Form 2 CTRP Amendment
The Clinical Trials Reporting Program (CTRP) Database (NCI)
Attachment_2B_FORM_CTRP Amendments
Amendment for the Clinical Trials Reporting Program (CTRP) Database (NCI)
OMB: 0925-0600
NCI CTRP Attachment 2B
NCI CTRP Amendment Portal Workflow and Screen Shots
Step 1: User accesses the NCI Clinical Trials Reporting Program website at http://trials.nci.nih.gov – see screenshot, page 2
Step 2: User clicks “Login”
Step 3: User enters “Email Address” and “Password” – see screenshot, page 3
Step 4: User reviews NCI Clinical Trials Reporting Program burden statement – see screenshot, page 4
Step 5: System displays “Search Submitted Clinical Trials” page – see screenshot, page 10
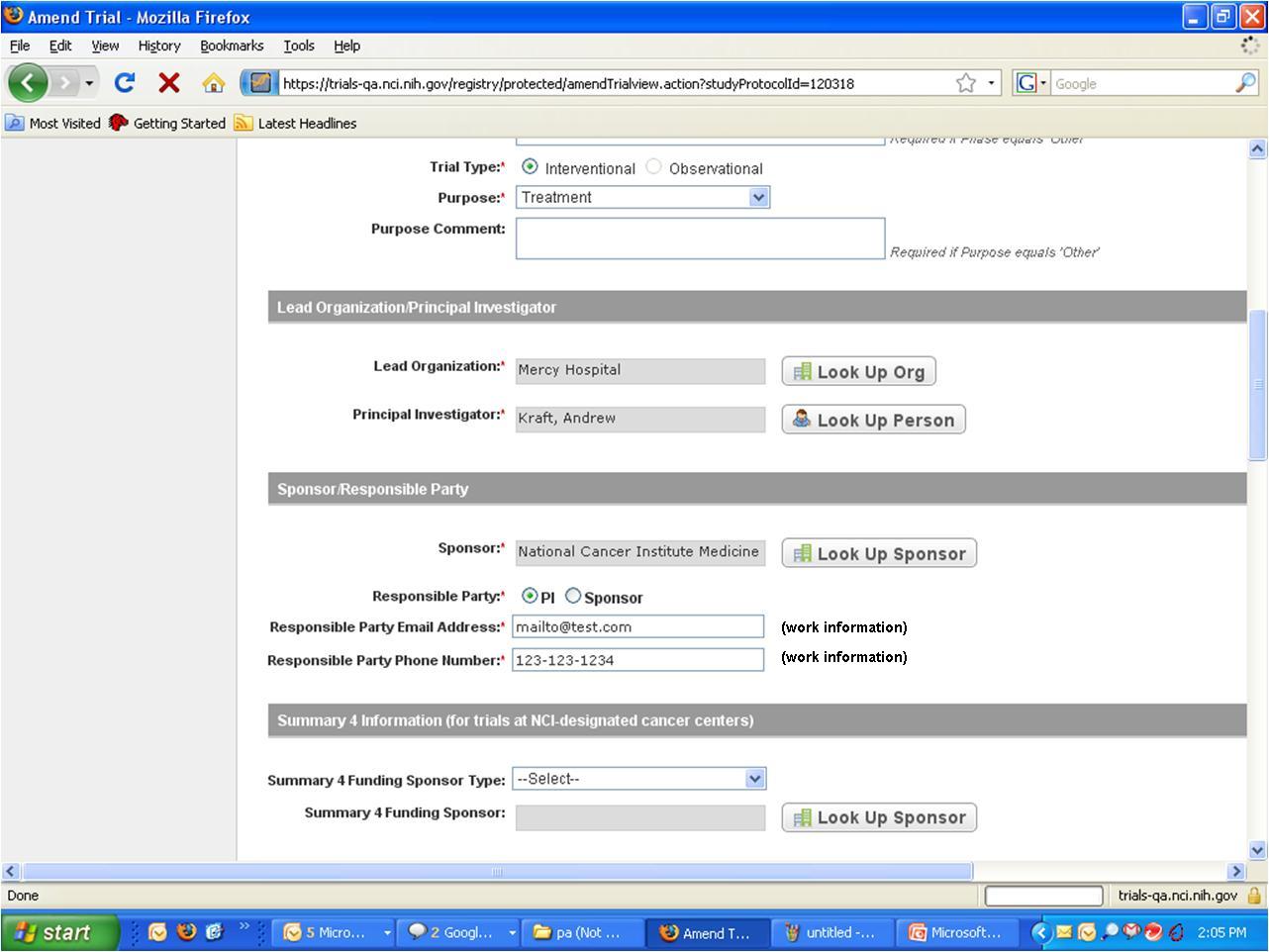
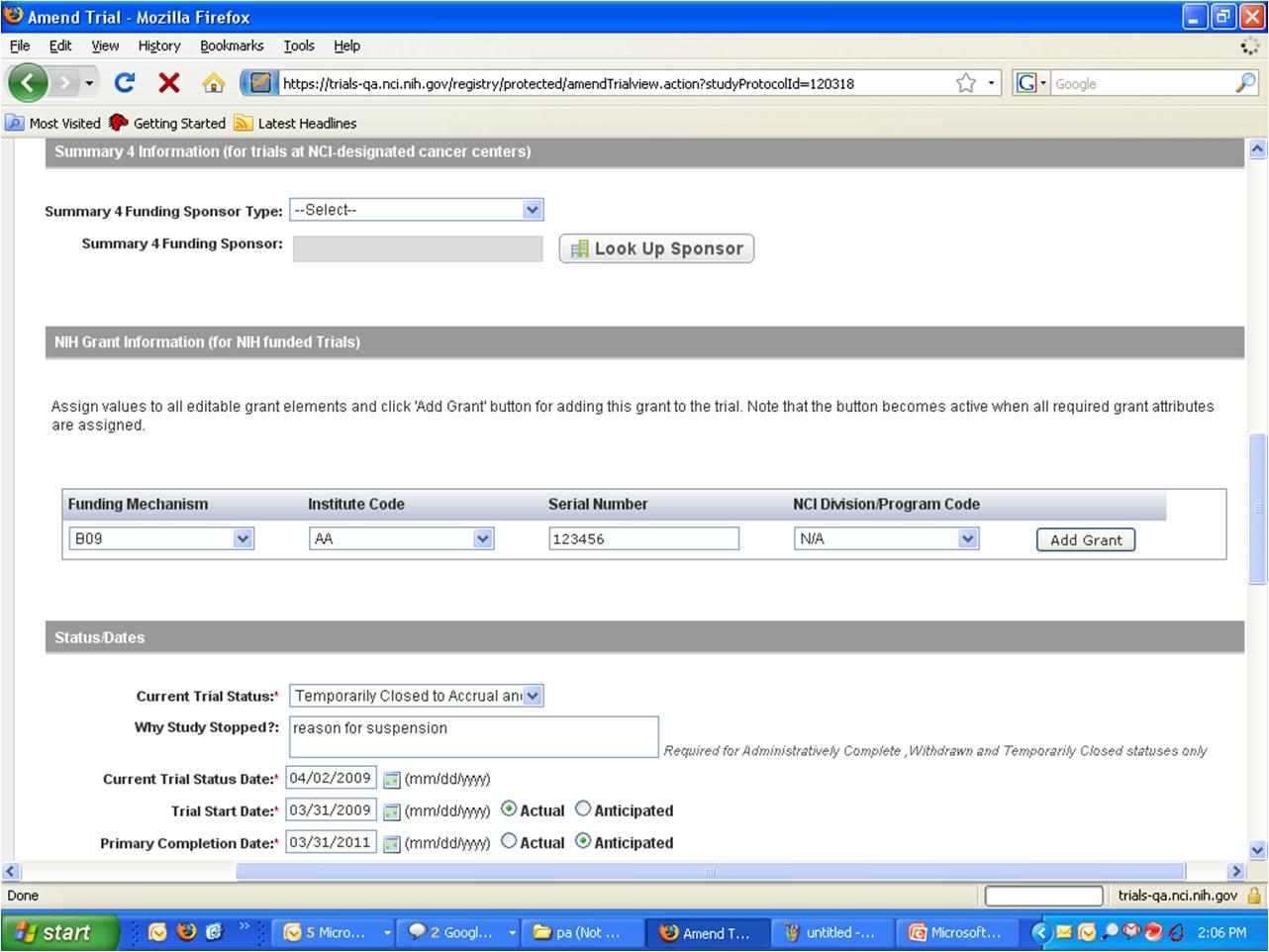
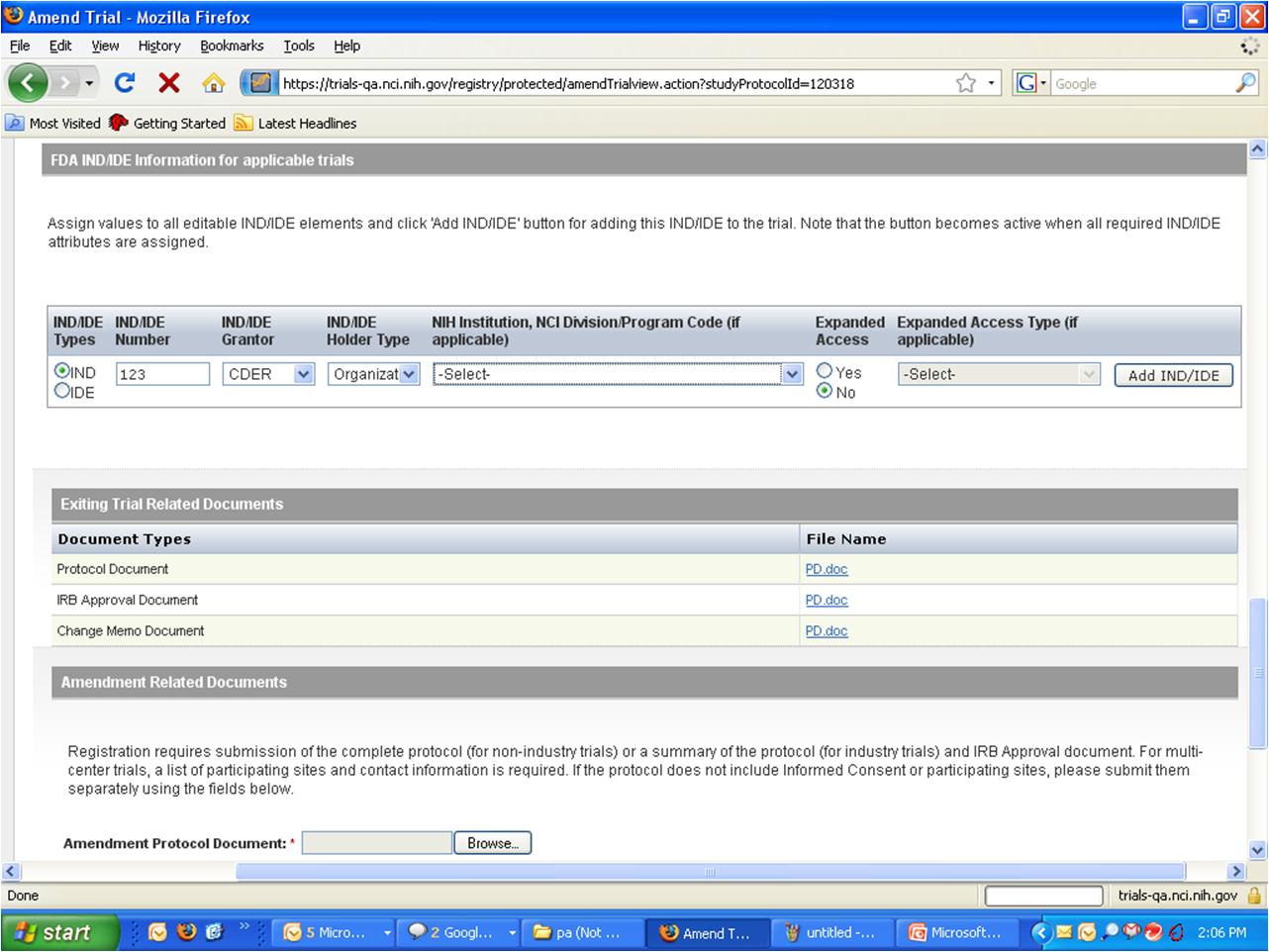
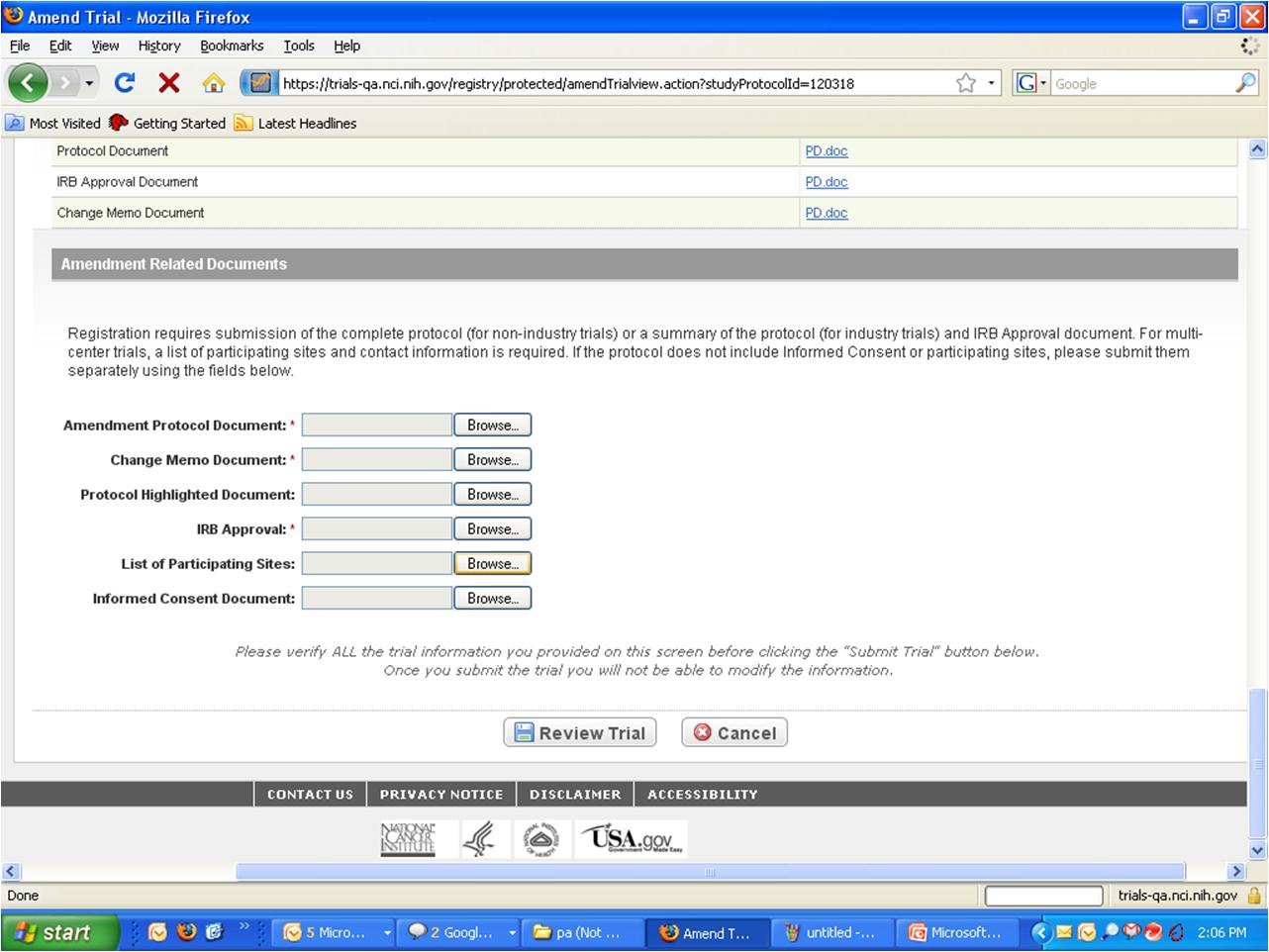
Step 6: User selects to “Submit Trial Amendment” and amends an existing trial