the six categories of research that are exempt under 45 CFR Part 46, the committee will evaluate: 1) the justification for the exemption, 2) human subjects involvement and characteristics, and 3) sources of materials.
Inclusion of Women, Minorities, and Children. When the proposed project involves clinical research, the committee will evaluate the proposed plans for inclusion of minorities and members of both genders, as well as the inclusion of children.
Vertebrate Animals. The committee will evaluate the involvement of live vertebrate animals as part of the scientific assessment according to the following five points: 1) proposed use of the animals, and species, strains, ages, sex, and numbers to be used; 2) justifications for the use of animals and for the appropriateness of the species and numbers proposed; 3) adequacy of veterinary care; 4) procedures for limiting discomfort, distress, pain and injury to that which is unavoidable in the conduct of scientifically sound research including the use of analgesic, anesthetic, and tranquilizing drugs and/or comfortable restraining devices; and 5) methods of euthanasia and reason for selection if not consistent with the AVMA Guidelines on Euthanasia.
Resubmission Applications. When reviewing a Resubmission application (formerly called an amended application), the committee will evaluate the application as now presented, taking into consideration the responses to comments from the previous scientific review group and changes made to the project.
Renewal Applications. When reviewing a Renewal application (formerly called a competing continuation application), the committee will consider the progress made in the last funding period.
Revision Applications. When reviewing a Revision application (formerly called a competing supplement application), the committee will consider the appropriateness of the proposed expansion of the scope of the project. If the Revision application relates to a specific line of investigation presented in the original application that was not recommended for approval by the committee, then the committee will consider whether the responses to comments from the previous scientific review group are adequate and whether substantial changes are clearly evident.
Biohazards. Reviewers will assess whether materials or procedures proposed are potentially hazardous to research personnel and/or the environment, and if needed, determine whether adequate protection is proposed.
Additional Review Considerations. As applicable for the project proposed, reviewers will address each of the following items, but will not give scores for these items and should not consider them in providing an overall impact score.
Budget and Period Support. Reviewers will consider whether the budget and the requested period of support are fully justified and reasonable in relation to the proposed research.
Select Agent Research. Reviewers will assess the information provided in this section of the application, including 1) the Select Agent(s) to be used in the proposed research, 2) the registration status of all entities where Select Agent(s) will be used, 3) the procedures that will be used to monitor possession use and transfer of Select Agent(s), and 4) plans for appropriate biosafety, biocontainment, and security of the Select Agent(s).
Applications from Foreign Organizations. Reviewers will assess whether the project presents special opportunities for furthering research programs through the use of unusual talent, resources, populations, or environmental conditions that exist in other countries and either are not readily available in the United States or augment existing U.S. resources.
Resource Sharing Plans. Reviewers will comment on whether the following Resource Sharing Plans, or the rationale for not sharing the following types of resources, are reasonable: 1) Data Sharing Plan (http://grants.nih/gov/grants/policy/data_sharing/data_sharing_guidance.htm); 2) Sharing Model Organisms (http://grants.nih.gov/grants/guide/notice-files/NOT-OD-04-042.html); and 3) Genome Wide Association Studies (GWAS) (http://grants.nih.gov/grants/guide/notice-files/NOT-OD-07-088.html).
Dual-Level Peer Review
The second level of review will usually be performed by the Advisory Council or Board of the potential awarding component (Institute, Center, or other unit). Council or Board recommendations are based not only on considerations of scientific merit, as judged by the SRGs, but also on the relevance of the proposed study to an Institute/Center’s mission, programs and priorities.
7. Supplemental Instructions to the SF 424(R&R) for Preparing an Individual Research Career Development Award (CDA) Application (“K” Series)
7.1 Introduction
All applicants must use the SF 424 R&R Application for Federal Assistance, following the instructional information in this Application Guide. The supplemental instructions found in this section (I.7) are for Individual Career Development Award (CDA) series applications and include guidance and instructional information only when there is a difference in the required information to be submitted or there is a need for more specificity for the individual “K” program. Therefore, these supplemental instructions must be used along with the information found in Parts I.1 – I.6 of this document.
These instructions do not cover applications for K12 and other institutional career development programs. Institutions planning such applications should consult the applicable Funding Opportunity Announcement (FOA) concerning eligibility, award criteria, and application procedures. Some K-series funded through Requests for Applications (RFAs) may have special instructions.
It is imperative that applicants become familiar with the “K” activity code for which support is being requested. Before applying for a “K” award, applicants should carefully review the applicable FOA for the career award of interest, noting especially the eligibility requirements, award provisions, requirements for a mentor, any special application instructions, and review criteria. Each FOA contains more specific information associated with the award mechanism and includes names of individuals that may be contacted prior to submission of an application for additional or clarifying information.
The eligibility criteria, support levels, and other important aspects of specific career awards, including availability, may vary among NIH Institutes or Centers and other PHS agencies. For this reason, it is strongly recommended that applicants consult with the NIH Scientific/Research contact of the appropriate awarding component prior to submitting an application. FOAs and other guidelines are available on the NIH K Kiosk website http://grants.nih.gov/training/careerdevelopmentawards.htm. Announcements for various career award opportunities are issued periodically in the NIH Guide for Grants and Contracts, a weekly electronic publication (http://grants.nih.gov/grants/guide/index.html).
Note: A few individual K-series programs supported by the NIH include a delayed-award activation and/or two award phases (e.g., K22, K99/R00). NIH intramural researchers may be eligible to apply for these awards. The FOA will include any additional and/or specific instructions that must be followed when applying for such support.
7.2 Individual Career Development Award Programs
The following chart provides a summary of the existing Career Development programs. Since this information is subject to change, prospective applicants are encouraged to review the K Kiosk for the most current program information. The K Kiosk includes information on NIH-wide Parent FOAs as well as IC-specific FOAs for a particular K program.
Summary of Research Career Development Award Programs
program |
description |
mentor |
reference letters |
K01 |
Mentored Research Scientist Development Award (see K Kiosk) |
Yes |
Yes |
K02 |
Independent Scientist Award (see K Kiosk) |
No |
No |
K05 |
Senior Scientist Award (see K Kiosk) |
No |
No |
K07 |
Academic Career Award (see K Kiosk) |
* |
* |
K08 |
Mentored Clinical Scientist Development Award (see K Kiosk) |
Yes |
Yes |
K18 |
Career Enhancement Award (see K Kiosk) |
Yes |
Yes |
K22 |
Career Transition Award (see K Kiosk) |
* |
Yes |
K23 |
K23 Mentored Patient-Oriented Research Career Development Award (see K Kiosk) |
Yes |
Yes |
K24 |
Mid-Career Investigator Award in Patient Oriented Research (see K Kiosk) |
No |
No |
K25 |
Mentored Quantitative Research Career Development Award (see K Kiosk) |
Yes |
Yes |
K26 |
Midcareer Investigator Award in Mouse Pathobiology Research (see K Kiosk) |
No |
No |
K99/R00 |
NIH Pathways to Independence (PI) Award (see K Kiosk) |
Yes |
Yes |
*Varies with career status and source of award. Check the Funding Opportunity Announcement (FOA).
7.3 Letters of Reference (must be submitted electronically through the eRA Commons)
At least three (but no more than five) Letters of Reference are required for all applications defined as new and resubmissions for mentored support as indicated in the table above. The letters should be from individuals not directly involved in the application, but who are familiar with the applicant’s qualifications, training, and interests. The mentor/co-mentor(s) of the application cannot be counted toward the three required references. It is important for the applicant to include the names of those individuals in the application so that the NIH staff will be aware of planned reference letter submissions. Within the application, the list of referees (including name, departmental affiliation, and institution) is included in the Other Project Information Component, Item 11. Other Attachments (see special K instructions below for section 4.4.11). In addition, applicants must include the same list and information in the PHS 398 Cover Letter.
