Attachment 8 - RPPR Instruction Guide
Attachment 8 - RPPR Instruction Guide.docx
PHS Research Performance Progress Report and Other Post-award Reporting
Attachment 8 - RPPR Instruction Guide
OMB: 0925-0002

Powering the Advancement of Science
NIH Research Performance Progress Report (RPPR) Instruction Guide
System Version 3.3.3.1
Document Version 2.0.0
June 8, 2012
Forms Approved Through XX/XX/20XX
OMB No. 0925-0002

Contact Us
Document Comments:
We value your feedback on this document. Please submit comments to your eRA Customer Relationship Manager.
For general questions about this module and associated business processes:
Please contact your IC Coordinator. A list of IC Coordinators is available online:
http://inside.era.nih.gov/techrep_list.cfm.
Troubleshooting support:
Please contact the eRA Help Desk:
Toll-free: 1-866-504-9552
Phone:
301-402-7469
TTY: 301-451-5939
Web: http://ithelpdesk.nih.gov/eRA/ (Preferred method of contact)
Email: [email protected] (for Commons Support)
Email: [email protected] (for IMPAC II Support)
Hours: Mon-Fri, 7:00 a.m. to 8:00 p.m. Eastern Time
DISCLAIMER STATEMENT
No data shown in illustrations represents any real account, project, or individual. Any resemblance to actual accounts, projects, or individuals is purely coincidental.
Document History
Date |
System Version |
Document Version |
Description of Change |
Author |
4/25/2012 |
3.3.3.1 |
1.0.0 |
Develop initial draft of document |
eRA Documentation Team |
6/8/2012 |
3.3.3.1 |
2.0.0 |
Updated for June 2012 |
eRA Documentation Team |
Table of Contents
1 Purpose 1
2 Background and Paperwork Burden 2
3 RPPR Due Dates 3
4 Data Entry, PDF Attachments, and Style 4
4.1 Data or Text Box, and PDF Size Limits 4
4.2 PDF Attachments and File Names 4
5 Navigation 6
5.6 Submit the RPPR to Agency 20
5.9 Viewing the Final RPPR in Commons 23
6 RPPR Component Instructions 26
6.2 Section B – Accomplishments 27
6.4 Section D – Participants 34
6.7 Section G – Special Reporting Requirements 43
6.8 Section H – Budget [applicable to non-SNAP awards only] 53
7 Supplemental Instructions 54
7.1 Individual Career Development (K) Awards 54
7.6.1 Subcomponent (Project/Core) Instructions 63
8 Assurances/Certifications 65
9 Government Use of Information Under the Privacy Act 67
List of Tables
List of Figures
Figure 1: RPPR Navigation Links from Cover Page 6
Figure 2: Status Screen and List of Applications/Grants Links 7
Figure 3: eSNAP Link on Status Result – List of Applications/Grants 7
Figure 4: Manage eSNAP List of Grant Applications 8
Figure 5: eSNAP/RPPR Options 8
Figure 6: RPPR Menu for Initiating the Report 11
Figure 7: RPPR Error Messages 12
Figure 8: Check for Errors Button on RPPR Menu 12
Figure 9: Status Screen and List of Applications/Grants Links 13
Figure 10: eSNAP Link on Status Result – List of Applications/Grants 14
Figure 11: Manage eSNAP List of Grant Applications 14
Figure 12: RPPR Menu Buttons 15
Figure 13: RPPR Cover Page and Component Navigation Links 16
Figure 15: Total Remaining Characters 17
Figure 16: RPPR Menu – Route Button 18
Figure 17: Route RPPR to Next Reviewer 18
Figure 18: PD/PI Assurance Statement 19
Figure 19: RPPR Successfully Routed 19
Figure 20: RPPR Menu – Recall Button 20
Figure 21: RPPR Successfully Recalled 20
Figure 22: Successful Submission Message 22
Figure 23: RPPR Menu – View Button 22
Figure 24: RPPR Menu – View Routing History Button 23
Figure 25: RPPR Routing History 23
Figure 26: Status Menu Option 24
Figure 27: Application ID hyperlink 24
Figure 28: Status Information Screen – e-Application Link 25
Figure 29: RPPR Section A. Cover Page 27
Figure 30: RPPR Section B. Accomplishments – Question B1 28
Figure 31: RPPR Section B. Accomplishments – Questions B2 & B3 29
Figure 32: RPPR Section B. Accomplishments – Question B4 30
Figure 33: RPPR Section B. Accomplishments – Question B5 30
Figure 34: RPPR Section B. Accomplishments – Question B6 31
Figure 35: RPPR Section C. Products – Question C1 32
Figure 36: RPPR Section C. Products – Questions C2 & C3 33
Figure 37: RPPR Section C. Products – Questions C4 & C5 34
Figure 38: RPPR Section D. Participants – Question D1 37
Figure 39: RPPR Section D. Participants – Questions D2a & D2b 38
Figure 40: RPPR Section D. Participants – Questions D2c – D2e 40
Figure 41: RPPR Section E. Impact – Questions E1 through E4 41
Figure 42: RPPR Section F. Changes – Questions F1 & F2 42
Figure 43: RPPR Section F. Changes – Question F3 43
Figure 44: RPPR Section G. Special Reporting Requirements – Questions G1 through G3 44
Figure 45: RPPR Section G. Special Reporting Requirements – Question G4 49
Figure 46: RPPR Section G. Special Reporting Requirements – Questions G5 through G7 50
Figure 47: RPPR Section G. Special Reporting Requirements – Question G8 51
Figure 48: RPPR Section G. Special Reporting Requirements – Question G9 52
Figure 49: RPPR Section G. Special Reporting Requirements – Questions G10 through G12 53
The purpose of this document is to provide those preparing the Research Performance Progress Report (RPPR) with an explanation of the RPPR module in the eRA Commons and the information required in the report. This document also provides the steps for accessing and completing the report in eRA Commons, navigation, validations, routing and submission to the NIH.
Progress reports to NIH must be submitted electronically through the NIH RPPR module in the eRA Commons, unless the award has not yet transitioned to use of the eSNAP or RPPR. See http://grants.nih.gov/grants/RPPR/ to determine whether the award requires an RPPR, eSNAP, PHS 2590 Non-competing Continuation Progress Report, or PHS 416-9 NRSA Individual Fellowship Progress Report for Continuation Support.
For progress reports for multi-year funded awards (project period and budget period are the same and are longer than one year), follow the instructions at: http://grants.nih.gov/grants/policy/myf.htm.
Note that the RPPR will appear in the eSNAP Commons module until all SNAP awards have transitioned to use of the RPPR. At that time the eSNAP module will be renamed RPPR.
The NIH Research Performance Progress Report (RPPR) implements the uniform reporting format for interim research progress reporting developed under the auspices of the National Science and Technology Council, through the Committee on Science and the Research Business Models Subcommittee, and established by the Office of Management and Budget for use by agencies that support research and research-related activities.
The RPPR replaces the Public Health Service (PHS) Non-competing Continuation Progress Report (PHS 2590) and the Ruth L. Kirschstein National Research Service Award Individual Fellowship Progress Report for Continuation Support (PHS 416-9). Other PHS agencies that utilize the NIH RPPR are the Food and Drug Administration, Centers for Disease Control and Prevention, and Agency for Healthcare Research and Quality. These agencies may have requirements that differ from those for NIH grantees; refer to the Notice of Award (NoA) or contact the Grants Management Specialist named in the NoA.
NIH is transitioning use of the PHS 2590, eSNAP, and the PHS 426-9 to the RPPR. To determine whether an award has transitioned, see http://grants.nih.gov/grants/RPPR/.
Progress reports are required to continue support of a PHS grant for each budget year within a competitive segment. The NIH RPPR is not used for submitting a Final Progress Report; instructions for submitting a Final Progress Report are at http://grants.nih.gov/grants/funding/finalprogressreport.pdf.
PHS estimates that it will take approximately 15 hours to complete this progress report. An agency may not conduct or sponsor, and a person is not required to respond to, a collection of information unless it displays a currently valid OMB control number. If you have comments regarding the burden estimate or other aspect of the collection of information, including suggestions for reducing the burden, send comments to: NIH, Project Clearance Office, 6705 Rockledge Drive MSC 7974, Bethesda, MD 20892-7974, ATTN: PRA (0925-0002). Do not send progress reports to this address.
Grantees can determine which progress reports are due through the website located at: http://era.nih.gov/commons/quick_queries/index.cfm#progress, and should periodically check the site, which is updated on/around the 30th of each month. Progress report due dates are also available in the eRA Commons Status system. In addition, automatic e-mail notifications are sent to the PD/PI prior to due date. If an award is issued under the SNAP (Streamlined Noncompeting Award Process) provisions, the progress report is due the 15th of the month preceding the month in which the budget period ends (e.g., if the budget period ends 11/30, the due date is 10/15). If the 15th falls on a weekend or Federal holiday, the due date is automatically extended to the next business day. If an award is not issued under the SNAP provisions, the progress report is due the first of the month preceding the month in which the budget period ends (e.g., if the budget period ends 11/30, the due date is 10/1). For Fellowships the progress report is due two months before the beginning date of the next budget period. Occasionally the Notice of Award (NoA) will indicate a different due date which will supersede these dates. Grantees should consult the NoA to determine when SNAP procedures apply.
