#13_Justif Memo_NightShift
#13_Justif Memo_NightShift.doc
Formative Research, Pretesting, and Customer Satisfaction of NCI's Communication and Education Resources (NCI)
#13_Justif Memo_NightShift
OMB: 0925-0046

Date: March 18, 2011
To: Office of Management and Budget (OMB)
Through: Seleda Perryman, DHHS Report Clearance Officer
Mikia Currie, Program Analyst, Project Clearance Branch, OPERA, NIH
Vivian Horovitch-Kelley, OMB Project Clearance Liaison, OMAA, NCI
From: Bu-Tian Ji, Staff Scientist
Occupational and Environmental Epidemiology Branch (OEEB),
Division of Cancer Epidemiology and Genetics (DCEG),
National Cancer Institute (NCI)/NIH
Subject: Bundled Generic Sub-study, “Nightshift Work and Sleep Pattern Questionnaire” under “Formative Research, Pretesting, and Customer Satisfaction of NCI’s Office of Communication and Education,”
OMB No. 0925-0046-13; Expiration Date 02/28/2013
Background, Need and Use of Information
The Division of Cancer Epidemiology and Genetics (DCEG) is an intramural research program of the National Cancer Institute (NCI), National Institutes of Health (NIH) that conducts population and multidisciplinary research to discover the genetic and environmental determinants of cancer and new approaches to cancer prevention. The Division conducts broad-based research; maintains a national and international perspective, giving priority to emergent issues identified through clinical, laboratory, and epidemiologic observations, as well as to public health concerns identified by the Institute, Congress, regulatory agencies, and other appropriate bodies; and develops infrastructures, resources, and strategic partnerships in molecular epidemiology across NCI, NIH, and the extramural community. As a branch of the DCEG, the Occupational and Environmental Epidemiology Branch (OEEB) conducts research projects that are designed to identify occupational, environmental, and other factors affecting cancer risk; to characterize exposure response relationships; to elucidate biological mechanisms of action; to identify susceptible populations and gene environment interactions; and to improve research methods for occupational investigations.
Night-shift work involving disruption of circadian rhythms has been classified as a probable cause of human cancer by the International Agency for Research on Cancer (IARC), based on sufficient animal evidence and limited human evidence1. The epidemiologic evidence supporting this link has been inconsistent. A weakness of most existing studies is the inadequate assessment of the history of shift work, such as nightshift hours and frequency and rotation of shift, and non-occupational exposure to light at night. Since the IARC designation of night-shift work as probable human carcinogen has important policy implications worldwide, including patient compensation among nightshift workers and workplace safety, we propose to initially conduct formative research and then possibly conduct the full-scare epidemiologic research to clarify this association. We have drafted a questionnaire that is designed to overcome the criticism on data collection in previous studies.1, 2 This questionnaire is designed by NCI to capture shift work exposure for each job held over one year in participants' lifetime, as well as light exposure at night during different periods of life (Attachment 13A).
Participants and Collaborations
Occupational cancer risk is a major area of OEEB’s research focus. We propose to conduct formative research to determine whether the draft questionnaire is effective in obtaining information as an initial step in further clarifying the association between nightshift work and cancer in the Shanghai Women’s Health Study (SWHS). The SWHS is a prospective cohort study of 75,000 healthy women in Shanghai, China, recruited during 1997-2000. The participants are being re-contacted every two years to update their health status, including newly diagnosed cancer, and limited lifestyle and environmental exposures, such as dietary changes and employment status. The next biennial survey will begin in January, 2012. The response rates for the baseline in-person interview and the first, second, and third follow-up surveys were 93%, 99.8%, 98.7%, and 96.7%, respectively. Given the large size of the cohort and the limited resources, including interview time, NCI requires accurate, timely, and useful information about the relevance, usefulness, and appropriateness of the questionnaire. The formative research process is being used to pretest the questionnaire on a small part of the large target population, ensuring feasibility of the questionnaire message, user-friendliness, and the time use in an in-person interview. Given this proposal, it clearly fits under the active generic submission titled, “Formative Research, Pretesting and Customer Satisfaction of NCI’s Communication and Education Resources,” (OMB No. 0925-0046) which was revised and approved by OMB in 2010.