The reference letters are critically important and should address the candidate's competence and potential to develop into an independent biomedical or behavioral investigator. Only those individuals who can make the most meaningful comments about the candidate's professional training and qualifications for a research career should be used as referees. Where possible, some referees who are not from the candidate's current department or organization, but are knowledgeable about their qualifications, should be selected.
The candidate should request reference letters only from individuals who will be able to submit them to the NIH within 5 days after the application submission due date.
Applications that are missing the required letters of reference may be delayed in review or may not be accepted.
Electronic submission of a letter of reference is a separate process from submitting an application electronically. Reference letters are submitted directly through the eRA Commons and do not use Grants.gov. Therefore, this process requires that the referee be provided information including (a) the PI’s (candidate’s) eRA Commons user name, (b) the PI’s first and last name as they appear on the PI’s eRA Commons account, and (c) the number assigned to this Funding Opportunity Announcement.
Confirmation emails will be sent to both the referee and the candidate following reference letter submission. The confirmation sent to the candidate will include the referee’s name and the date the letter was submitted. The confirmation sent to the referee will include the referee and applicant’s names, a confirmation number, and the date the letter was submitted.
The candidate may check the status of submitted letters by logging into their Commons account and accessing the “check status” screen for this application. The candidate is responsible for reviewing the status of submitted reference letters and contacting referees to ensure that letters are submitted by the receipt deadline. While the candidate is able to check on the status of the submitted letters, the letters are confidential and he/she will not have access to the letters themselves. Note: Because email can be unreliable, it is the candidate’s responsibility to check the status of his/her letters of reference in the Commons.
Candidates should provide the following instructions to their referees.
Instructions for Referees: (these instructions are also found at: http://grants.nih.gov/grants/funding/424/Referee_Instructions_Mentored_Career_Awards.doc)
Name of Candidate (First & Last Name as shown in the eRA Commons): _______________
Candidate’s eRA Commons UserName: _______________
FOA Number: __________________________
The candidate is applying to the NIH for a Career Development Award. The purpose of this award is to develop the research capabilities and career of the candidate. These awards provide salary support and guarantee them the ability to devote at least 9 person-months (75% of their total professional effort) to research for the duration of the award. Many of these awards also provide funds for research and career development costs. The award is available to persons who have demonstrated considerable potential to become independent researchers, but who need additional supervised research experience in a productive scientific setting, as well as to newly independent researchers.
In two pages or less (PDF format), describe the qualities and potential of the candidate for the career development award program for which support is being requested. This should include your evaluation with special reference to:
potential for conducting research;
evidence of originality;
adequacy of scientific background;
quality of research endeavors or publications to date, if any;
commitment to health-oriented research; and
need for further research experience and training
any additional related comments that the referee may wish to provide
Please put the name of the candidate at the top of the letter. Also, be sure to include your name and title in the letter.
Submitting Reference Letters
Letters may be submitted directly to the NIH eRA commons at: https://commons.era.nih.gov/commons/reference/submitRefereeInformation.jsp and must be submitted within 5 days after the application receipt due date.
You will be requested to enter the following information on-line at the time of submission:
Referee Information:
Referee First Name (Required)
Referee Last Name Required)
Referee MI Name (Not Required)
Referee Email (Required)
Referee institution/affiliation (Required)
Referee department (Required)
Candidate Information:
PI Commons User ID (Required)
PI’s last name, as it appears on the PI’s Commons account (Required) (will be validated to ensure they match)
Funding Opportunity Announcement (FOA) Number (Required)
Reference letter confirmation number (Required only if resubmitting a letter; not required otherwise)
Reference letter – two pages maximum; PDF format
After you have submitted your letter, both you and the candidate will receive a confirmation of receipt by email. The confirmation sent to the candidate will include your name and the date your letter was submitted. However, the letters are confidential and the candidate will not be able to access the letters themselves. Your email confirmation will include a Reference Letter Submission Confirmation Number. The Confirmation Number will be required when resubmitting letters. Please print the confirmation email for your records.
Revised reference letters may be submitted within 7 days of the application receipt date.
7.4 “K”- Specific Instructions for “K” Applications using the SF424 (R&R) Application
Standard Instructions found in Parts I.1 – I.6 should be followed with the exceptions found in this section. Section numbers referenced below (e.g. 4.2 – 5.6) reflect those found in Part I.
7.4.1 Special Instructions for 4.2 Cover Component
Item 8. Type of Application: Unless stated in the applicable FOA, individual “K” awards are usually not renewable nor are they supplemented/revised (contact awarding component staff if clarification is needed). Therefore, the applicant should generally check “new” or “resubmission.” “Renewal” applications are accepted only for a few K programs; thus this value should only be checked if a specific FOA states Renewals are accepted.
Item 13. Proposed Project (Start and Ending Dates): The requested period of support must be within specified limits for the type of “K” award requested.
Item 15. Project Director/Principal Investigator (PD/PI) Contact Information: Provide the name of the individual candidate (considered the PD/PI for “K” award programs). If the candidate is not located at the applicant organization at the time the application is submitted, the information in Item 15 should reflect where the candidate can be reached prior to the requested award start date in item 13. If the PD/PI is not located at the applicant organization at the time of submission, the Commons account for the PD/PI must be affiliated with the applicant organization. For additional information on creating affiliations for users in the eRA Commons, see: https://commons.era.nih.gov/commons-help/175.htm.
Note: For some career transition award programs (e.g., K22) the applicant may apply WITHOUT an institutional affiliation. These individuals should refer to the specific funding opportunity announcement (FOA) for application instructions.
7.4.2 Special Instructions for 4.3 Research & Related Project/Performance Site Locations Component
Indicate where the work described in the Research and Career Development Plans will be conducted.
7.4.3 Special Instructions for 4.4 Other Project Information Component
Item 6. Project Summary/Abstract (Do not exceed 1 page): Provide an abstract of the entire application (candidate, environment, and research). Include the candidate's immediate and long-term career goals, key elements of the research career development plan, and a description of the research project, as indicated in Part I.4.4.6.
Item 9. Facilities & Other Resources: Provide in the Attachment a detailed description of the institutional facilities and resources available to the candidate, following the instructions in Part I.4.4.9. The information provided is of major importance in establishing the feasibility of the goals of the career development plan.
Item 11. Other Attachments: All mentored K applications must include a list of Referees here. The list should include the name of the referee, departmental affiliation and institution. This same list is also provided in the Cover Letter.
7.4.4 Special Instructions for 4.5 Senior/Key Person Profile(s) Component
7.4.4.1 The Candidate
For all “K” applications the “K” candidate is considered the Project Director/Principal Investigator (PD/PI). Therefore the candidate must be registered in the eRA Commons and be assigned the PI role within the Commons. Follow the instructions in Part I.2 which provides information regarding required registration in the eRA Commons.
Note that agency policies concerning “Multiple PIs” are not applicable to “K” applications. Therefore, do not use the PD/PI role for any other senior/key personnel.
Candidate’s Biographical Sketch
A biographical sketch attachment (limited to 4 pages) is required for the “K” candidate.
A biosketch for the “K” applicant should follow the instructions below:
Position Title: If the candidate is not currently located at the applicant organization, include both “current” and “projected” position titles, labeling each accordingly.
Education: Complete the educational block at the top of the format page beginning with baccalaureate or other initial professional education, such as nursing, and include postdoctoral training; separately referencing residency training when applicable. For each entry provide the name and location of the institution, the degree received (if applicable) the month and year the degree was received; and the field of study. For residency entries, the Field of Study section should reflect the area of residency. For non-degree education, indicate the time period covered. List professional certifications received within the last 10 years.