RPPR data is either entered directly into data or text boxes or uploaded as text attachment PDF files. Data fields and text entry boxes only support ASCCII characters: Greek letters, mathematical equations, images, and so-called “rich” text (including bold, italic, underlined, superscript, subscript, strikethrough, etc.) are not permitted. Although you may enter non-ASCCII characters in RPPR text fields, upon submission of the RPPR a PDF of the report is generated and the non-ASCCI characters will not appear correctly. PDF attachments may include non-ASCCII characters, “rich” text, and/or images. Grantees should review the final RPPR prior to submission.
Most text entry boxes have an 8,000 character limit (3 pages); this limit is standardized across federal agencies implementing the RPPR and entry of more than 8,000 characters is prevented by the system. In an effort to reduce grantee burden and encourage concise responses NIH prefers that grantees limit text responses to no more than one page, or approximately 2800 characters. Text boxes with a limit of less than 8,000 characters are agency specific items.
Warning: Text exceeding 8,000 characters is cut to 8,000 when using the cut and paste feature.
PDF file uploads (attachments) do not have page limits, but may not be more than 6 megabytes (6MB). PDF attachments are utilized when there may be a need for a grantee to provide considerable detail (e.g., change in human subject protocols that requires a new or revised Protection of Human Subjects section as described in Part II of the competing application instructions). Even when developing PDF responses, grantees are encouraged to be concise and avoid unnecessary detail.
Applicants should generate text attachments using any word processing software and then convert those files to PDF before attaching the files to the appropriate component in the progress report. The PDF format is used to preserve document formatting. All PDF attachments must be submitted as individual files. Although some software packages allow bundling of multiple PDFs into a single file, eRA systems cannot support “Bundling” or “Portfolio” features at this time. Use of these features may result in delays in agency acceptance of the progress report.
Save all files with descriptive file names of 50 characters or less and be sure to only use standard characters in file names: A through Z, a through z, 0 through 9, and underscore (_). Do not use any special characters (example: &, -, *, %, /, and #) or spacing in the file name, and for word separation use an underscore (e.g., My_Attached_File.pdf).
Style
Use English and avoid jargon. Abbreviations and language that may not be known to the broader scientific community should be avoided unless clearly defined. Internet Web site addresses (URLs) should not be used unless provided under C.2.
The RPPR is completed using the eRA Commons system. The report in Commons consists of separate screens for each reporting component as listed below:
A. Cover Page
B. Accomplishments
C. Products
D. Participants
E. Impact
F. Changes
G. Special Reporting Requirements
H. Budget
Users may work on the screens in any order, however, it is important to click the Save button in the navigation bar before leaving a screen in order to retain data entered on that screen. Upon submission to NIH, the system will generate a PDF of the progress report, which may be viewed from the RPPR Menu screen using the View button.
Once submitted, the final RPPR, in PDF format, is accessible in Commons via the Status Information screen. Refer to the section titled View the Final RPPR in Commons for detailed steps.
Note that a link to a site outside the RPPR (e.g., U.S. Select Agency Registry in F.3.d, ClinicalTrials.gov in G.4.c, or the NIH human embryonic stem cell Registry in G.6) opens a site in a new browser window. You must close that window to return to the RPPR. Do not close the browser or use the browser’s back button.
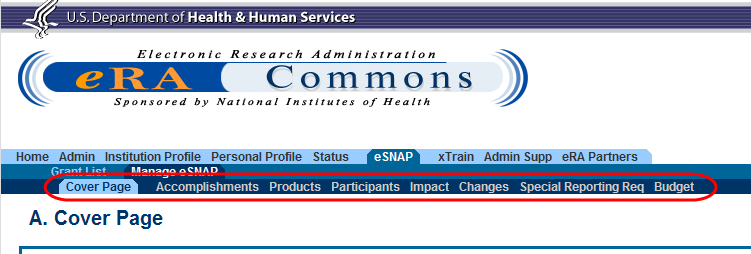
Figure 1: RPPR Navigation Links from Cover Page
Only the PD/PI or the PD/PI delegate may initiate an RPPR. When there are multiple PIs (MPI), only the Contact PI or the PD/PI delegate of the Contact PI may initiate the report. To initiate, the user can choose from one of two ways to access the RPPR functionality:
Access RPPR from Status:
Select the Status tab from the Commons menu options.
Select the List of Applications/Grants link from the Status screen or from the menu options.
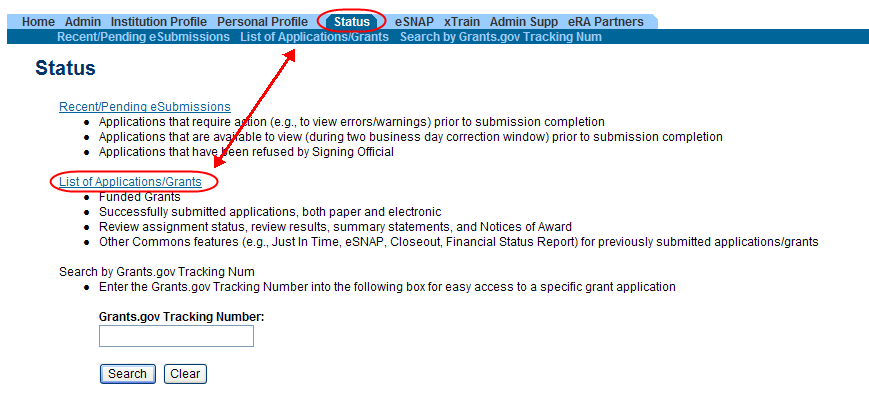
Figure 2: Status Screen and List of Applications/Grants Links
From the Status Result – List of Applications/Grants screen, locate the application and select the eSNAP link from the Action column for the specific application.
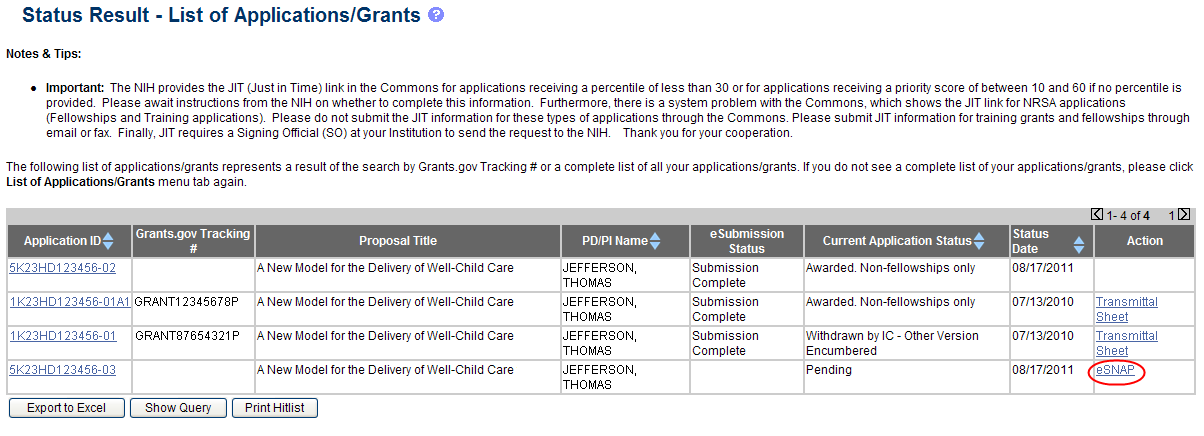
Figure 3: eSNAP Link on Status Result – List of Applications/Grants
NOTE: If the award is a Fellowship, it is eligible for RPPR only, and the RPPR link displays.
–OR–
Access RPPR from eSNAP tab:
Select the eSNAP tab from the Commons menu options.
The Manage eSNAP screen displays. Manage eSNAP is used to view the progress reports to which the user has access and allows the user to select a progress report in order to perform various actions. PD/PIs or users delegated PD/PI updating authority uses the Manage eSNAP screen to view their own progress reports. SOs and AOs use the screen to search for grants from their institutions and/or for grants routed to them for review.
Select the specific grant by clicking the hyperlink in the Grant Number column on the Manage eSNAP screen.
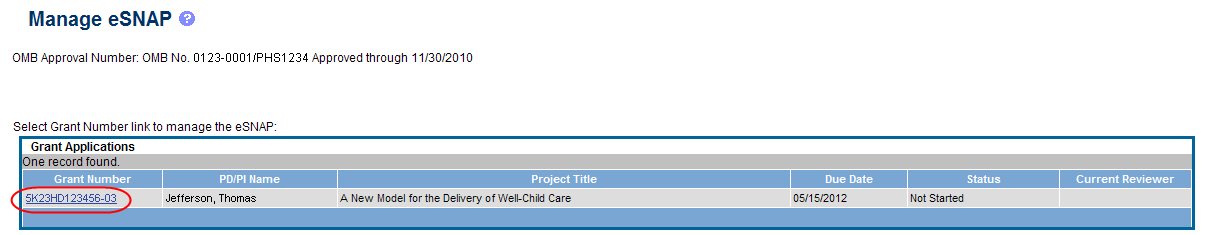
Figure 4: Manage eSNAP List of Grant Applications
During the pilot phase the following message displays:
Your organization has agreed to participate in the NIH RPPR pilot. You may choose to submit either an RPPR or eSNAP. You may not submit both.