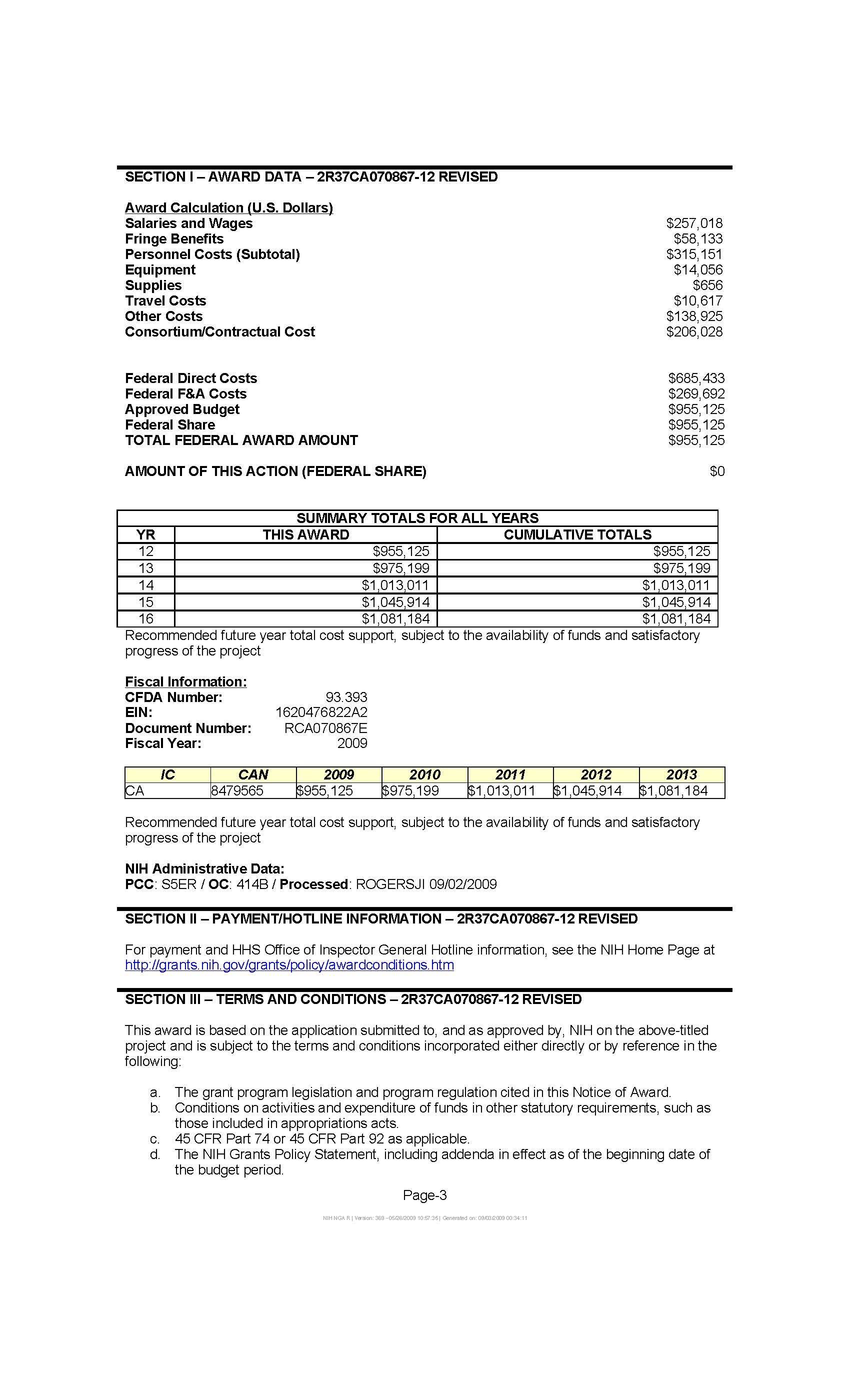
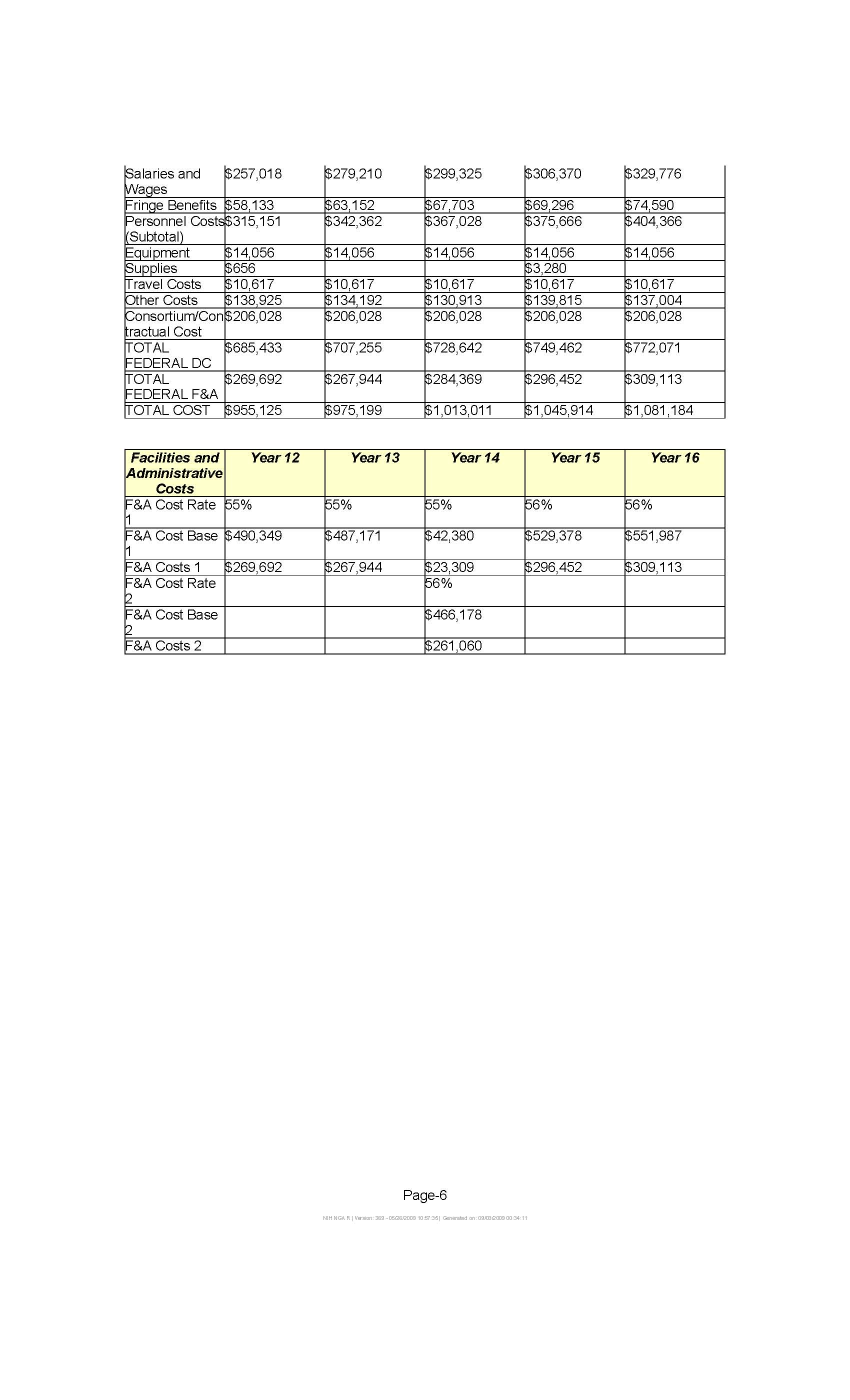
The SWHS is a collaborative project between NCI, Vanderbilt University and the Shanghai Cancer Institute. An NCI extramural grant to Vanderbilt University supported the baseline and follow-up in-person interviews of the SWHS participants, and collection of blood and urine from 20,000 of them at baseline (Attachment 13D). To expand the molecular component of this cohort, the OEEB/DCEG contributed intramural funds to support the collection of blood and urine samples from the remainder of the cohort. The biological samples from this cohort are also used in research jointly conducted by the Centers for Disease Control and Prevention and NCI, and other international research and academic institutions.
Given the existing collaboration, the rapport developed between the investigators and the cohort participants over the years, and the lifetime occupational history already collected from the participants, we propose to pretest the nightshift work and light exposure at night questionnaire on 300 of the cohort participants. This formative research/pretesting helps ensure that messages included have the potential to be received, understood, and accepted by the participants involved in the SWHS in China. If the pretest is successful, we plan to incorporate the shift-work questionnaire with the Vanderbilt survey questions to be collected at the next biennial update starting in January, 2012. We will seek separate OMB review and approval for the final questionnaire before we begin the main data collection.
A lifetime occupational history of all jobs held for one year or more outside the home was collected from each cohort participant at baseline, including job title, type of industry, and year started and ended each job. However, no information was collected on whether each job involved night shift, frequency of shift-work, hours of each shift, and pattern of shift, i.e., whether the shift work was rotating or permanent. The proposed questionnaire will take advantage of the lifetime occupational history already collected, and append the questions regarding nightshift work for each job reported in the history.