Personal Statement: Briefly describe why your experience and qualifications make you particularly well-suited to receive the "K" for which you are applying.
Research and/or Professional Experience:
Use the headings given below instead of the instructions on the Biographical Sketch Format Page. Identify each heading.
Employment
Start with the first position held following the baccalaureate and give a consecutive record to date. Indicate the department and organization, department head or supervisor, rank, tenured or non-tenured, status (full- or part-time), and inclusive dates (month and year). When applicable, include information on military service, and, if not referenced under Education above, internships, research assistantships, fellowships, etc. If the candidate is not currently located at the applicant organization, include the projected employment position in this section as well.
Honors
List academic and professional honors chronologically, including research grants and competitive fellowships awarded to the candidate.
Professional Societies and Public Advisory Committees
Identify professional societies and related organizations in which membership has been held within the last 10 years, giving dates. Include present membership on any Federal Government public advisory committee.
Publications
List up to 15 publications divided into the following three groups: 5 most recent publications, 5 best publications, and 5 publications most relevant to the application. To avoid listing publications more than once, each publication may be identified by one or more categories (use (R) for most recent, (B) for best, and (M) for most relevant to the application), e.g., "Author. Author. And Author. Title. Citation. (B, M)." Candidates without 15 publications may substitute the following in lieu of publications:
Original research and theoretical treatises;
Non-experimental articles, e.g., review of literature in field, book chapters, etc.;
Books, pamphlets, etc.
The list should include the title and complete references. If a copy of a publication is being submitted with the application, indicate with an asterisk and footnote "copies sent." For Renewal applications (when applicable), also identify with a double asterisk and appropriately footnote all papers published during the previous period of support.
Do not include manuscripts submitted or in preparation.
When citing articles that fall under the Public Access Policy, were authored or co-authored by the applicant and arose from NIH support, provide the NIH Manuscript Submission reference number (e.g., NIHMS97531) or the PubMed Central (PMC) reference number (e.g., PMCID234567) for each article. If the PMCID is not yet available because the Journal submits articles directly to PMC on behalf of their authors, indicate "PMC Journal - In Process." A list of these Journals is posted at: http://publicaccess.nih.gov/submit_process_journals.htm. Citations that are not covered by the Public Access Policy, but are publicly available in a free, online format may include URLs or PMCID numbers along with the full reference (note that copies of publicly available publications are not accepted as appendix material.)
Research Support
List both selected ongoing and completed (during the last three years) research projects (Federal or non-Federal support). Begin with the projects that are most relevant to the research proposed in this application. Briefly indicate the overall goals of the projects and responsibilities of the key person identified on the Biographical Sketch. Do not include number of person months or direct costs.
Don’t confuse “Research Support” with “Other Support.” Though they sound similar, these parts of the application are very different. As part of the biosketch section of the application, “Research Support” highlights your accomplishments, and those of your colleagues, as scientists. This information will be used by the reviewers in the assessment of each individual’s qualifications for a specific role in the proposed project, as well as to evaluate the overall qualifications of the research team. In contrast, “Other Support” information is required for all applications that are selected to receive grant awards. NIH staff will request complete and up-to-date “other support” information from you after peer review. This information will be used to check that the proposed research has not already been Federally-funded.
7.4.4.2 Mentor, Co-mentor, and Other Senior/Key Persons
The mentored “K” awards require a primary mentor, and there may be co-mentor(s), consultants and contributors. All individuals who have committed to contribute to the scientific development and execution of the project, including mentors and co-mentors, should be identified as Senior/Key personnel, even if they are not committing any specified measurable effort to the proposed project. Mentors and co-mentors should be assigned the Project Role of “Other Professional” and then enter “Mentor” or “Co-mentor” in the Other Project Role Category field.
Consultants should also be assigned the Other Professional” role even if they are not committing any specified measurable effort. Then, enter the specific project role under “Other Project Role Category.”
Any individuals identified as Senior/Key personnel who are committing specified measurable effort should be appropriately assigned under Project Role (and Other Project Role Category, if necessary). Additional information can be found in Section 4.5.1.
Current and Pending Support for Mentors/Co-mentors: For Mentored Career Development Awards, as part of the application submission modified Current and Pending Support pages must be submitted for the mentor and co-mentor(s), but not for the candidate, on the R&R Senior/Key Person Profile (Expanded) page. Provide information on the following selected items for the mentor’s and co-mentor’s current and pending research support relevant to the candidate’s research plan. Each attachment is limited to 4 pages. Note, Current and Pending Support for the Candidate will be requested on a Just-In-Time basis.
Special Instructions for Selected Items of Current & Pending Support for Mentor/Co-Mentors
Project Number: If applicable, include a code or identifier for the project.
Source: Identify the agency, institute, foundation, or other organization that is providing the support.
Major Goals: Provide a brief statement of the overall objectives of the project, subproject, or subcontract.
Dates of Approved/Proposed Project: Indicate the inclusive dates of the project as approved/proposed. For example, in the case of NIH support, provide the dates of the approved/proposed competitive segment.
Annual Direct Costs: In the case of an active project, provide the current year’s direct cost budget. For a pending project, provide the proposed direct cost budget for the initial budget period.
Do not include information on overlap and level of effort.
For non-mentored CDAs: Candidates for non-mentored CDAs should not submit Other Support Pages at the time of application unless specified to do so in the applicable FOA.
Updated information on all active support for the candidate, mentor(s), co-mentor(s), and Senior/Key Personnel may be requested by the awarding component prior to award.
Biographical Sketch for Mentor/Co-mentor and Other Senior/Key Person: For the biographical sketch for all individuals other than the candidate, follow the biographical sketch instructions found in Part I.4.5.
Note: K22 and K99/R00 candidates should follow instruction in the specific FOA regarding key personnel.
7.4.5 Special Instructions for 4.6 Selecting the Appropriate Budget Component
“K” award mechanisms are not modular; therefore, only the R&R budget component is applicable and only a few budget categories are actually used. Information regarding allowable costs for the candidate and any allowable research development or other costs is included in each K program FOA. Candidates are advised to contact the targeted awarding component if uncertain about allowable amounts for the applicable K award mechanism, keeping in mind that amounts vary with awarding components. The application forms package associated with CDA funding opportunities includes the R&R Budget Component.
Instructions for completing the R&R Budget Component are provided below. Additional guidance may also be provided in the specific funding opportunity announcement.
Note: NIH intramural candidates applying for transitional career award support (e.g., K22, K99/R00) should follow instructions in the applicable FOA. For the mentored phase of these awards, budgets are negotiated with the sponsoring intramural laboratory. For awardees who receive approval to transition to the extramural phase, a budget will be required as part of the extramural sponsored application.
7.4.6 Special Instructions for 4.7 R&R Budget Component
Follow the instructions provided in Part I.4.7 with the following exceptions:
4.7.1. A. Senior/Key Person: In general this section should include the name of the candidate only. Do not include the mentor(s) or any other Senior/Key persons. For the candidate, provide the base salary, person months, and requested salary and fringe benefits. For person months, be reminded that “K” programs include a minimum effort requirement, usually 75% or 9 academic person months. For the salary column, most NIH ICs limit the amount of salary provided for K programs. However, applicants should include information on actual institutional base salary and the actual amount of salary and fringe being requested. ICs may request updated salary information prior to award. Any adjustments based on policy limitations will be made at the time of the award.
4.7.1. B. Other Personnel: In general, leave this section blank.
4.7.2. C – E: Leave these sections blank.
4.7.3 F. Other Direct Costs: In the Material and Supplies field (F.1), enter the total research development support being requested for the initial year of the K award. Usually, a specific total amount is allowed for research development and other costs (tuition, fees, research supplies, equipment, computer time, travel, etc) that do not require individual cost category identification. Unless instructed differently in the applicable FOA, applicants should enter only the total requested research development support amount in this box. All remaining budget fields in this section should be left blank.