NOTE: If the award is a Fellowship, it is eligible for RPPR only and this message does not display.
If an RPPR exists already, Commons displays the report for editing.
Select the RPPR button.

The RPPR Menu screen displays. The options for the uninitiated report are Initiate and Cancel. Once an RPPR is in progress, the buttons for other options are enabled. These options are discussed later, following the steps for initiation.
The RPPR Menu screen includes the following fields:
Grant Number
This is the complete number of the grant
Grantee Institution
This field contains the name of the applicant’s institution
PD/PI Name
The PD/PI of the grant award for which the progress report is being prepared. In the case of MPIs, a list of PD/PI names displays with the contact PD/PI indicated by the word Contact.
Project Title
The project title of the grant
Due Date
The electronic due date. The due date for awards issued under the SNAP (Streamlined Noncompeting Award Process) provisions is the 15th of the month preceding the month in which the budget period ends (e.g., if the budget period ends 11/30, the due date is 10/15). If the award is not issued under SNAP provisions, the progress report is due the first of the month preceding the month in which the budget period ends (e.g., if the budget period ends 11/30, the due date is 10/1). If the due date falls on a weekend or federal holiday, the due date is automatically extended to the next business day. Progress reports for Fellowships are due two months before the beginning date of the next budget period. Occasionally the Notice of Award (NoA) will indicate a different due date which will supersede these dates. Grantees should consult the NoA to determine when SNAP procedures apply.
Current Reviewer
The name of the current reviewer or organization (e.g., PD/PI name, NIH). This value is blank before the RPPR is initiated.
Status
The current state of the progress report. Possible values are as follows: Not Started, PD/PI Work in Progress, Reviewer Work in Progress, and Submitted to Agency.
Buttons
The displayed and enabled buttons vary depending on the status of the RPPR and/or the limitations of the current user’s role. The possible available actions include the following:
Initiate: Begins the RPPR process. Available for grants with a status of Not Started. Access is granted to PD/PIs and PD/PI delegates. An RPPR can be initiated even if required information in the Personal Profile and Institution Profile sections is missing. If any of this information is incorrect or missing, a prompt will appear to correct/complete the information after initiating the report. Processing may continue on the RPPR without making the corrections; however, the RPPR will not pass validations for submission to the agency until the errors are corrected.
Edit: Opens the RPPR for edits. Available for progress reports with a status of Work in Progress (WIP). Access is granted to PD/PIs or PD/PI delegates when the PD/PI is the current reviewer, AOs when the AO is the current reviewer, and SOs when the SO is the current reviewer. The Edit button allows the user to view and edit RPPR information.
View: Opens the RPPR report in PDF format, as it will be seen by the agency. Available for progress reports with a status of Work in Progress (WIP) or Submitted to Agency. Access is granted to PD/PIs, PD/PI delegates, and reviewers. Until the RPPR is submitted to agency, the PDF report shows a status of Draft and a blank submission date.
Check for Errors: Checks the RPPR for any errors or warnings. Available for progress reports with a status of WIP. Access is granted to any user with access to the grant. The RPPR can be validated at any time while in the status of WIP and can be validated multiple times.
View Routing History: Opens a page that displays a routing history table. Available for progress reports with a status of Work in Progress (WIP) or Submitted to Agency. Access is granted to PD/PIs, PD/PI delegates, and reviewers.
Route: Routes the RPPR to the next reviewer for further review or corrections. Available for progress reports with a status of Work in Progress (WIP). Access is granted to the current reviewer. A PD/PI delegate cannot route an RPPR to the next reviewer.
Recall: Recalls RPPRs that have been forwarded to another reviewer and resets the user as the current reviewer. Available for reports with a status of Work in Progress (WIP). Access is granted to the last reviewer (who recalls the report from the current reviewer). Signing Officials and PD/PIs can recall an RPPR even if they are not the last reviewer whenever it has a status of Reviewer Work in Progress. This is useful in situations when a RPPR has been routed to the wrong person or to someone who is unavailable.
Submit: Submits the RPPR to the Agency. Available for reports with a status of Work in Progress (WIP). Access is granted to the SO when the SO is the current reviewer and to the PD/PI when the PD/PI has been delegated eSNAP award submit authority.
Cancel: Closes the RPPR Menu screen and returns the user to the previous screen.
Select the Initiate button to begin the RPPR.
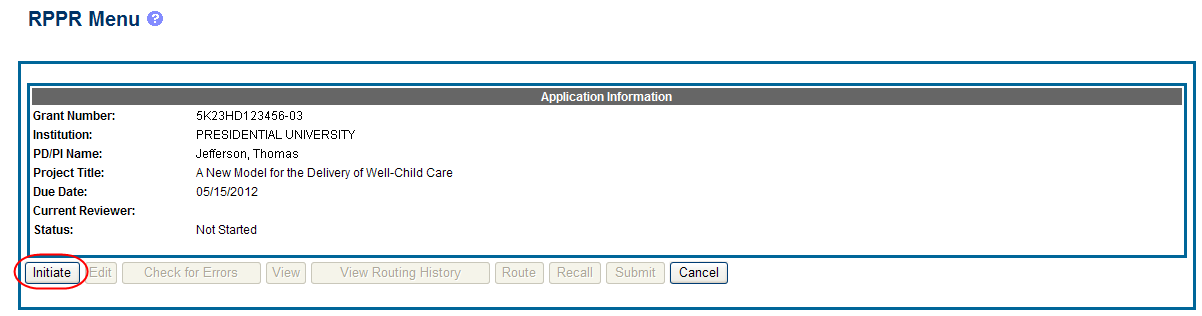
Figure 6: RPPR Menu for Initiating the Report
Once initiated, Commons creates the report in a PD/PI Work in Progress status and sets the current reviewer. A message displays as follows:
The RPPR has been successfully initiated.
NOTE: If at any time initiation fails due to business rules validations, error or warning messages display on the screen.
At any time before an RPPR is submitted to agency, an error check can be performed to verify that the report passes the business rules and system validations in place. Any user who has access to the RPPR may perform the error check.
If errors or a warning exist, the appropriate error or warning message displays for each failed occurrence.

If all validations pass, a message displays indicating: No errors found on validation.
To perform an error check on the RPPR, select the Error Check button from the RPPR Menu screen.
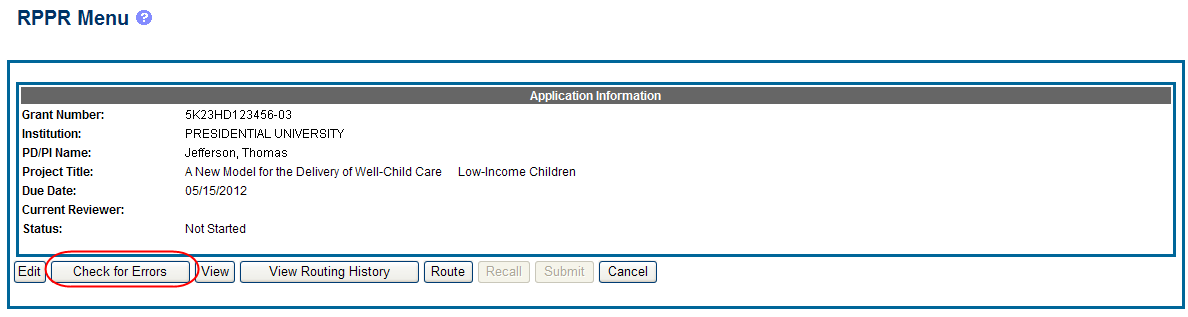
Figure 8: Check for Errors Button on RPPR Menu
All errors must be corrected prior to submission; the system will prevent submission of an RPPR containing errors. The only warning in the RPPR occurs when a publication is associated with the progress report in C.1. Publications that is not in compliance with the Public Access Policy (see 6.3 Section C). The Public Access compliance warning appears for the grantee’s information; the system will not prevent submission of an RPPR with this warning.
Once an RPPR is initiated, its status becomes PD/PI Work in Progress and it becomes available for editing. The PD/PI or delegate uses the Edit option for viewing and completing the report. Additionally, this option is available to the SO or AO when that user is the current reviewer of the report.
NOTE: For applications with multiple PD/PIs (MPI applications), only the Contact PD/PI has access to the Edit feature unless the Contact PD/PI has granted progress report authority to other PD/PIs. Without this authority, MPIs can only view the RPPR PDF and its routing history.
There are two means of accessing the progress report for editing. These are similar methods used for initiating the report and are as follows:
Access RPPR from Status:
Select the Status tab from the Commons menu options.
Select the List of Applications/Grants link from the Status screen.

Figure 9: Status Screen and List of Applications/Grants Links
From the Status Result – List of Applications/Grants screen, locate the application and select the RPPR link from the Action column for the specific application.

