REVISED #7 Justification Memo and attachments
#7_REV_Justif Memo Attach 7C 7D 7F_PSA_1-21-2011.docx
Formative Research, Pretesting, and Customer Satisfaction of NCI's Communication and Education Resources (NCI)
REVISED #7 Justification Memo and attachments
OMB: 0925-0046

Date: January 21, 2011 (Revised based on OMB’s comments)
December 14, 2010 (originally submitted)
To: Office of Management and Budget (OMB)
Through: Seleda Perryman, Report Clearance Officer, DHHS
Mikia Currie, Program Analyst, OPERA, NIH
Vivian Horovitch-Kelley, OMB Project Clearance Liaison, NCI
From: Nina Goodman, Project Office
National Cancer Institute (NCI)/NIH
Subject: Bundled Generic Sub-studies under “Formative Research, Pretesting, and Customer Satisfaction of NCI’s Office of Communications and Education”, OMB No. 0925-0046, Expiry Date 02/28/2013
Sub-Study #7, “Prostate-Specific Antigen (PSA) Decision-Making Focus Groups,” Proposed OMB No. 0925-0046-07
Background, Need and Use of Information
Recommendations for cancer screening have recently shifted from uniformly promoting their use to promoting a process of informed decision making about whether or not to get screened. This shift has been coupled with an increasing number of controversies over the risks and benefits of cancer screening tests, such as the Prostate-Specific Antigen (PSA) test to detect prostate cancer and mammograms for women in their 40’s. These controversies, combined with forces such as direct marketing of medical tests to consumers, have compelled this shift in perspective. In fact, the Institute of Medicine’s Quality Chasm report (2001)1 highlighted patient participation in decision making as a key component to improving the effectiveness of the health care system; the US Preventive Services Task Force (Sheridan, 2004) has also endorsed informed decision making as a necessary component of medical decisions, such as screening, that may have benefits for some but not for others.
These recent trends have resulted in cancer screening intervention research and practice now frequently considering an informed decision – rather than screening uptake – as the outcome of interest. However, little is still known about the process of informed decision making, and how that process between a patient and a health care provider may be influenced by the larger context of the health care system in which the decision is being made.
The purpose of this study is to assess individuals’ perceptions of their role (or lack thereof) in decision making with their health care providers, as well as their preferences for that process. A second purpose is to examine individuals’ beliefs about the larger societal implications of their health care decisions as they relate to their decision for health care. These research questions will be studied in the context of older males’ (55 -74 years of age) decision of whether or not to have the PSA test. The current screening guidelines for Prostate Cancer are for men to have a discussion with their provider about the PSA test; however the factors that play into this discussion and the ultimate decision for PSA testing are largely unexplored.
This study will explicate patients’ attitudes and misperceptions about screening as well as their desire for involvement in decision making. This information will help guide development of future measures assessing patient-provider communication and decision making. Study results may also help to identify points of intervention within the patient-provider interaction in order to promote more optimal (and informed) decision making in general, as well as in regard to screening decisions. The information collected can ultimately help to promote the appropriate use of screening through informed decision making between patients and providers. This process will take into account both the risks and benefits of screening based on an individual’s profile and desires and consequently reduce the burden of unnecessary testing. That reduction has implications for the individual and for the health care system as a whole. Finally, the information collected can be compared against studies in other countries with varying health care systems. Similar protocols and methods can be used to examine the effect of the national health care context on individuals’ attitudes toward and preference for participation in decision making.
This project fits with the mission of NCI’s Division of Cancer Control and Population Sciences (DCCPS),
Applied Cancer Screening Research Branch by examining the process of patient decision making and patient-provider communication discussions surrounding screening as part of the decision making process. This is in alignment with the Office of Communications and Education’s (OCE) generic clearance purposes of formative research to, “inform the design and development of NCI resources and ensure that they are appropriate and effective, and reach the intended audiences… [and to] assess the impact of resources and activities" (OMB No. 0925-0046, Supporting Statement A, December 30, 2009, p. 1).