4.7. 3. H. Indirect Costs: For all “K” applications, F&A/indirect costs are reimbursed at 8% of modified total direct costs (exclusive of tuition and fees and expenditures for equipment) rather than on the basis of a negotiated rate agreement. Follow the instructions in the chart below for completing this section.
Field Name |
Instructions |
Indirect Cost Type |
Indicate the Indirect Cost type as Modified Total Direct Costs. |
Indirect Cost Rate (%) |
Indicate the indirect cost rate (also known as Facilities & Administrative Costs [F&A]) as 8%. |
Indirect Cost Base ($) |
Enter the amount of the base for the indirect cost type. |
Funds Requested |
Enter the funds requested for the indirect cost type. |
Total Indirect Costs |
The total funds requested for indirect costs. |
Cognizant Federal Agency |
Enter “Not Applicable” |
4.7.3.K Budget Justification: Use this to provide a detailed description and justification for specific items within the Research Development Support costs; e.g., all equipment, supplies, and other personnel that will be used to help achieve the career development and research objectives of this award.
7.4.7 Special Instructions for 5. Completing PHS398 Components
5.1 Overview
In conjunction with the SF424 (R&R) components, NIH and other PHS agencies grants applicants should also complete and submit additional components titled “PHS398.” Note the PHS398 components include additional data required by the agency for a complete application. While these are not identical to the PHS398 application form pages, the PHS398 reference is used to distinguish these additional data requirements from the data collected in the SF424 (R&R) components. A complete application to NIH and other PHS agencies will include SF424 (R&R) and PHS398 components. The PHS398 components for the individual K programs include:
PHS398 Cover Letter Component (I.5.2): required for mentored K awards – must include a list of references; optional for independent K awards, however applicants are strongly encouraged to include this component
PHS398 Cover Page Supplement(I.5.3): this supplements the data requirements in the R&R Cover component. Note there are no “K” specific instructions for this component. Follow the instructions found in Part I.5.3.
PHS398 Checklist Component (I.5.6): See “K” specific instructions below.
PHS398 Career Development Award Supplemental Form (I.7.5)
Complete each component using the instructions found in Part I.5 and the “K” specific instructions provided below.
5.2 Cover Letter Component
Mentored CDA applicants must include a cover letter. Applicants for independent CDAs are encouraged to include a cover letter with the application. The cover letter is only for internal use and will not be shared with peer reviewers. For mentored CDA applications, the cover letter must contain the same list of Referees (including name, departmental affiliation, and institution) that is included in the Other Project Information Component Item 11. Other Attachment.
For both mentored and non-mentored “K” applications, the cover letter can also include the information found in Part I.5.2.
5.6 Checklist Component
2. Change of Investigator / Change of Institution Questions: A change in PD/PI is not allowed for K awards.
7.5 PHS398 Career Development Award Supplemental Form
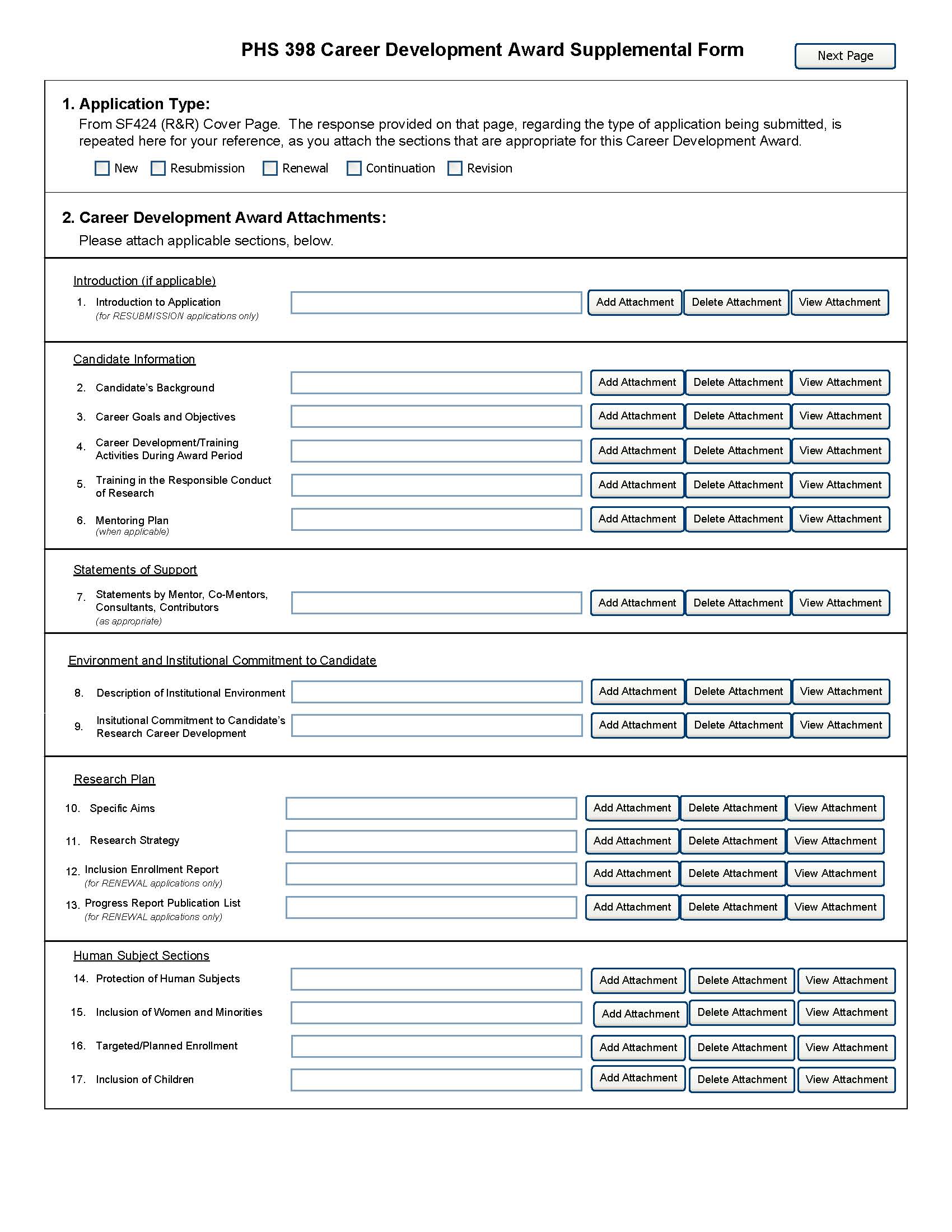

The PHS398 CDA Supplemental Form should include sufficient information needed for evaluation of the project, independent of any other document (e.g., previous application). Be specific and informative, and avoid redundancies. Some sections are required for all K award applications and some sections are only to be used when required by the FOA. Be sure to read all instructions in the FOA before completing this section since errors could lead to incomplete or rejected applications.
1. Application Type
This field is pre-populated from the SF424 (R&R) Cover Component. Corrections to this field must be made in that component.
2. Career Development Award Attachments (See also Section 2.3.2 Creating PDFs for Text Attachments)
Although many of the sections of this application are separate PDF attachments, page limits referenced in the instructions and/or funding opportunity announcement must still be followed. Agency validations will include checks for page limits (and use of appropriate font). Some accommodation will be made for sections that, when combined, must fit within a specified limitation.
Text attachments should be generated using word processing software and then converted to PDF using PDF generating software. Avoid scanning text attachments to convert to PDF since that causes problems for the agency handling the application. In addition, be sure to save files with descriptive file names.