Figure 10: eSNAP Link on Status Result – List of Applications/Grants
–OR–
Access RPPR from eSNAP tab:
Select the eSNAP tab from the Commons menu options.
Select the specific grant by clicking the hyperlink in the Grant Number column on the Manage eSNAP screen. SOs/AOs must perform a query first.

Figure 11: Manage eSNAP List of Grant Applications
The RPPR Menu screen displays with buttons for the following available options:
Edit
Check for Errors
View
View Routing History
Route
Cancel
NOTE: Once an RPPR has been routed for review, the Recall and Submit buttons are enabled. These functions are covered in subsequent chapters.
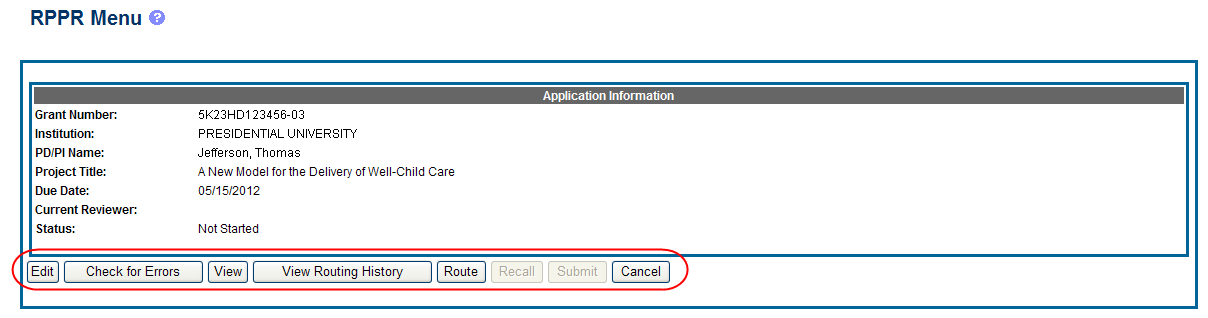
Select the Edit button to open the RPPR for editing.
The RPPR section A. Cover Page displays with information about the grant, PD/PI, signing and administrative officials, organization, and project/reporting/budget periods. For more information on the Cover Page, refer to the section of this document titled Section A – Cover Page located in the RPPR Component Instructions.
Update the information as necessary and select the Save button.
The Cover Page includes tabs at the top and links bottom of the page for navigating to the other reporting components (e.g., Participants, Accomplishments), which may be completed in any order. Before navigating to and from any of these component pages, it is always necessary to select the Save button to save all changes on the current page. Navigating away from any page on the RPPR without selecting Save results in the loss of any information entered prior to the last save.
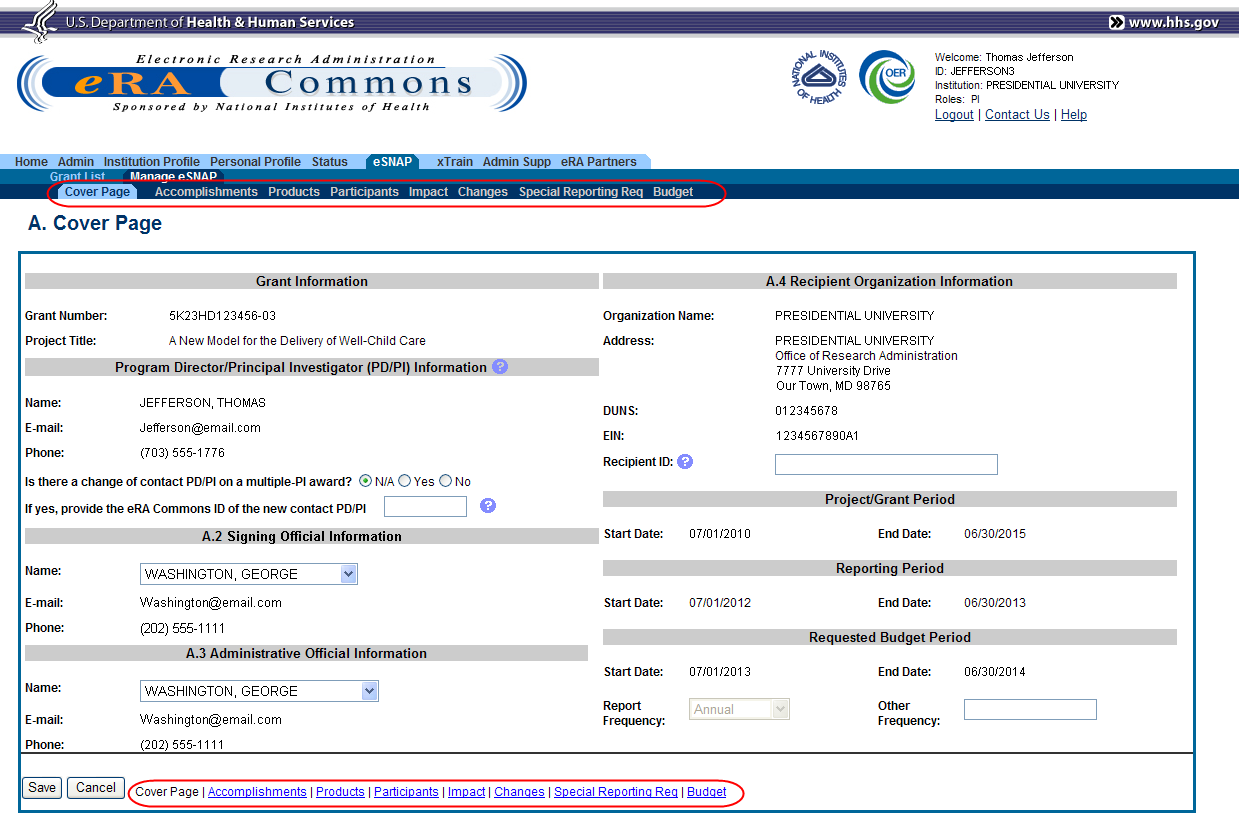
Figure 13: RPPR Cover Page and Component Navigation Links
Components can be completed in any order. To navigate and populate the other components of the RPPR, select the appropriate link from the top or bottom of the page.
The same navigational links appear on each component page of the RPPR. For information on the specific fields in each component refer to Chapter 6 RPPR Component Instructions.
Complete the appropriate fields of the report components.
Details for completing each component page are discussed later in this document. Many of the fields on these pages, however, behave in a similar manner and are discussed below.
Add/New
To use the Add/New feature, enter or select data into the appropriate fields. Select the Add/New button to add the data to a table.

Items can be edited or deleted from the table using the Action links.
Text Box
All text boxes on the RPPR have character limits. The number of characters available is reflected beneath each text box as characters are entered.
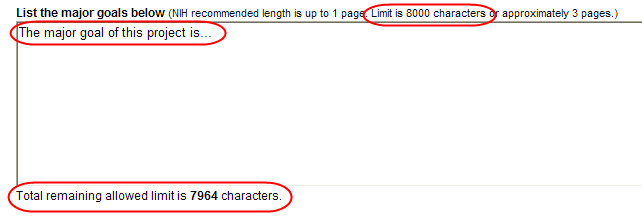
Figure 15: Total Remaining Characters
Changing Saved Responses
While in WIP status, answers may be changed. A warning message displays as follows:
The entered/uploaded response will be deleted. Do you wish to continue?
The user editing the information can choose to Continue or Cancel the action. Choosing Continue deletes the previous response, removes any attachments, and disables the relevant fields associated with the question. Choosing Cancel cancels the change.
Select the Save button before navigating to the next component page.
To return to the RPPR Menu, select the Cancel button.
When an RPPR is ready for review and submission, it is routed to the next reviewer. Refer to the section of this document titled Route the RPPR for steps on routing to the next reviewer.
Progress reports in Work in Progress (WIP) status can be routed to others for review or corrections by the current reviewer of the report. The routing feature is found on the RPPR Menu screen.
NOTE: A PD/PI delegate cannot route an RPPR to the next reviewer.
To route an RPPR to the next reviewer:
Select the Route button from the RPPR Menu screen.

Figure 16: RPPR Menu – Route Button
The Route RPPR to Next Reviewer screen displays. From this screen, the next reviewer can be chosen from a list of reviewers, and comments can be added.
Select a reviewer from the Next Reviewer drop-down list.
Optional: Enter comments in the Comments text box to provide information to the next reviewer.
Select the Submit button.
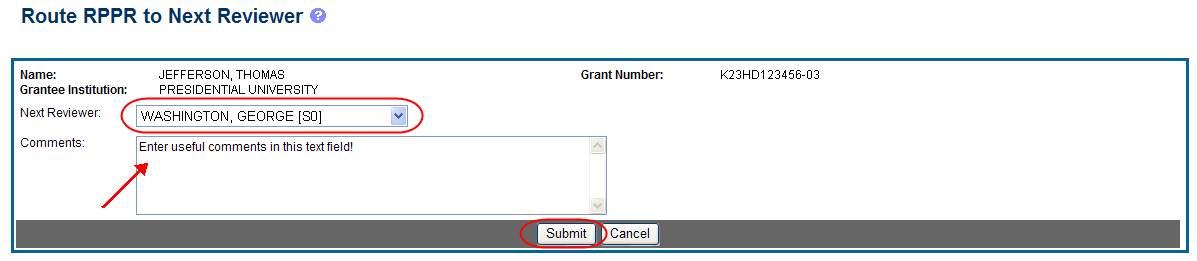
Figure 17: Route RPPR to Next Reviewer
When routed by the PD/PI only: The PD/PI Assurance statement displays. Select the I Agree button to continue.