The information gathered through this project will help guide development of future measures assessing patient-provider communication, decision making in a clinical context, as well as help to identify points of intervention within the patient-provider interaction to promote more optimal (and informed) decision making in general, as well as in regard to screening decisions.
To identify duplication and use of similar information, we performed a literature search in PubMed and PsycINFO. The majority of similar studies examining decision making in this area have either been cross-sectional secondary data analysis using large national surveys (Han et al., 2006; Hoffman et al., 2009; McFall, 2006), examined only socio-demographic correlates of informed decision making (McFall, 2006), focused on a specific sub-population, such as African Americans (Sanchez et al., 2007), or focused on treatment rather than screening (Gattellari et al., 2002). Other qualitative studies have mainly been based in countries (Chapple et al., 2008; Rai et al., 2007) which operate under very different health care systems and have different guidelines and procedures for screening (Ustun & Ceber, 2004), specifically for screening for prostate cancer using Prostate-Specific Antigen (PSA) (Burford et al., 2008). This project differs from previous projects in that it employs in-depth qualitative methods focused on a US population. In addition, this project focuses on the process of informed decision making of men with their health care providers in making the decision to get screened, rather than simply examining if any informed decision making has occurred and correlates thereof. This project differs from previous studies on similar topics in both its methods and focus, and therefore does not duplicate existing information.
The conceptualization of this project and the development of the screener (see Attachment 7A) and the focus group protocol/moderator guide (see Attachment 7B) have been guided in part by consultations with others outside of the main project team. Within NIH, Gordon Willis (NIH/NCI/DCCPS/APR) and Helen Meissner (NIH/OD/OBSSR) have contributed. Outside of NIH, we have received guidance from Paul Han (Maine Medical Center) as well as international colleagues Joan Austoker (University of Oxford/Cancer Research UK), Paul Hewitson (University of Oxford), and Julietta Patnick (University of Oxford/NHS Cancer Screening Programme, UK). Other researchers who may eventually wish to compare final results of this project or conduct similar studies have also been contacted in Canada and elsewhere.
A pilot focus group with nine men aged 56-70 was conducted on January 7, 2010 to test the procedures and methods to be undertaken for the main study. The focus group ran smoothly. Minor changes to the wording, ordering, and formatting of the focus group protocol were made based on the process and results of the pilot focus group.
Once OMB approval is secured, the focus groups will commence within 4 weeks. We anticipate that it will take no more than 2 months to collect information from a maximum of 45 participants. Results for this study will be summarized within 4 weeks after the completion of the study.
Participants
Recruitment for the focus groups will be by means of advertisements (newspaper or online) (see Attachment 7C). A total of five focus groups will be conducted nationwide with up to 9 participants in each group. Respondents will be males between the ages of 55 and 74 who have heard of the PSA test, have no personal history of prostate cancer, have visited a doctor in the past year, have health insurance, and were born in the United States. Potential participants will include men residing in various geographic regions across the United States, and representing all racial, ethnic, and educational backgrounds. Participation will be voluntary and users will be asked to participate in the focus group only once.
Because the purpose of the current study is to collect information for formative research, we will recruit respondents with diverse backgrounds, though the results will not be representative of nor generalized to a national sample. Recruitment will continue until the target sample size for each focus group is reached.
Methodology
Screening and scheduling procedures.
Experience with focus groups and other intensive interviewing techniques has shown that advertisements in local newspapers and flyers attract a large pool of potential participants (Willis, 2005). Other recruitment procedures will be developed, as necessary, to identify participants (e.g., soliciting support from seniors’ organizations). The first contact with potential research participants occurs in response to flyers or advertisements.
Interested persons call a listed telephone number, and leave contact information (name and telephone number) on an answering machine. The Project Recruiter will contact all potential respondents, provide a brief description of the study (see Attachment 7A), its purposes and, where the interview will take place, audio taping procedures, and the incentive to be offered.