Do not include any information in a header or footer of the attachments. A header will be system-generated that references the name of the PD/PI. Page numbers for the footer will be system-generated in the complete application, with all pages sequentially numbered.
Since a number of reviewers will be reviewing applications as an electronic document and not a paper version, applicants are strongly encouraged to use only a standard, single-column format for the text. Avoid using a two-column format since it can cause difficulties when reviewing the document electronically.
Full-sized glossy photographs of material such as electron micrographs or gels must only be included within the page limits of the Career Development Award application. The maximum size of images to be included should be approximately 1200 x 1500 pixels using 256 colors. Figures must be readable as printed on an 8.5 x 11 inch page at normal (100%) scale.
Candidates must use image compression such as JPEG or PMG. Do not include figures or photographs as separate attachments either in the Appendix or elsewhere in the application.
Separate Attachments
Separate attachments have been designed for the Career Development Award Supplemental Form sections to maximize automatic validations conducted by the eRA system. When the application is received by the agency, all of the CDA Supplemental Form sections will be concatenated in the appropriate order so that reviewers and agency staff will see a single cohesive application.
While each section of the CDA Supplemental Form needs to eventually be uploaded separately, applicants are encouraged to construct the Candidate Information and Research Plan as a single document, separating sections into distinct PDF attachments just before uploading the files. In this way the applicant can better monitor formatting requirements such as page limits. When validating for page limits, the eRA Commons will not count the white space created by breaking the text into separate files for uploading.
When attaching a PDF document to the actual forms, please note you are attaching an actual document, not just pointing to the location of an externally stored document. Therefore, if you revise the document after it has been attached, you must delete the previous attachment and then reattach the revised document to the application form. Use the “View Attachment” button to determine if the correct version has been attached.
Page Limits
The total number of pages for Items 2 – 5 (Candidate's Background, Career Goals and Objectives, Career Development/Training Activities During Award Period, and Training in the Responsible Conduct of Research) and Item 11 (Research Strategy) combined may not exceed 12 pages. All tables, graphs, figures, diagrams, and charts must be included within the 12-page limit.
Exception: If the page limits in the Funding Opportunity Announcement differ from these instructions, follow the limits as specified in the FOA.
All applications and proposals for NIH funding must be self-contained within specified page limits. Agency validations will include checks for page limits. Some accommodation will be made for sections that when combined must fit within a specified limitation. Note that while these computer validations will help minimize incomplete and/or non-compliant applications, they do not replace the validations conducted by NIH staff. Applications found not to comply with the requirements may be delayed in the review process. Unless otherwise specified in an NIH solicitation, Internet website addresses (URLs) may not be used to provide information necessary to the review because reviewers are under no obligation to view the Internet sites. Moreover, reviewers are cautioned that they should not directly access an internet site as it could compromise their anonymity.
Notice of Proprietary Information
Applicants are discouraged from submitting information considered proprietary unless it is deemed essential for proper evaluation of the application. However, when the application contains information that constitutes trade secrets, or information that is commercial or financial, or information that is confidential or privileged, make sure you have checked the “Yes” box of question #3 in the “Other Project Information” component. Identify the pages in the application that contain this information by marking those paragraphs or lines with an asterisk (*) in the left-hand margin. Include a legend at the beginning of Section 2, similar to “The following sections marked with an asterisk contain proprietary/privileged information that (name of Applicant) requests not be released to persons outside the Government, except for purposes of review and evaluation.”
When information in the application constitutes trade secrets or information that is commercial or financial, or information that is confidential or privileged, it is furnished to the Government in confidence with the understanding that the information shall be used or disclosed only for evaluation of this application. If a grant is awarded as a result of or in connection with the submission of this application, the Government shall have the right to use or disclose the information to the extent authorized by law. This restriction does not limit the Government’s right to use the information if it is obtained without restriction from another source.
Research Plan
A Research Plan is required for all types of individual K awards. The Research Plan is a major component of the research career development plan. It is important to relate the research to the candidate's scientific career goals. Describe how the research, coupled with other developmental activities, will provide the experience, knowledge, and skills necessary to achieve the objectives of the career development plan and launch and conduct an independent research career, or enhance an established research career. For mentored K awards, explain the relationship between the candidate’s research on the CDA and the mentor’s ongoing research program.
For most types of research, the plan should include: a specific hypothesis; a list of the specific aims and objectives that will be used to examine the hypothesis; a description of the methods/approaches/techniques to be used in each aim; a discussion of possible problems and how they will be managed; and, when appropriate, alternative approaches that might be tried if the initial approaches do not work.
The Research Plan of a CDA is expected to be appropriate for, and tailored to the experience level of the candidate, and allow him/her to develop the necessary skills needed for further career advancement, and reviewers will evaluate the plan accordingly. The plan should be achievable within the requested time period. Pilot or preliminary studies and routine data gathering are generally not appropriate as the sole component(s) of a CDA research plan. Although candidates for mentored K awards are expected to write the Research Plan, the mentor should review a draft of the plan and discuss it in detail with the candidate. Review by other knowledgeable colleagues is also helpful. Although it is understood that CDA applications do not require the extensive detail usually incorporated into regular research applications, a fundamentally sound Research Plan and a reasonably detailed Approach section should be provided.
In general, less detail will be expected in descriptions of research planned for the future years of the proposed CDA. However, sufficient detail should be provided to enable the peer reviewers to determine that the plans for those years, including the approach to be used, are worthwhile and are likely to enable the candidate to achieve the objectives of the Research Plan.
The PHS398 Career Development Award Supplemental Form is comprised of sections for: Candidate Information; Statement of Support (Mentors); Environment & Institutional Commitment to the Candidate; and the Research Plan (including Human Subjects and Other Research Plan Sections).
Begin each text section of the Candidate Information and Research Plan with a section header (e.g., Introduction, Specific Aims, Background & Significance, etc). See Specific FOA for additional information.