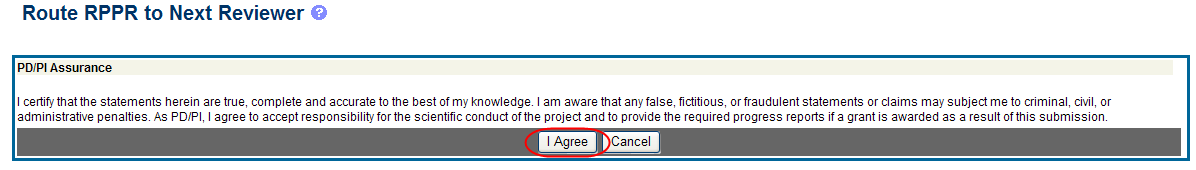
Figure 18: PD/PI Assurance Statement
The RPPR Menu displays once again. If the routing is successful, the message on the screen reads as follows:
The RPPR was successfully routed to [Selected Reviewer User ID], [Selected Reviewer Name].
The person who routed the RPPR can no longer edit the report (Edit button becomes disabled). The editing feature is now available only to the new reviewer. The RPPR status is updated to Reviewer Work in Progress.
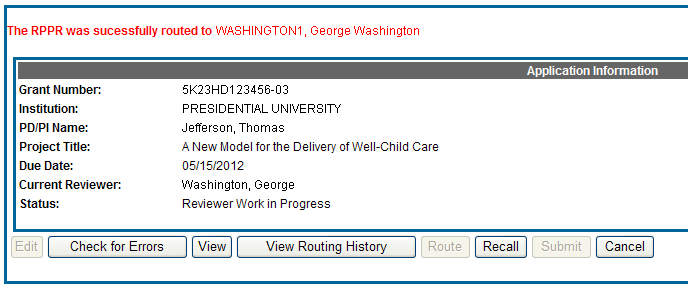
Figure 19: RPPR Successfully Routed
RPPRs that have been routed to a reviewer can be recalled by the person who performed the routing action. This is useful in situations when the report was routed to the wrong person or the reviewer is unavailable. The last reviewer of the report is able to recall it; however, Signing Officials at the Institution and the Contact PD/PI who are not the last reviewer can also recall the report when it is in a status of Reviewer Work in Progress.
NOTE: A PD/PI delegate does not have the ability to recall the RPPR.
To recall an RPPR, select the Recall button from the RPPR Menu screen.

Figure 20: RPPR Menu – Recall Button
A message displays on the screen indicating: The RPPR has been successfully recalled. You have been set as the Current RPPR Reviewer.
The status of the RPPR is updated to PD/PI Work in Progress or Reviewer Work in Progress, the reviewer from whom the RPPR is recalled receives an email informing him of the action, and the RPPR routing audit history is updated to reflect the action.
Additionally, the Edit and Route buttons are enabled, providing the new reviewer with the ability to continue editing the RPPR or to route it to another reviewer.
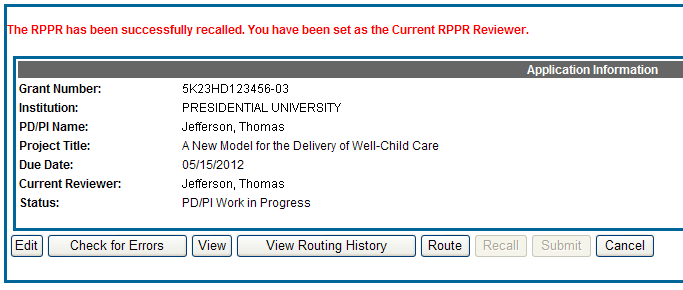
Figure 21: RPPR Successfully Recalled
Grantees are strongly encouraged to view the RPPR prior to submission to the NIH to ensure that the correct information and attachments are provided (see 5.7 View the RPPR).
Completed and validated RPPRs in a status of Work in Progress can be submitted to the Agency for acceptance. This act is performed by the Signing Official (SO) when the SO is the current reviewer of the report. PD/PIs may also submit the report if they have been delegated submit authority by the SO for SNAP awards.
To submit the RPPR to agency:
Select the Submit button from the RPPR Menu screen.
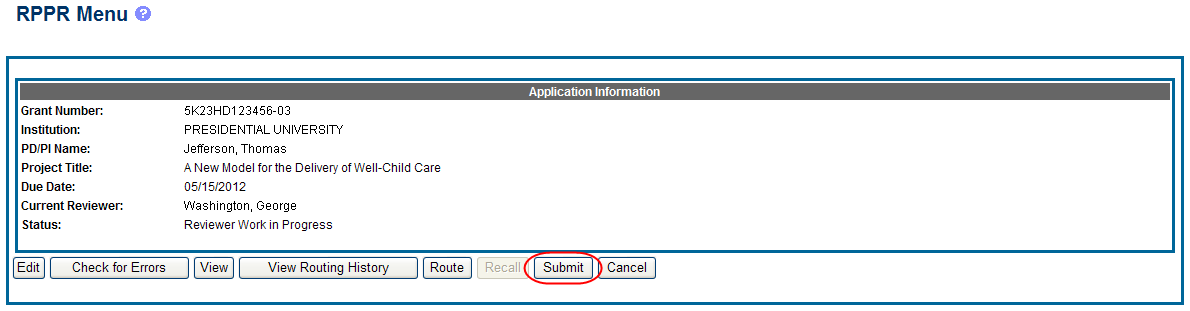
The Submit RPPR screen displays a certification statement as follows:
In submitting this RPPR, the SO (or PD/PI with delegated authority), certifies that the grantee organization is in compliance with the terms and conditions specified in the Notice of Award and Grants Policy Statement, and verifies the accuracy and validity of all administrative, fiscal, and scientific information in the progress report. The SO (or PD/PI with delegated authority) further certifies that the grantee organization will be accountable for the appropriate use of any funds awarded and for the performance of the grant-supported project or activities resulting from the progress report. Deliberate withholding, falsification, or misrepresentation of information could result in administrative actions such as withdrawal of a progress report, suspension and/or termination of an award, debarment of individuals, as well as possible criminal penalties. The grantee institution may be liable for the reimbursement of funds associated with any inappropriate or fraudulent conduct of the project activity.
Select the I Agree button to sign off on the certification.
The RPPR is validated for systemic and business rules. If there are any validation failures, they are indicated by error messages on the RPPR Menu screen. Errors must be corrected in order to submit the RPPR.
If warnings exist, they are displayed on the RPPR Menu screen. Although the RPPR can be submitted with warnings present, the warning messages should be reviewed to determine if an issue should be addressed.
If Warnings Exist: To address issues associated with warnings, select the Cancel button, correct the issue, and resubmit the RPPR again. To continue with submission despite the warnings, select the OK button.
If all validations pass, the RPPR Menu screen displays the following message: The RPPR has been successfully submitted to PHS.

Figure 22: Successful Submission Message
The current reviewer is updated to NIH, the RPPR status is updated to Submitted to Agency, and the RPPR Submission date is recorded. The routing history is updated to reflect the submission to Agency.
Any citations associated with the RPPR in C.1. Publications are officially associated with the award in MyNCBI.
If Inclusion Enrollment data was reported in the RPPR, this information is updated into the eRA Population Tracking system for NIH staff review and acceptance. The data then becomes the data of record for the particular grant year.
When an RPPR is submitted to Agency, email notification is sent to the PD/PI (Contact PI) on the grant and the SO and AO assigned to the RPPR.
As indicated in 5.6, grantees are strongly encouraged to view the RPPR prior to submission to the NIH to ensure that the correct information and attachments are provided (see 5.6 Submit the RPPR to Agency).
PD/PIs, PD/PI delegates, and reviewers can view a PDF version of an RPPR in Work in Progress (WIP) or Submitted to Agency status to see how it will be seen by the Agency. Until the RPPR is submitted to agency, the PDF report shows a status of Draft and a blank submission date.
To view the RPPR form, select the View button from the RPPR Menu screen.
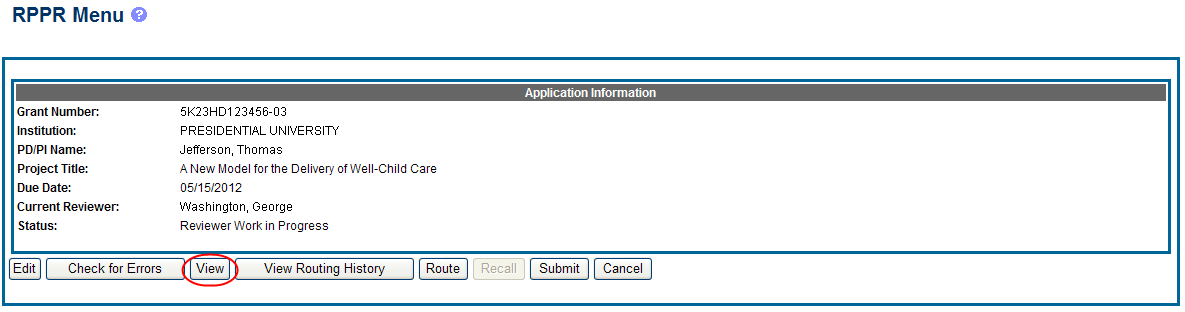
Figure 23: RPPR Menu – View Button
From initiation to submission to Agency, the routing of an RPPR is captured for auditing purposes. PD/PIs, PD/PI delegates, and reviewers can view the routing history for Work in Progress or Submitted to Agency RPPRs at any time, even when not they are not the current reviewer.