If the respondent is interested, then the recruiter determines through a brief series of screener questions (see Attachment 7A) whether the volunteer possesses the desired research characteristics (e.g., gender, age, and educational level to avoid interviewing multiple people with very similar demographic characteristics). If the person does and would like to participate, he is scheduled for a focus group. Telephone numbers and the minimal demographic information listed earlier are obtained for all scheduled volunteers. For those callers who are ineligible for the study, or no longer interested after hearing a description of the research, no identifying information will be retained.
Focus groups procedures.
If a participant is scheduled, he will travel to the location of the activity (contractor office or another location). When participants arrive they are greeted by staff working on the project and directed to the interviewing location, where they are individually greeted by the focus group Moderator. Participants will be given a packet containing the consent form (see Attachment 7D) and the Participant Demographics Questionnaire (see Attachment 7E). The Participant Demographics Questionnaire will be used to ask men about aspects of their lives that research has already shown to be related to participation in informed decision making. Assessing these items will provide context for data analysis. No record is kept of responses potential participants give during the recruitment process. Therefore we propose to ask these questions at the time of the focus group in order to link the responses with the participant – no identifying information is kept, but the questionnaire and participant data can be linked. Once the forms have been completed, they will be returned to the Focus Group Moderator. In the rare instance that consent is not granted and an individual cannot therefore participate, he will be excused, but will still receive the monetary incentive.
Participants will receive $75 as a thank you for their participation. For intensive forms of interviews (that is, cognitive interviews, focus groups, and usability tests), participants generally receive remuneration, for several reasons: (1) Eligibility criteria for participants are usually specific, and receiving an incentive will help attract participants; (2) Intensive forms of interviews require an unusual level of mental effort and a significant amount of time; and (3) Participants are being asked to travel to a focus group facility, which involves transportation and possibly parking expenses.
After a discussion with the contractors, they are concerned that anything less than $75 will have a substantial impact on the ability to successfully recruit participants for the focus groups. They indicate that the focus groups will be conducted during the day, which mean participants may miss work. Thus a low incentive may reduce the recruitment for younger, still working men to participate and therefore bias the findings. They also indicate that reducing the incentive will mean that more people will need to be recruited in order to get enough people to show up. Because there are costs associated with recruiting each participant, even for no-shows, needing to recruit more would substantially increase the cost of the project.
A project staff member will moderate the focus group. Before discussion begins, the Moderator will distribute name tags. Only first names are written on the name tags. The Moderator will then and reiterate the purpose of the focus group (see Attachment 7B, Section A1) and describe the process of the focus group and ask if there are questions. After all questions are answered, the Moderator will begin the focus group discussion following the Moderator guide (see Attachment 7B).
Analysis.
Analysis of the focus group data will occur in a series of steps. First, we will perform a content analysis of the data to inventory all issues mentioned. Subsequent analysis will identify patterns and themes that emerge in the data and then cluster these patterns or themes into broader categories. During subsequent analyses we will also look for patterns, themes, similarities and differences between subgroups of respondents using demographic data from the questionnaire. We will also develop data displays, matrices, and decision models to help us better understand the relationships between respondents, their attitudes, and their self-reported behaviors related to informed decision making.
Other Considerations
Personally identifying information (PII) in the form of names and phone numbers of individual respondents is collected for the sole purpose of confirming the respondents’ focus group participation after the screener is conducted. The PII will be kept in a separate file and will not be shared with the moderators or data analysts. To maintain anonymity during the focus groups only first names will be used. The NIH Privacy Act Officer will review this information to confirm that the Privacy Act applies.
Individual respondents will not be identified and participation will be strictly voluntary. Only people authorized to work on this project will have access to information obtained as part of this study, and the report summarizing the findings will not contain any names or identifying information. PII will not be retained after scheduling and study participation has been completed. Respondents will be assured that neither their participation/non-participation nor any responses to items will have any effect on their eligibility for, or receipt of, services. No questions will be asked of a personal or sensitive nature. Participants may choose to withdraw from the focus group at any time or choose not to offer an answer to a question posed by the moderator.