The design of the proposed questionnaire was based on the IARC’s extensive review of the literature and expert assessment on the information needed to adequately address the health effects of night shift work.1,3
Methodology and Research Instrument
An in-person interview will be conducted to complete the nightshift work and sleep pattern questionnaire. Based on experience from previous follow-up surveys, the responses from the participants were positive when we visited them at their homes. Therefore, for this proposed formative research, we plan to visit the participants at their homes and explain this survey to them. We will seek their consent (Attachment 13B) prior to the interview.
The questionnaire included questions concerning (Attachment 13A):
Type of shift work for jobs held for one year or longer reported as of the last follow-up (pre-printed the information of job history for use at the pretest)
Duration and frequency of shift work during each job held
Sleep pattern (e.g., usual bed time) during ages <=25, 25-55, and >55 years old
Light exposure levels during bed time at night during ages <=25, 25-55, and >55 years old
The entire pretest of interviewing 300 subjects is expected to complete within two months. Each questionnaire will be pre-assigned with a participant study ID number. When the interview is completed, the responses will be coded and keyed into a computer data file at the Shanghai Cancer Institute. Statistical analysis will be conducted by NCI to review for completeness and consistency of the responses. Logic and range checks will be conducted to detect any problems in responding to the questions. Interviewers’ comments will be reviewed to identify any difficulties that respondents might have with the understanding or responding to specific questions.
Other Considerations
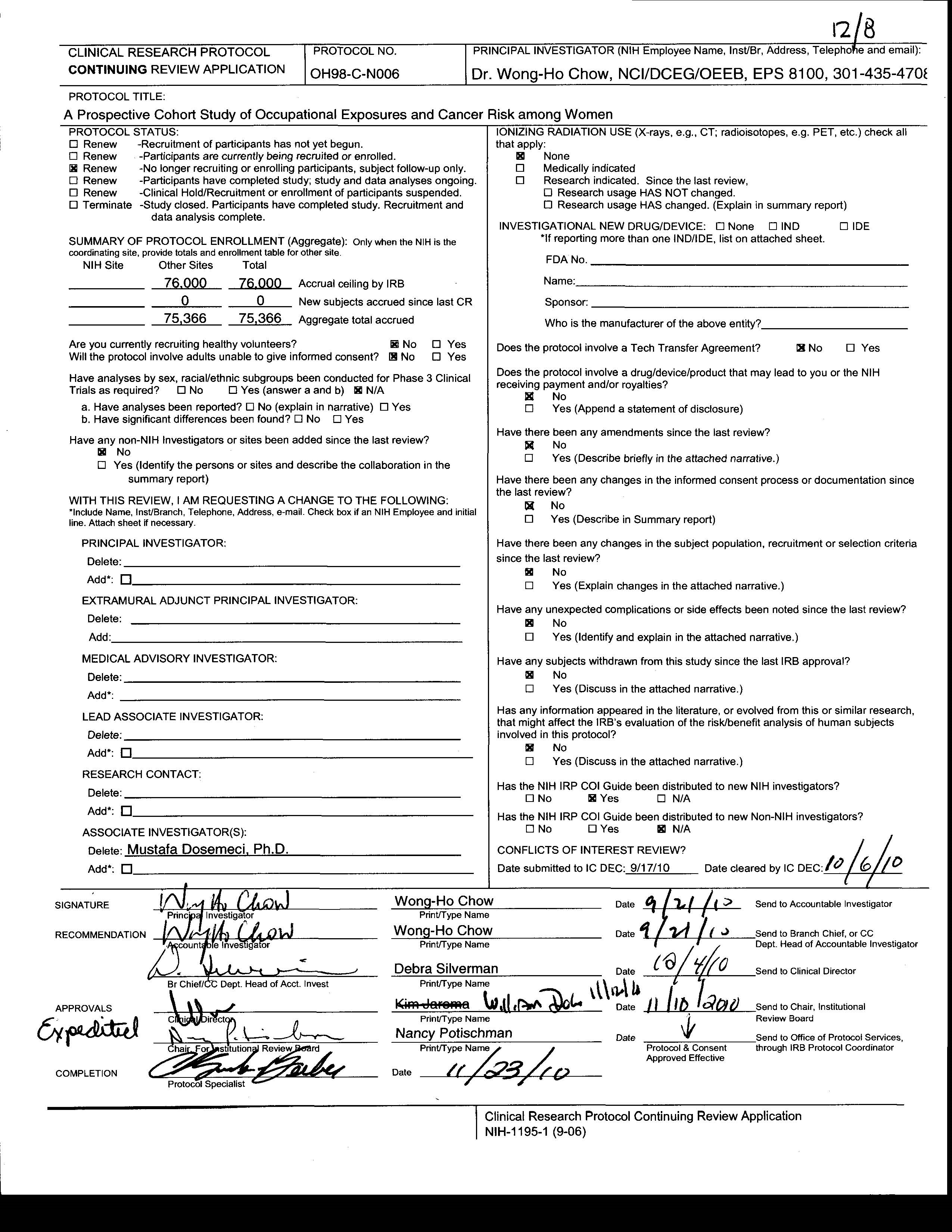
The IRB for the SWHS has been approved by NCI SSIRB (Protocol ID: OH-98-C-N006) (See Attachment 13C for the IRB approval). All information provided by participants will be kept secure to the extent permitted by law. Additionally, the SWHS data kept under lock and key, and in password protected data files at the Shanghai Cancer Institute. NCI received only anonymous data for research purposes only. No personally identifiable information (PII) will be collected in the present questionnaire.