To view the routing history:
Select the View Routing History button from the RPPR Menu screen.
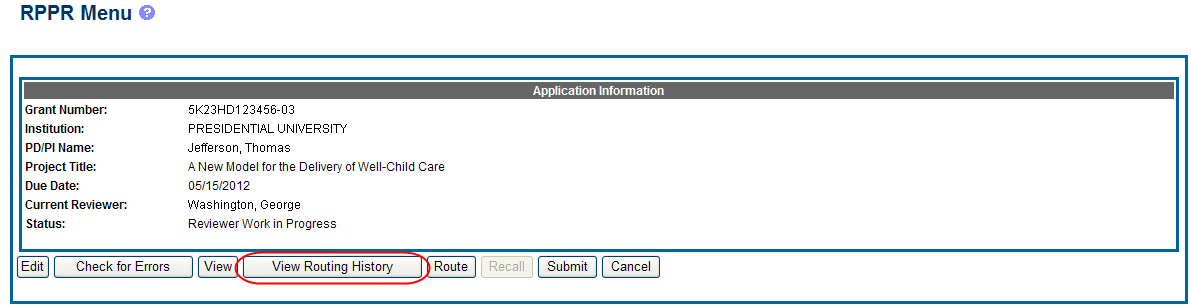
Figure 24: RPPR Menu – View Routing History Button
The Routing History screen displays showing the Reviewer Name, Action, Notification Sent (date and time), Date of Action, Next Reviewer Name, and Comments (when available).
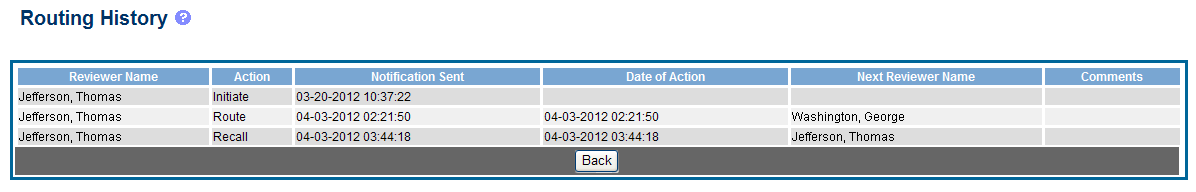
Figure 25: RPPR Routing History
To close the screen, select the Back button.
The final RPPR, in PDF format, is accessible in Commons within the Status Information screen. To view the final RPPR, perform the following steps:
From Commons, select the Status menu option.
Select the link for List of Applications/Grants.

From the Status Result – List of Applications/Grants screen, select the hyperlink for the specific Application ID.
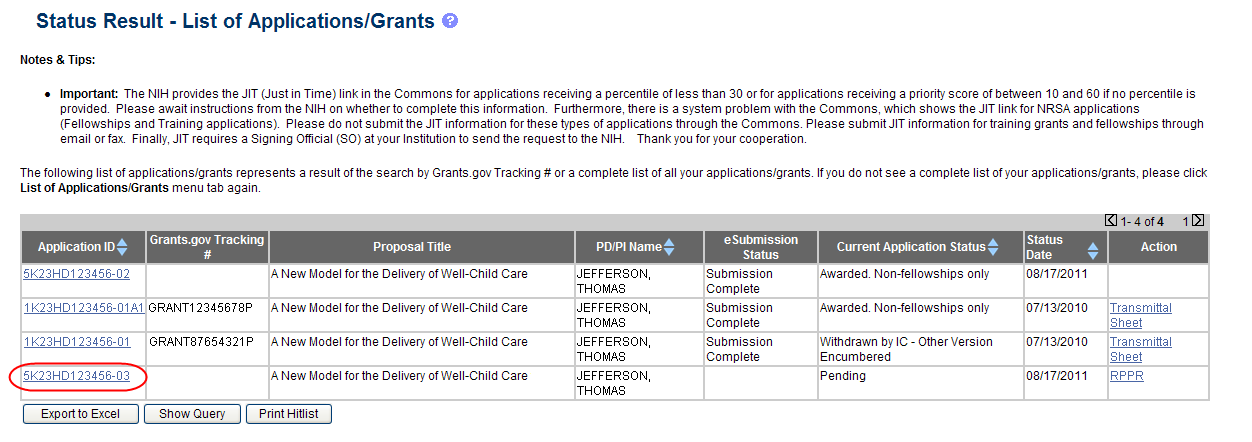
Figure 27: Application ID hyperlink
The Status Information screen displays with the Other Relevant Documents section in the top right corner.
Select the e-Application link from the Other Relevant Documents section of the Status Information screen.
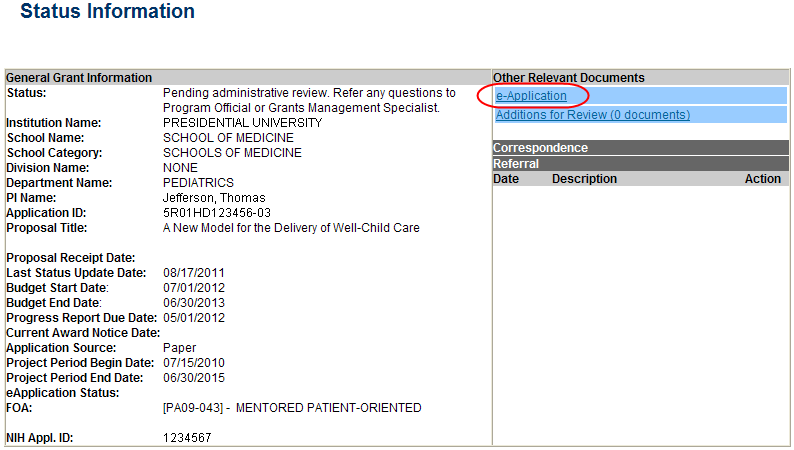
Figure 28: Status Information Screen – e-Application Link
The PDF version of the RPPR opens in a separate window.
NOTE: The submitted RPPR can also be accessed from the RPPR Menu screen. The View button opens the PDF version of the RPPR.
The instructions in this section apply to the following awards: D71, DP1, DP5, G08, G11, G13, P40, R00, R01, R03, R18, R21, R33, R34, R36, R37, R56, RC1, RC2, RL1, S10, S21, S22, SC1, SC2, SC3, UB1, UC2, UH1, UH2, UH3, UP5. Many of these instructions apply to other awards but there may be exceptions (items that are not applicable, replace, or are in addition) for awards not listed above. Refer to the table in chapter 7 Supplemental Instructions and follow the appropriate instruction for the applicable activity code of other awards. The electronic RPPR display is dynamic and shows the appropriate questions and instructions based on the activity code and Streamlined Non-competing Award Process (SNAP) status of the award.
![]() Agency-specific
reporting requirements and instructions are denoted by the DHHS logo
displayed to the left of the requirement or instruction, as
illustrated here.
Agency-specific
reporting requirements and instructions are denoted by the DHHS logo
displayed to the left of the requirement or instruction, as
illustrated here.
Not Applicable next to a particular item indicates that item does not apply to the particular kind of award, and the item should be ignored.
References to competing application instructions means either the SF424 (R&R) Application Guides (http://grants.nih.gov/grants/funding/424/index.htm) or the PHS 398 Grant Application (http://grants.nih.gov/grants/funding/phs398/phs398.html).
The RPPR may not be used for prior approval requests, with the exception of requesting prior approval for a reduction in the level of effort of the PD/PI or other senior/key personnel named in the Notice of Award under D.2 of the RPPR. All other prior approval requests must be submitted directly to the Grants Management Officer of the awarding component in accord with the Grants Policy Statement, 8.1.2.
The RPPR section A. Cover Page includes information about the award, PD/PI, organization, and project/reporting/budget periods. Much of this information is pre-populated from data in NIH systems, but certain fields are editable.
The addresses, emails and phone numbers are pre-populated from the Commons Profile. To update contact information as displayed, go to the Commons Profile and save the changes there.
To select a Signing Official and Administrative Official, choose a name from the associated drop-down box. The SO and AO may be the same individual. The SO need not be the SO that submits the RPPR.
If there is a change to the Contact PD/PI (Multiple-PD/PI awards only), select the Yes radio button and enter the Commons ID of the new Contact PD/PI in the associated field. The change in Contact PD/PI does not take effect until the agency accepts the report and issues an NoA. The Contact PD/PI must have a PD/PI role in the eRA Commons and must be associated with the grantee institution. The RPPR is not an appropriate vehicle for a prior approval request to change, add, or delete PD/PIs.