record – see screenshots, pages 5 - 9
CTRP Home page
CTRP Login Screen
CTRP Burden Statement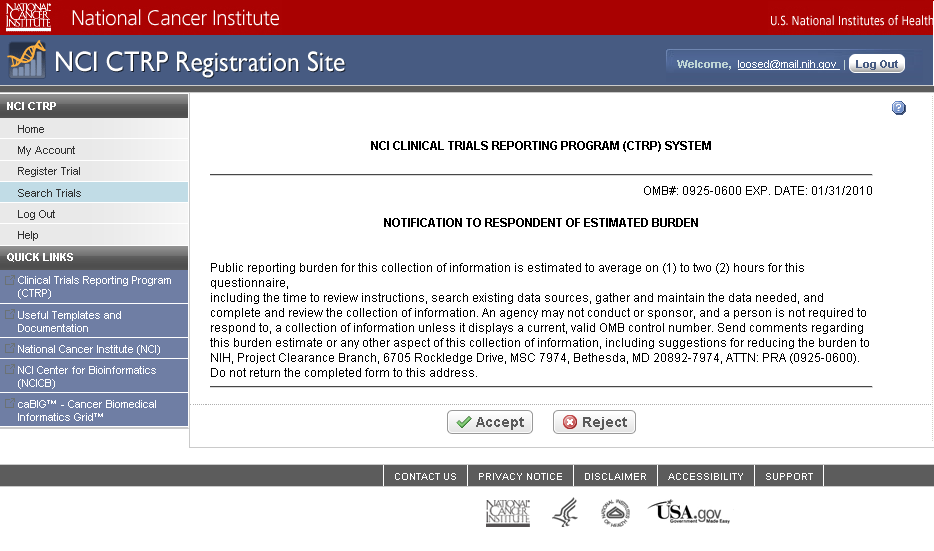
Submit Trial Amendment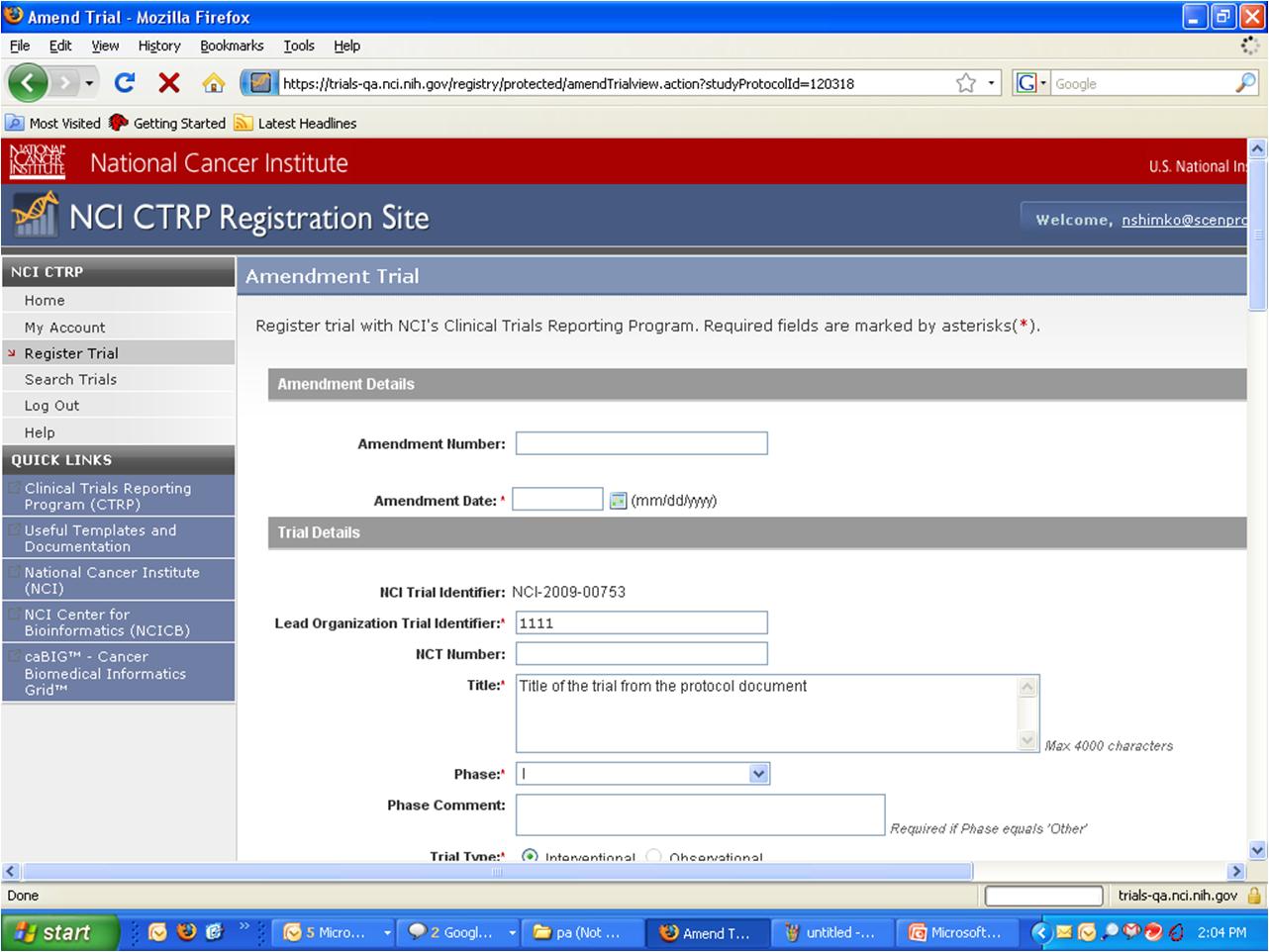
Search Submitted Clinical