Burden
We aim to complete each interview within an average of 10 minutes. In a pilot test of the proposed questionnaire in 5 participants, the interviews varied around 10-15 minutes. Therefore we expect the total respondent burden for this proposed effort to be 50 hours. This effort will account for less than 1 percent of the total burden hours granted in the full generic OMB clearance package. To date, a total of 1758 burden hours have been used of the 7050 hours that were requested.
Types of Respondents |
Number of Respondents |
Frequency of Response |
Average Response Time (Minutes/Hour) |
Annual Hour Burden |
Shanghai Women’s Health Study Participants |
300 |
1 |
10/60 (0.1667) |
50 |
Totals |
300 |
|
50 |
|
References:
International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Volume (8. Painting, Firefighting, and Shift-work. 2010. p, 562-763.
Pronk A, Ji BT, Shu XO, et al., Night-shift work and breast cancer risk in a cohort of Chinese women. Am J Epidemiol, 2010 (9): 953-959.
Stevens RG, Hansen J, Costa G, et al., Considerations of circadian impact for defining “shift work” in cancer studies: IARC Working Group Report. Occup Environ Med, 2011; 68: 154-162.
List of Attachments (attached in a separate file)
13A: Nightshift Work and Sleep Pattern Questionnaire
List of Attachments (attached below)
13B. Informed Consent Form
13C: IRB approval from SSIRB
13D: Notice of Grant Award to Vanderbilt University
Attachment 13B: Informed Consent From for the cohort study
Shanghai Women's Health Study
Follow-up Survey
CONSENT FORM
You have been invited to participate in the Shanghai Women’s Health Study being conducted by the Shanghai Cancer Institute, Vanderbilt University, and the U.S. National Cancer Institute since 1997-2000. We are now conducting the 5th follow-up survey and hope you will continue to be a participant of this important health study.
If you agree to complete the survey, you will be asked to answer some questions regarding nightshift work and sleep patterns from a standardized questionnaire. The interview will take about 10 minutes. These are questions relating to the type, frequency, and duration of shift work for jobs you held as of the last follow-up 2 years ago; and sleep pattern (e.g., usual bed time) and light exposure levels during bed time.
All information we obtain from you will be used exclusively for scientific research only. Any personal information that can identify you as an individual will be kept secure to the extent permitted by law. Your name will never be used in any study reports. Your data will be put together with those from other participants to make totals, averages, and other descriptive statistics.
You may be contacted in the future to obtain missing or additional information, or to inquire about your interest in other studies; however, you have the right to refuse to participate in the study at any time without jeopardizing your relationships with the Shanghai Cancer Institute or Hospitals. If you have any questions about this study, you may contact Dr. Yu-Tang Gao at 64043057.
Your continuing participation in the study is completely voluntary. Your signature below indicates that you have read the information provided, and have agreed to participate. You will be offered a copy of this form to keep.
Date: ____________________
Participant's signature: __________________________
Attachment 13C: IRB approval from SSIRB
Attachment 13D: Notice of Grant Award to Vanderbilt University




Page
| File Type | application/msword |
| File Title | DEPARTMENT OF HEALTH & HUMAN SERVICES |
| Author | krs0 |
| Last Modified By | Ji, Bu-Tian (NIH/NCI) |
| File Modified | 2011-03-25 |
| File Created | 2011-03-25 |
© 2026 OMB.report | Privacy Policy