The Recipient ID field allows the grantee to record an internal tracking number or identifier for its own use. It is not a mandatory field and NIH will disregard the information.
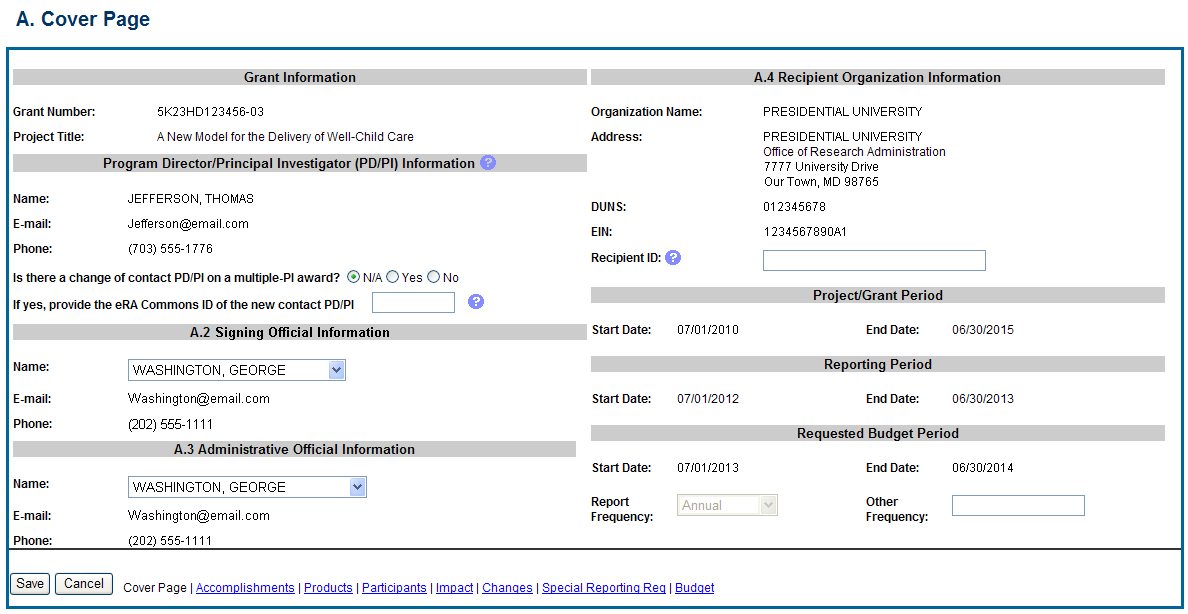
Figure 29: RPPR Section A. Cover Page
The RPPR section B. Accomplishments allows the agency to assess whether satisfactory progress has been made during the reporting period.
PD/PIs are reminded that the grantee is required to obtain prior written approval from the awarding agency grants official whenever there are significant changes in the project or its direction. See agency specific instructions for submission of these requests.
B.1 What are the major goals of the project?
List the major goals of the project as
stated in the approved application or as approved by the agency. If
the application lists milestones/target dates for important
activities or phases of the project, identify these dates and show
actual completion dates or the percentage of completion.
Generally,
the goals will not change from one reporting period to the next.
However, if the awarding agency approved changes to the goals during
the reporting period, list the revised goals and objectives. Also
explain any significant changes in approach or methods from the
agency approved application or plan.
![]() Goals
are equivalent to specific aims. Significant changes in
objectives and scope require prior approval of the agency (e.g., NIH
Grants Policy Statement, 8.1.2).
Goals
are equivalent to specific aims. Significant changes in
objectives and scope require prior approval of the agency (e.g., NIH
Grants Policy Statement, 8.1.2).
The specific aims must be provided in the initial RPPR (i.e., first non-competing type 5 submission). In subsequent RPPRs this section will pre-populate with the aims/goals previously entered, and may be amended by answering Yes to question B.1.a.
![]() B.1.a
Have the major goals changed since the initial competing award or
previous report?
B.1.a
Have the major goals changed since the initial competing award or
previous report?
Select Yes if the major goals/specific aims have changed since the initial competing award or previous report, and provide a revised description of major goals/specific aims. Remember that written prior approval from the awarding agency grants official is required for significant changes in the project or its direction. The RPPR is not an appropriate vehicle to request such a change.
The first year that an RPPR is submitted any revised goals should be entered into the text box for B.1. In subsequent years, if the user selects Yes the text box under B.1.a for entering revised major goals will be provided.
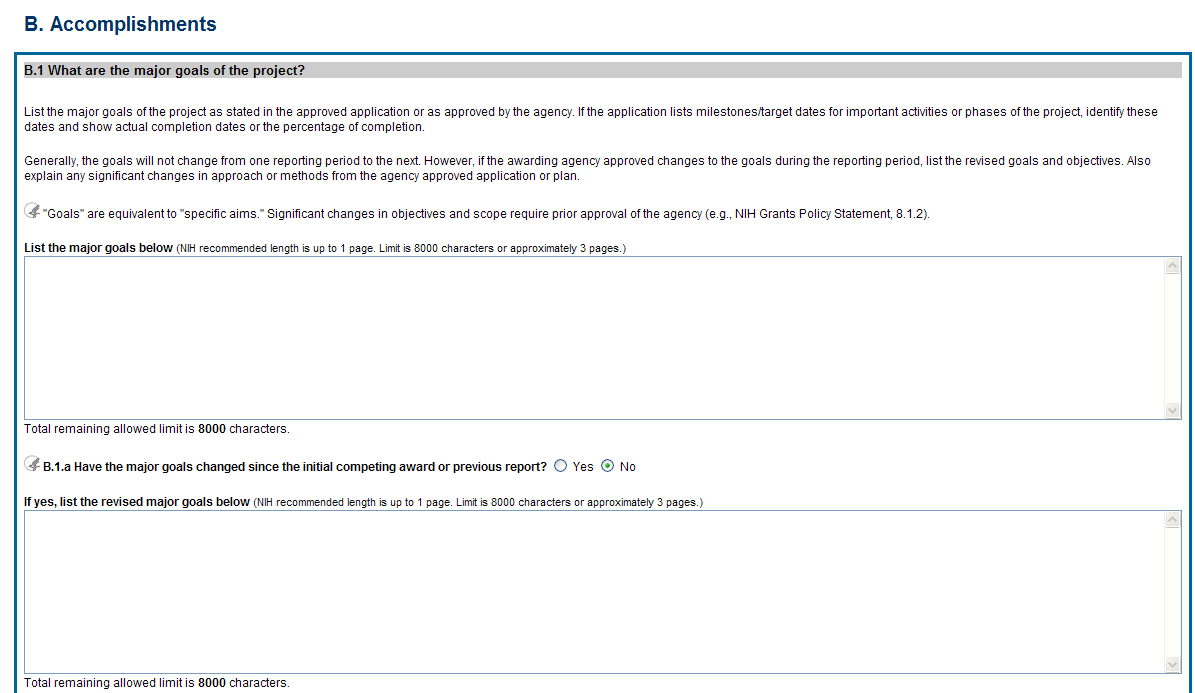
Figure 30: RPPR Section B. Accomplishments – Question B1
B.2 What was accomplished under these goals?
For this reporting period describe: 1) major activities; 2) specific objectives; 3) significant results, including major findings, developments, or conclusions (both positive and negative); and 4) key outcomes or other achievements. Include a discussion of stated goals not met. As the project progresses, the emphasis in reporting in this section should shift from reporting activities to reporting accomplishments.
![]() Goals
are equivalent to specific aims. In the response, emphasize
the significance of the findings to the scientific field. For most
NIH awards the response should not exceed 2 pages.
Goals
are equivalent to specific aims. In the response, emphasize
the significance of the findings to the scientific field. For most
NIH awards the response should not exceed 2 pages.
![]() B.3
Competitive Revisions/Administrative Supplements.
B.3
Competitive Revisions/Administrative Supplements.
For this reporting period, is there one or more Revision/Supplement associated with this award for which reporting is required?
If yes, identify the Revision(s)/Supplements(s) by grant number (e.g., 3R01CA098765-01S1) or title and describe the specific aims and accomplishments for each Revision/Supplement funded during this reporting period. Include any supplements to promote diversity or re-entry, or other similar supplements to support addition of an individual or a discrete project.
The NoA will indicate any reporting requirements. Be advised that the NoA incorporates requirements of the FOA that may also include reporting requirements.