Field Name |
Instructions |
1. Introduction to Application (for Resubmissions only) |
There is no time limit for the submission of a resubmission application (A1). See NIH Notice NOT-OD-09-00-003 and NOT-OD-09-016 for additional information/clarification of NIH policy. Resubmission applications must include an Introduction to Resubmission Application, not to exceed one page unless the FOA specifies otherwise. The Introduction must include responses to the criticisms and issues raised in the Summary Statement. Summarize the substantial additions, deletions, and changes. In the body of the application, highlight paragraphs with significant changes by bracketing and changing typography. Save this information in a single file in a location you remember. Click Add Attachment, browse to where you saved the file, select the file, and then click Open. |
Candidate Information
Field Name |
Instructions |
2. Candidate’s Background |
Use this section to provide any additional information not described in the Biographical Sketch Format Page such as research and/or clinical training experience. (Note that the total number of pages for Items 2-5 and Item 11 (Research Strategy) combined may not exceed 12 pages.) Save this information in a single file in a location you remember. Click Add Attachment, browse to where you saved the file, select the file, and then click Open. |
3. Career Goals and Objectives |
Describe your past scientific history, indicating how the award fits into past and future research career development. If there are consistent themes or issues that have guided previous work, these should be made clear; if your work has changed direction, the reasons for the change should be indicated. It is important to justify the award and how it will enable you to develop or expand your research career. You may include a timeline, including plans to apply for subsequent grant support. (Note that the total number of pages for Items 2-5 and Item 11 (Research Strategy) combined may not exceed 12 pages.) Save this information in a single file in a location you remember. Click Add Attachment, browse to where you saved the file, select the file, and then click Open. |
4. Career Development/ Training Activities During Award Period |
Stress the new enhanced research skills and knowledge you will acquire as a result of the proposed award. If you have considerable research experience in the same areas as the proposed research, reviewers may determine that the application lacks potential to enhance your research career. For mentored awards, describe structured activities, such as course work or technique workshops, which are part of the developmental plan. If course work is included, provide course numbers and descriptive titles. Briefly discuss each of the activities, except research, in which you expect to participate. Include a percentage of time involvement for each activity by year, and explain how the activity is related to the proposed research and the career development plan. Note that recipients of mentored “K” awards may receive concurrent support from an NIH research grant award or cooperative agreement only under certain conditions (see NIH Notice NOT-OD-08-065). (Note that the total number of pages for Items 2-5 and Item 11 (Research Strategy) combined may not exceed 12 pages.) Save this information in a single file in a location you remember. Click Add Attachment, browse to where you saved the file, select the file, and then click Open. |
5. Training in the Responsible Conduct of Research |
All CDA applications must describe a plan to acquire training (or provide training in the case of independent awards, e.g., K05, K24) in the responsible conduct of research. There are no specific curriculum or formal requirements for this instruction; however, conflict of interest, responsible authorship, policies for handling misconduct, policies regarding the use of human and animal subjects, data management, and data-sharing are areas that are strongly suggested for consideration. Applicants may wish to consult the NIH web site (http://www.nih.gov/sigs/bioethics/researchethics.html) for additional guidance. (Note that the total number of pages for Items 2-5 and Item 11 (Research Strategy) combined may not exceed 12 pages.) Save this information in a single file in a location you remember. Click Add Attachment, browse to where you saved the file, select the file, and then click Open. |
6. Mentoring Plan (Include only when required by the specific FOA, e.g., K24 and K05) |
The plan should provide information about the candidate’s commitment to serve as a mentor to other investigators, and describe previous mentoring activities. The plan should describe the setting and provide information about the available pool of mentees with appropriate backgrounds and interests in the same field of science. It should also include information on the candidate’s past and proposed mentees sufficient to evaluate the quality of prior mentoring experiences, including the professional levels of mentees, and the frequency and kinds of mentoring interactions between the candidate and the mentees. Describe the productivity of the mentoring relationship for the scientific development of the new scientists as judged by their publications and current research activities. Senior level (K05) candidates should describe any financial and material support from their own funded research and research resources that will be available to their mentees. The candidate’s proposed percent effort commitment to the mentoring plan should also be stated. Save this information in a single file in a location you remember. Click Add Attachment, browse to where you saved the file, select the file, and then click Open. |
Statement of Support
Field Name |
Instructions |
7. Statements by Mentor, Co-mentor(s), Consultants, Contributors |
This section is to be completed by the mentor, co-mentor(s), consultant(s), and contributor(s), as appropriate. The letters must be appended together and uploaded as a single pdf file. For mentored awards (see Summary of Career Development Award Mechanisms table), the mentor must explain how they will contribute to the development of the candidate's research career. This statement should include all of the following: 1. The plan for the candidate's training and research career development. This description must include not only research, but also other developmental activities, such as seminars, scientific meetings, training in the responsible conduct of research, and presentations. It should discuss expectations for publications over the entire period of the proposed project and define what aspects of the proposed research project the candidate will be allowed to take with him/her to start their own research program. 2. The source of anticipated support for the candidate’s research project for each year of the award period. 3. The nature and extent of supervision and mentoring of the candidate, and commitment to the candidate's development that will occur during the award period. 4. The candidate's anticipated teaching load for the period of the award (number and types of courses or seminars), clinical responsibilities, committee and administrative assignments, and the portion of time available for research. 5. A plan for transitioning the candidate from the mentored stage of his/her career to the independent investigator stage by the end of the project period of the award. The mentor should describe previous experience as a mentor, including type of mentoring (e.g., graduate students, career development awardees, postdoctoral students), number of persons mentored, and career outcomes. All mentored career development applications should identify all co-mentors, consultants and collaborators involved with the proposed research and career development program. Briefly describe their roles and anticipated contributions. A co-mentor must specifically address the nature of his/her role in the career development plan and how the responsibility for the candidate’s development is shared with the mentor. Describe respective areas of expertise and how they will be combined to enhance the candidate’s development. Also describe the nature of any resources that will be committed to this CDA. Letters from the mentor(s), co-mentor(s), consultant(s), advisory committee members (if applicable), and contributor(s) documenting their role and willingness to participate in the project must be included in this section of the application. Do not place these letters in the Appendix. Non-mentored career development award applications should list any contributors or consultants. Briefly describe research materials, data, guidance, or advice they will provide. Letters from consultant(s) and contributor(s), documenting their willingness to participate in the project and describing their roles, must be included in this section of the application. Save this information in a single file in a location you remember. Click Add Attachment, browse to where you saved the file, select the file, and then click Open. |
Environment and Institutional Commitment to the Candidate
Field Name |
Instructions |
8. Description of Institutional Environment |
The sponsoring institution must document a strong, well-established research program related to the candidate's area of interest, including the names of key faculty members relevant to the candidate's proposed developmental plan. Referring to the resources description (See section 4.4.9 Facilities and Other Resources), indicate how the necessary facilities and other resources will be made available for career enhancement as well as the research proposed in this application. Describe opportunities for intellectual interactions with other investigators, including courses offered, journal clubs, seminars, and presentations. Save this information in a single file in a location you remember. Click Add Attachment, browse to where you saved the file, select the file, and then click Open. |
9. Institutional Commitment to Candidate’s Research Career Development |