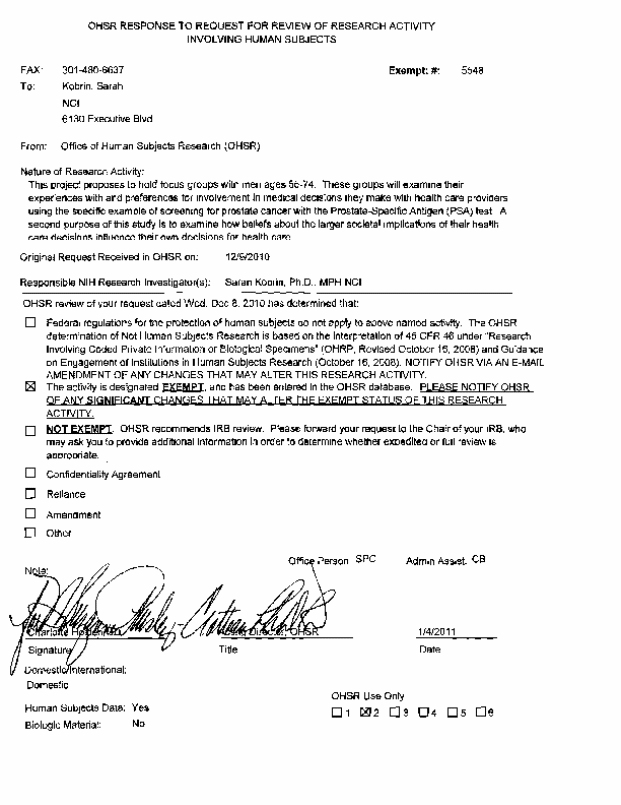
All data collection and analysis will be performed in compliance with the Privacy Act and Protection of Human Subjects requirements. The Office of Human Subject Research Exemption (OHSR) request was submitted on December 9, 2010 and was approved January 4, 2011 (see Attachment 7F).
Participant Burden
There will be a maximum of 45 respondents (9 participants each in a total of 5 focus groups) with an average response time of 100 minutes to complete the screener, demographic questionnaire and focus group, which culminates in a total burden of 79 hours (Table A.12-1). The total burden approved for this generic study was 7050 hours and this sub-study accounts for approximately 1% of the burden for this package.
Table A.12-1 Estimates of Hour Burden |
|||||
Type of Respondents |
Instruments |
Number of Respondents |
Frequency of Response |
Average Response Time (minutes/hour) |
Hour Burden |
Males between the ages of 55-74 |
Screener (Attach 7A) |
80 |
1 |
5/60 (0.08) |
7 |
Focus Group (Attach 7B) |
45 |
1 |
90/60 (1.5) |
68 |
|
Questionnaire (Attach 7E) |
45 |
1 |
5/60 (0.08) |
4 |
|
Total |
|
170 |
|
|
79 |
Thank you for your consideration of this proposed sub-study.
Attachments (attached below):
7C) Newspaper/Online Recruiting Advertisements
7D) Consent Form
7F) Office of Human Subject Research (OHSR) Exemption approval
Attachments (in a separate file):
7A) Informed Decision-Making Screener
7B) Informed Decision-Making Protocol/Moderator Guide
7E) Participant Questionnaire
References
Burford D, Kirby M, Austoker J. Prostate Cancer Risk Management Programme: an information pack for primary care. Sheffield: NHS Cancer Screening Programmes; 2008.
Chapple A, Ziebland S, Hewitson P, McPherson A. Why men in the United Kingdom still want the prostate specific antigen test. Qualitative Health Research 2008;18(1):56.
Gattellari M, Voigt K, Butow P, Tattersall M. When the treatment goal is not cure: are cancer patients equipped to make informed decisions? Journal of Clinical Oncology 2002;20(2):503.
Han PK, Coates RJ, Uhler RJ, Breen N. Decision making in prostate-specific antigen screening National Health Interview Survey, 2000. American Journal of Preventive Medicine 2006 May;30(5):394-404.
Hoffman R, Couper M, Zikmund-Fisher B, Levin C, McNaughton-Collins M, Helitzer D, et al. Prostate cancer screening decisions: results from the National Survey of Medical Decisions (DECISIONS study). Archives of Internal Medicine 2009;169(17):1611.