Trials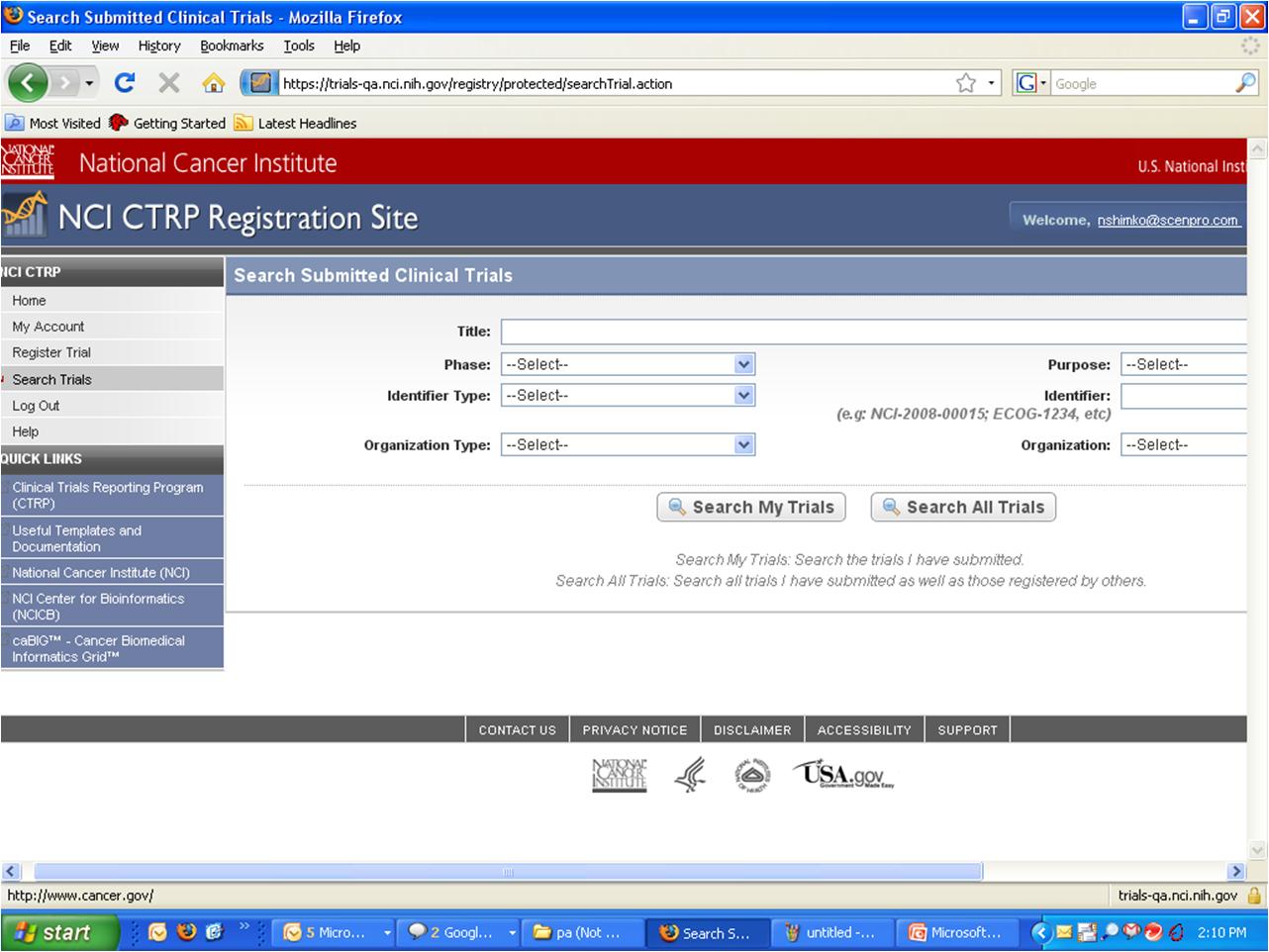
| File Type | application/msword |
| Author | David Loose |
| Last Modified By | Vivian Horovitch-Kelley |
| File Modified | 2009-10-16 |
| File Created | 2009-08-28 |
© 2026 OMB.report | Privacy Policy