IntroductionThe institution should provide a document on institutional letterhead that describes its commitment to the candidate and the candidate’s career development, independent of the receipt of the CDA. The document should include the institution’s agreement to provide adequate time and support for the candidate to devote the proposed protected time to research and career development for the entire period of the proposed award. The institution should provide the equipment, facilities, and resources necessary for a structured research career development experience. It is essential to document the institution's commitment to the retention, development and advancement of the candidate during the period of the award. Because of the diverse types of K awards, applicants should contact the appropriate awarding component Scientific/Research contact listed in the specific FOA to determine the level of commitment required for this application. AgreementThe applicant organization must: a. Agree to release the candidate from other duties and activities to devote the required percentage of time for development of a research career. For most K awards, commitment of at least 75 percent of time is required. Describe actions that will be taken to ensure this; e.g., reduction of the candidate's teaching load, committee and administrative assignments, and clinical or other professional activities for the current academic year. (For example, describe the actions that will be taken to compensate for the reduction in clinic responsibilities of the candidate, e.g., hiring of additional staff). Describe the candidate's academic appointment, bearing in mind that it must be full-time, and that the appointment (including all rights and privileges pertaining to full faculty status if in an academic setting) and the continuation of salary should not be contingent upon the receipt of this award. Describe the proportion of time currently available for the candidate's research experience and what the candidate's institutional responsibilities will be if an award is made. b. Provide the candidate with appropriate office and laboratory space, equipment, and other resources and facilities (including access to clinical and/or other research populations) to carry out the proposed Research Plan. c. Provide appropriate time and support for any proposed mentor(s) and/or other staff consistent with the career development plan. SignaturesThe institutional commitment must be dated and signed by the person who is authorized to commit the institution to the agreements and assurances listed above. In most cases, this will be the dean or the chairman of the department. The signature must appear over the signer's name and title at the end of the statement. If the candidate will be working away from the home institution, signatures from both the home and the host institution are required. The sponsoring institution, through the submission of the application and in the institutional commitment section, certifies that all items outlined above will be provided and that the institution will abide by the applicable assurances and PHS policies. See: NOT-OD-06-054. Create a single file of the institutional letter and save it in a location you remember. Click Add Attachment, browse to where you saved the file, select the file, and then click Open. |
Research Plan
Field Name |
Instructions |
10. Specific Aims |
State concisely the goals of the proposed research, summarize the expected outcome(s), and describe how the research will contribute to the objectives of the career development plan. List succinctly the specific objectives of the research proposed, e.g., to test a stated hypothesis, create a novel design, solve a specific problem, challenge an existing paradigm or clinical practice, address a critical barrier to progress in the field, or develop new technology. Limited to one page. Save this information in a single file in a location you remember. Click Add Attachment, browse to where you saved the file, select the file, and then click Open. |
11. Research Strategy |
Provide a Research Strategy in the specified order using the instructions provided below. Start each section with the appropriate section heading—Significance, Innovation, Approach. These sections will be evaluated taking into consideration the experience level of the candidate. (Note that the total number of pages for Items 2-5 and Item 11 (Research Strategy) combined may not exceed 12 pages.) (a) Significance
(b) Innovation
(c) Approach
Save this information in a single file in a location you remember. Click Add Attachment, browse to where you saved the file, select the file, and then click Open. |
12. Inclusion Enrollment Report (Renewal applications only) |
If the renewal involves clinical research, then you must report on the enrollment of research subjects and their distribution by ethnicity/race and sex/gender. See Part II, Section 4.3 for more detailed instructions on which Target and Enrollment Report or Table to use |
13. Progress Report Publication List (Renewal applications only) |
List the titles and complete references to all appropriate publications, manuscripts accepted for publication, patents, and other printed materials that have resulted from the project since it was last reviewed competitively. When citing articles that fall under the Public Access Policy, were authored or co-authored by the applicant and arose from NIH support, provide the NIH Manuscript Submission reference number (e.g., NIHMS97531) or the Pubmed Central (PMC) reference number (e.g., PMCID234567) for each article. If the PMCID is not yet available because the Journal submits articles directly to PMC on behalf of their authors, indicate “PMC Journal – In Process.” A list of these journals is posted at: http://publicaccess.nih.gov/submit_process_journals.htm. Citations that are not covered by the Public Access Policy, but are publicly available in a free, online format may include URLs or PMCID numbers along with the full reference (note that copies of these publications are not accepted as appendix material). |
Human Subjects Sections
Field Name |
Instructions |
14. Protection of Human Subjects |
This section covers only the initial information regarding the Protection of Human Subjects. Follow the instructions in Part II, Supplemental Instructions for Preparing the Human Subjects Section of the Research Plan. See separate sections below for other human subjects related sections that may apply. Do not use the human subjects section to circumvent the page limits of the Research Strategy. Save this information in a single file in a location you remember. Click Add Attachment, browse to where you saved the file, select the file, and then click Open. Unless an explanation is necessary, if Human Subjects research is not involved, and you have checked the box marked “No” on the Other Project Information Component, you need not include any additional information in this section. |
15. Inclusion of Women and Minorities |
To determine if Inclusion of Women and Minorities applies to this application, follow the instructions in Part II, Supplemental Instructions for Preparing the Human Subjects Section of the Research Plan. Save this information in a single file in a location you remember. Click Add Attachment, browse to where you saved the file, select the file, and then click Open. |
16. Targeted/Planned Enrollment |
If this application involves the Inclusion of Women and Minorities, complete the Targeted/Planned Enrollment Table. Save this information in a single file in a location you remember. Click Add Attachment, browse to where you saved the file, select the file, and then click Open. |
17. Inclusion of Children |
To determine if Inclusion of Children applies to this application, follow the instructions in the Supplemental Instructions for Preparing the Human Subjects Section of the Research Plan. Save this information in a single file in a location you remember. Click Add Attachment, browse to where you saved the file, select the file, and then click Open. |
Other Sections
Field Name |
Instructions |
18. Vertebrate Animals |
If you indicated that Vertebrate Animals are involved in this project, address the following five key points. In addition, when research involving vertebrate animals will take place at collaborating site(s) or other performance site(s), provide this information before discussing the five points. Although no specific page limitation applies to this section of the application, be succinct. 1. Provide a detailed description of the proposed use of the animals in the work outlined in the Research Strategy section. Identify the species, strains, ages, sex, and numbers of animals to be used in the proposed work. 2. Justify the use of animals, the choice of species, and the numbers to be used. If animals are in short supply, costly, or to be used in large numbers, provide an additional rationale for their selection and numbers. 3. Provide information on the veterinary care of the animals involved. 4. Describe the procedures for ensuring that discomfort, distress, pain, and injury will be limited to that which is unavoidable in the conduct of scientifically sound research. Describe the use of analgesic, anesthetic, and tranquilizing drugs and/or comfortable restraining devices, where appropriate, to minimize discomfort, distress, pain, and injury. 5. Describe any method of euthanasia to be used and the reasons for its selection. State whether this method is consistent with the recommendations of the American Veterinary Medical Association (AVMA) Guidelines on Euthanasia. If not, include a scientific justification for not following the recommendations. If the involvement of animals is indefinite, provide an explanation and indicate when it is anticipated that animals will be used. If an award is made, prior to the involvement of animals the grantee must submit to the NIH awarding office detailed information as required in 1-5 above and verification of IACUC approval. If the grantee does not have an Animal Welfare Assurance then an appropriate Assurance will be required (see Part III, 2.2). Do not use the vertebrate animal section to circumvent the page limits of the Research Strategy. Save this information in a single file in a location you remember. Click Add Attachment, browse to where you saved the file, select the file, and then click Open. |
|
For those applicants familiar with the PHS398, please note that the Literature Cited section of the Research Plan is now captured as “Bibliography & References Cited.” Refer to Item 8 in the Other Project Information Component for instructions. |
19. Select Agent Research |
Select Agents are hazardous biological agents and toxins that have been identified by DHHS or USDA as having the potential to pose a severe threat to public health and safety, to animal and plant health, or to animal and plant products. CDC maintains a list of these agents. See http://www.cdc.gov/od/sap/docs/salist.pdf. If the activities proposed in your application involve only the use of a strain(s) of Select Agents which has been excluded from the list of select agents and toxins as per 42 CFR 73.4(f)(5), the Select Agent requirements do not apply. Use this section to identify the strain(s) of the Select Agent that will be used and note that it has been excluded from this list. The CDC maintains a list of exclusions at http://www.cdc.gov/od/sap/sap/exclusion.htm. If the strain(s) is not currently excluded from the list of select agents and toxins but you have applied or intend to apply to DHHS for an exclusion from the list, use this section to indicate the status of your request or your intent to apply for an exclusion and provide a brief justification for the exclusion. If any of the activities proposed in your application involve the use of Select Agents at any time during the proposed project period, either at the applicant organization or at any other performance site, address the following three points for each site at which Select Agent research will take place. Although no specific page limitation applies to this section, be succinct. 1. Identify the Select Agent(s) to be used in the proposed research. 2. Provide the registration status of all entities* where Select Agent(s) will be used.
*An “entity” is defined in 42 CFR 73.1 as “any government agency (Federal, State, or local), academic institution, corporation, company, partnership, society, association, firm, sole proprietorship, or other legal entity.” 3. Provide a description of all facilities where the Select Agent(s) will be used.