Institute of Medicine. Crossing the quality chasm: a new health system for the 21st century. Washington, DC: National Academy Press, 2001. Available from URL: http://www.nap.edu/books/0309072808 /html/index.html
McFall S. US men discussing prostate-specific antigen tests with a physician. The Annals of Family Medicine 2006;4(5):433.
Rai T, Clements A, Bukach C, Shine B, Austoker J, Watson E. What influences men's decision to have a prostate-specific antigen test? A qualitative study. Family Practice 2007;24(4):365-71.
Sanchez M, Bowen D, Hart Jr A, Spigner C. Factors influencing prostate cancer screening decisions among African American men. Ethnicity & Disease 2007;17(2):374-80.
Sheridan S, Harris R, Woolf S. Shared decision making about screening and chemoprevention. American Journal of Preventive Medicine 2004;26(1):56-66.
Ustun C, Ceber E. Ethical issues for cancer screenings Five countries—four types of cancer. Preventive Medicine 2004;39(2):223-9.
Willis G. Cognitive interviewing: a tool for improving questionnaire design: Sage Publications; 2005.
Attachment 7C: Newspaper/Online Recruiting Advertisements
Newspaper Ad:
Focus Group about Health: $75

Males between 55-75 are needed to participate in a 1 ½ hour focus group in ____ (city) about how men make health care decisions with their doctors. Those who are eligible and attend the focus group will receive $75 cash as an incentive for your participation. Call Sarita at 800------- .
Ad for Craigslist:
Focus
group about health: $75
WESTAT, a social science research organization, is looking for males between 55 and 75 years of age who are willing to participate in a focus group. The focus group will take about an hour and a half, and you will receive $75 cash as an incentive for your participation.
Men will discuss how they make health care decisions with their doctors.
All information you give us will be kept private under the Privacy Act. No information about you will be shared with others, and you will not be put on a marketing/mailing list.
If you want to know more about Westat, please visit our website: www.westat.com
For more information or if you are interested in participating, please send an email to: [email protected]. In your email, please provide your name, phone number, and a good time to reach you.
Someone from Westat may then contact you and ask a few additional questions to determine if you meet certain eligibility requirements. If you do, we will tell you the date and time of the focus group.
Please understand that we may not be able to use everyone who contacts us. Thank you.
Attachment 7D:
Consent Form for Volunteer Participants: Medical Decision-Making Focus Groups
Westat is conducting research for the National Cancer Institute (NCI). The purpose of this focus group is to discuss how people make decisions about medical tests and procedures with their doctors.
The discussion will be audio taped and notes will be taken. Project researchers may also observe the discussion.
The discussion should take up to 1½ hours.
Your participation is completely voluntary. You may stop at any time, and you do not have to answer any questions you do not wish to answer.
This research does not involve any foreseeable risks.
All information obtained from this study will be kept private under the Privacy Act and will only be seen by people authorized to work on this project. The report summarizing the findings will not contain any names or identifying information. We will destroy any identifying information when the project ends.
There are no direct benefits to participants in this research.
Participants will receive $75 in cash as a thank you for your participation.
If you have questions about this research please contact Sarah Kobrin, the Principal Investigator at NCI (301-435-8662; [email protected]). If you have questions about your role as a research participant, please contact Sharon Zack, the Westat Institutional Review Board Administrator (301-610-8828; [email protected]).
A copy of this consent form has also been provided for your records.
If you agree to participate in this focus group, please read the following statement and sign your name below:
I have read the above information about this project and my rights as a participant. I consent to participate in this research and to have this discussion audio taped.
Signature Date
Printed Name
Attachment 7F: Office of Human Subject Research (OHSR) Exemption approval
1 All references are cited below.
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| File Title | DEPARTMENT OF HEALTH & HUMAN SERVICES |
| Author | krs0 |
| File Modified | 0000-00-00 |
| File Created | 2021-02-02 |
© 2026 OMB.report | Privacy Policy