If you are responding to a specific Funding Opportunity Announcement, address any requirements specified by the FOA. Reviewers will assess the information provided in this Section, and any questions associated with Select Agent research will need to be addressed prior to award. Save this file in a location you remember. Click Add Attachment, browse to where you saved the file, select the file, and then click Open. |
20. Consortium/Contractual Arrangements |
Explain the programmatic, fiscal, and administrative arrangements to be made between the applicant organization and the consortium organization(s). If consortium/contractual activities represent a significant portion of the overall project, explain why the applicant organization, rather than the ultimate performer of the activities, should be the grantee. The signature of the authorized organizational official on the SF424 (R&R) cover component (Item 18) signifies that the applicant and all proposed consortium participants understand and agree to the following statement: The appropriate programmatic and administrative personnel of each organization involved in this grant application are aware of the agency’s consortium agreement policy and are prepared to establish the necessary inter-organizational agreement(s) consistent with that policy. A separate statement is no longer required. Save this information in a single file in a location you remember. Click Add Attachment, browse to where you saved the file, select the file, and then click Open. |
21. Resource Sharing Plan(s) |
NIH considers the sharing of unique research resources developed through NIH-sponsored research an important means to enhance the value and further the advancement of the research. When resources have been developed with NIH funds and the associated research findings published or provided to NIH, it is important that they be made readily available for research purposes to qualified individuals within the scientific community. See Part III, 1.5 Sharing Research Resources. 1. Data Sharing Plan: Investigators seeking $500,000 or more in direct costs (exclusive of consortium F&A) in any year are expected to include a brief 1-paragraph description of how final research data will be shared, or explain why data-sharing is not possible. Specific Funding Opportunity Announcements may require that all applications include this information regardless of the dollar level. Applicants are encouraged to read the specific opportunity carefully and discuss their data-sharing plan with their program contact at the time they negotiate an agreement with the Institute/Center (IC) staff to accept assignment of their application. See Data-Sharing Policy or http://grants.nih.gov/grants/guide/notice-files/NOT-OD-03-032.html. 2. Sharing Model Organisms: Regardless of the amount requested, all applications where the development of model organisms is anticipated are expected to include a description of a specific plan for sharing and distributing unique model organisms or state why such sharing is restricted or not possible. See Sharing Model Organisms Policy, and NIH Guide NOT-OD-04-042. 3. Genome Wide Association Studies (GWAS): Applicants seeking funding for a genome-wide association study are expected to provide a plan for submission of GWAS data to the NIH-designated GWAS data repository, or an appropriate explanation why submission to the repository is not possible. GWAS is defined as any study of genetic variation across the entire genome that is designed to identify genetic associations with observable traits (such as blood pressure or weight) or the presence or absence of a disease or condition. For further information see Policy for Sharing of Data Obtained in NIH Supported or Conducted Genome-Wide Association Studies, NIH Guide NOT-OD-07-088, and http://grants.nih.gov/grants/gwas/. 4. Human Specimen and/or Data Research Resource Repositories: Applicants seeking funding for human specimen and/or data research resource repositories are expected to provide a brief description of the procedures and policies that will govern the collection, storage, and use of human specimens and/or data for research. For the purposes of this policy, a research resource repository is defined as an entity that collects, stores or distributes human biological specimens and/or data expressly for the purpose of sharing of specimens and data for current and/or future research. For further information, see NIH Guide NOT-OD-XX-XXX, and http://XXXXXXXXX. Save this information in a single file in a location you remember. Click Add Attachment, browse to where you saved the file, select the file, and then click Open. |
22. Appendix |
Only one copy of appendix material is necessary. Use the add attachments button to the right of this field to complete this entry. A maximum of 10 PDF attachments is allowed in the Appendix. If more than 10 appendix attachments are needed, combine the remaining information into attachment #10. Note that this is the total number of appendix items, not the total number of publications. When allowed there is a limit of 3 publications that are not publicly available (see below for further details and check the FOA for any specific instructions), though not all grant mechanisms allow publications to be included in the appendix. Do not use the appendix to circumvent the page limit of the candidate information (items 2-5) and the Research Strategy (item 11). Appendix material may not appear in the assembled application in the order attached, so it is important to use filenames for attachments that are descriptive of the content. A summary sheet listing all of the items included in the appendix is also encouraged but not required. When including a summary sheet, it should be included in the first appendix attachment. Applications that do not follow the appendix requirements may be delayed in the review process. New, resubmission, renewal, and revision applications may include the following materials in the Appendix:
Items that must not be included in the appendix:
|
3. Citizenship
The candidate must provide information regarding citizenship status. Other than for the K99/R00 award program, the candidate must be a citizen or non-citizen national of the United States or its possessions and territories, or must have been lawfully admitted to the United States for permanent residence by the time of award.
For those K award programs with a citizenship requirement, an individual who has applied for Permanent Residence and expects to have obtained such status prior to the time award, may submit an application recognizing that no award will be made until legal verification of permanent resident status is provided. If a candidate’s citizenship status changes after submission of the application, the new status should be reported in the candidate’s Personal Profile in the eRA Commons. Before an award is issued, a permanent resident will be required to submit a notarized statement that a licensed notary has seen the candidate’s Alien Registration Receipt Card or some other valid verification from the U.S. Immigration and Naturalization Service of legal admission to the U.S. as a permanent resident.
It is the responsibility of the sponsoring institution to determine and retain documentation indicating that the individual candidate’s visa will allow him/her to reside in the proposed research training/career development setting for the period of time necessary to complete the approved career development program. Information may be requested by the NIH prior to issuance of an award.
Each candidate must check the applicable box, check only one:
U.S. Citizen or non-citizen national: Check this box if the candidate is a U.S. Citizen or Noncitizen national. Noncitizen nationals are people, who, although not citizens of the United States, owe permanent allegiance to the United States. They generally are people born in outlying possessions of the United States (e.g., American Samoa and Swains Island).;
Permanent Resident of the U.S.: Check this box if the candidate has been lawfully admitted for permanent residence; i.e., is in the possession of an Alien Registration Receipt Card or other legal verification of such status. If the candidate has applied for Permanent Residence and expects to have obtained such status prior to the time award, this box should also be checked. In all cases, a notarized statement will be required as part of the pre-award process.
Non-U.S. citizen with temporary U.S. visa: This box is applicable only to specific programs that do not require U.S. citizenship or permanent residency; e.g. K99/R00. The NIH awarding component may request verifying information as part of the pre-award process.
![]()
Once all data have been entered, click the Close Form button at the top of the form or use the scroll bar to scroll up. You will be returned to the Grant Application Package screen. To remove a document from the Submission box, click the document name to select it and then click the Move Form to Delete button. This will return the document to the Mandatory Documents Submission List or Optional Documents Submission List.
7.6 “K” Peer Review Process
The goal of NIH-supported career development programs is to help ensure that diverse pools of highly trained scientists are available in adequate numbers and in appropriate research areas to address the Nation’s biomedical, behavioral, and clinical research needs. Each application must be tailored to the individual candidate.
The general process information (Overview, Streamlining, and Dual-Level Peer Review) found in Part I.6 applies to “K” applications as well. However, the actual review criteria and other review considerations are different. For “K” applications, the scientific review group will address individual career development award applications by considering information provided for each of the following elements in the application:
Review Criteria:
Candidate
Career Development Plan
Research Plan
Mentoring Plan (non-mentored), Mentor’s Statement (mentored), Collaborator(s), Consultant(s)
Environment and Institutional Commitment to the Candidate
Additional Review Criteria include the following:
Training in the Responsible Conduct of Research
Protection of Human Subjects from Research Risk
Inclusion of Women, Minorities, and Children in Research
Care and Use of Vertebrate Animals in Research
Resubmission Applications
Biohazards
Additional Review Considerations include the following:
Budget and Period of Support
Candidates should carefully review the applicable FOA for complete information associated with the peer review process. The FOA will describe essential information to be submitted for each of the above elements.
| File Type | application/msword |
| File Title | NIH SF424 R&R Application Guide for Adobe Forms Version A |
| Subject | Guide for preparing/submitting SF424 (R&R), Adobe Forms Version A |
| Author | NIH, Office of Director, Office of Extramural Research |
| Last Modified By | Leslie Dorman |
| File Modified | 2009-03-11 |
| File Created | 2009-03-11 |
© 2026 OMB.report | Privacy Policy