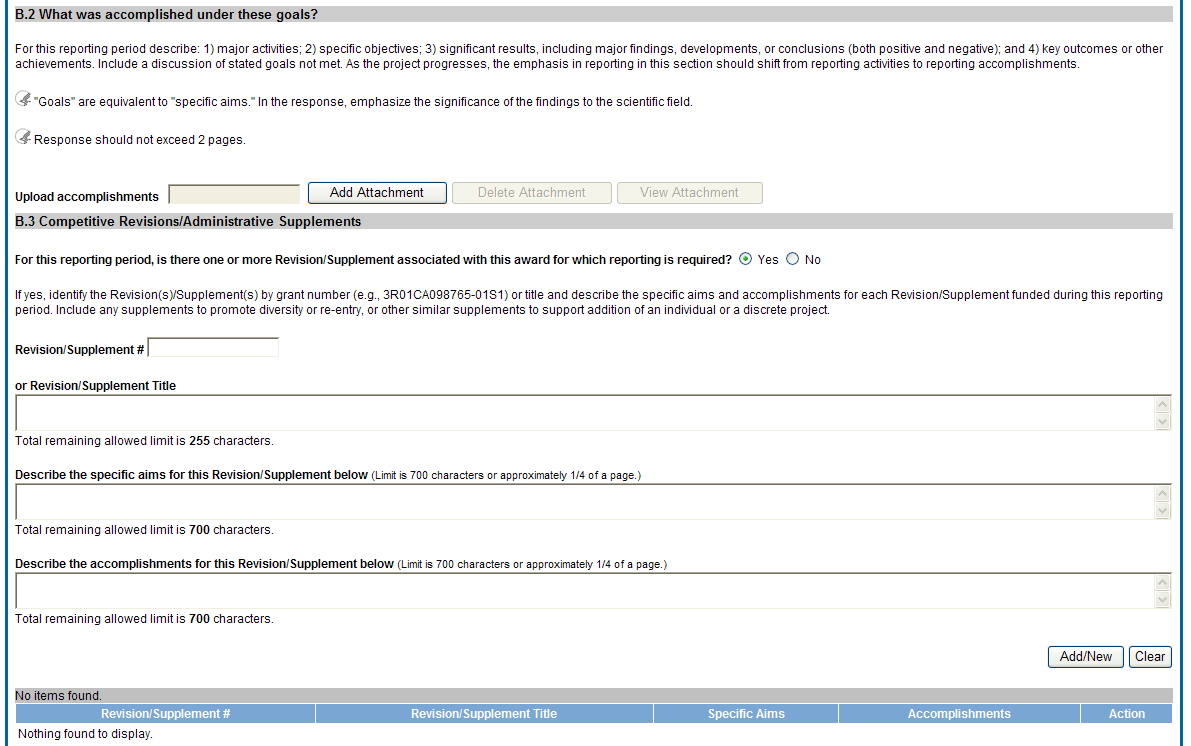
Figure 31: RPPR Section B. Accomplishments – Questions B2 & B3
B.4 What opportunities for training and professional development has the project provided?
If the research is not intended to provide training and professional development opportunities or there is nothing significant to report during the reporting period, select Nothing to Report.
Describe opportunities for training and professional development provided to anyone who worked on the project or anyone who was involved in the activities supported by the project. Training activities are those in which individuals with advanced professional skills and experience assist others in attaining greater proficiency. Training activities may include, for example, courses or one-on-one work with a mentor. Professional development activities result in increased knowledge or skill in one’s area of expertise and may include workshops, conferences, seminars, study groups, and individual study. Include participation in conferences, workshops, and seminars not listed under major activities.
![]() For
T, F, K, R25, R13, D43 and other awards or award components designed
to provide training and professional development opportunities, a
response is required. Do not reiterate what is reported under
Accomplishments. Limit the response to this reporting period.
For
T, F, K, R25, R13, D43 and other awards or award components designed
to provide training and professional development opportunities, a
response is required. Do not reiterate what is reported under
Accomplishments. Limit the response to this reporting period.

Figure 32: RPPR Section B. Accomplishments – Question B4
B.5 How have results been disseminated to communities of interest?
Describe how the results have been disseminated to communities of interest. Include any outreach activities that have been undertaken to reach members of communities who are not usually aware of these research activities, for the purpose of enhancing public understanding and increasing interest in learning and careers in science, technology, and the humanities.
![]() Reporting
the routine dissemination of information (e.g., websites, press
releases) is not required. For awards not designed to disseminate
information to the public or conduct similar outreach activities, a
response is not required and the grantee should select Nothing to
Report. A detailed response is only required for awards or award
components that are designed to disseminate information to the public
or conduct similar outreach activities. Note that scientific
publications and the sharing of research resources will be reported
under Products.
Reporting
the routine dissemination of information (e.g., websites, press
releases) is not required. For awards not designed to disseminate
information to the public or conduct similar outreach activities, a
response is not required and the grantee should select Nothing to
Report. A detailed response is only required for awards or award
components that are designed to disseminate information to the public
or conduct similar outreach activities. Note that scientific
publications and the sharing of research resources will be reported
under Products.

Figure 33: RPPR Section B. Accomplishments – Question B5
B.6 What do you plan to do for the next reporting period to accomplish the goals?
Describe briefly what you plan to do during the next reporting period to accomplish the goals and objectives.
![]() Remember
that significant changes in objectives and scope require prior
approval of the agency (e.g., NIH Grants Policy Statement, 8.1.2.).
Remember
that significant changes in objectives and scope require prior
approval of the agency (e.g., NIH Grants Policy Statement, 8.1.2.).
Include any important modifications to the original plans. Provide a scientific justification for any changes involving research with human subjects or vertebrate animals. A detailed description of such changes must be provided under Section F. Changes.
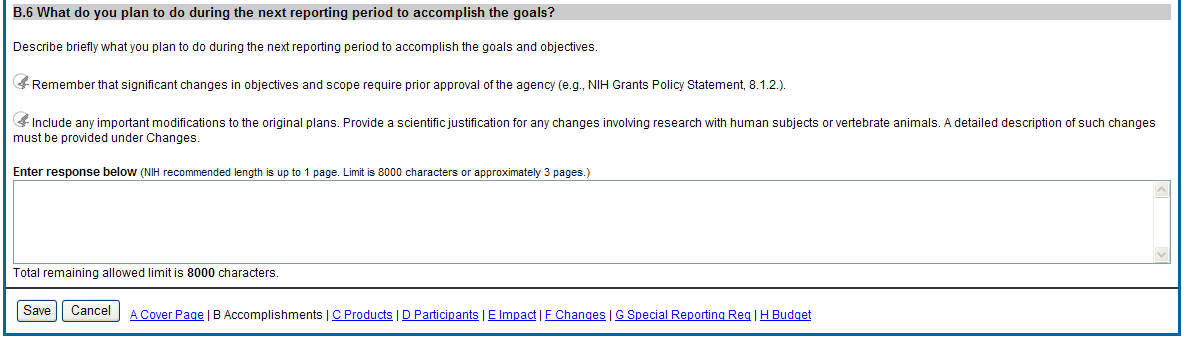
Figure 34: RPPR Section B. Accomplishments – Question B6
The RPPR section C. Products allows agencies to assess and report both publications and other products to Congress, communities of interest, and the public.
C.1 Publications.
Are there publications or manuscripts accepted for publication in a journal or other publication (e.g., book, one-time publication, monograph) during the reporting period resulting directly from the award?
PD/PIs are required to report all publications that arise from their NIH award in this section. Publications listed in other parts of the RPPR will not be tracked as award products. If there are publications to report select Yes and ensure that the Associate with this RPPR box is checked as appropriate. If there are no publications to report select No. The tables draw information from the PD/PI’s My NCBI account. PD/PIs can log in to their My NCBI account via the My NCBI link at the top of C.1. Any changes they make to their My Bibliography collection will be reflected in the RPPR once the screen is refreshed (i.e., by clicking the Save button).
For more information on My NCBI, see:
Get Started with My NCBI: Access My NCBI, Register, and Sign In
Edit Your My Bibliography Settings (Add a Delegate)
The first table, All Publications Associated with this Project in My NCBI, lists all publications that are in the PD/PI’s My Bibliography collection, are associated with this award, and have not been reported in previous electronic progress reports for this award.
The first column Associate with this RPPR is automatically checked. Leaving the box checked upon submission associates the publication with this progress report, results in the publication being displayed in RePORT, and makes the award-publication association in My NCBI permanent and the association will be reported in PubMed. Unchecking the box disassociates the publication with this progress report and, upon submission of the RPPR to NIH, removes the award-publication association in My NCBI .
The second column, NIH Public Access Compliance, indicates the current compliance status with the NIH Public Access Policy. This information is from My NCBI. Publications that fall under the Public Access Policy and are non-compliant still must be reported. Generally, publications can be brought into compliance within 10 business days; PD/PIs are advised to do so as soon as possible to ensure their award is renewed in a timely manner. For more information, see Manage Compliance with the NIH Public Access Policy in My NCBI and the NIH Public Access website.
The second table, Publications not associated with this project in MyNCBI, lists all other publications that are in the PD/PI’s My Bibliography collection but do not have an association with this award. Checking Associate with this RPPR box will associate a publication with the award both in the progress report and in My NCBI. Refreshing this screen (i.e., clicking the Save button) will also move the newly associated publications from this table to the first table. Similarly, publications disassociated in the first table will appear in this table when the screen is refreshed.
The final table, Publications previously reported for this project, lists publications reported in a previous electronic progress report for this award. Grantees are responsible for ensuring that these publications comply with the Public Access policy even if they were provisionally compliant (listed as in Progress) when previously reported.
Publications listed in other parts of a progress report are not captured electronically. They will not be included in this table, and may not be listed as resulting from this award in RePORT.
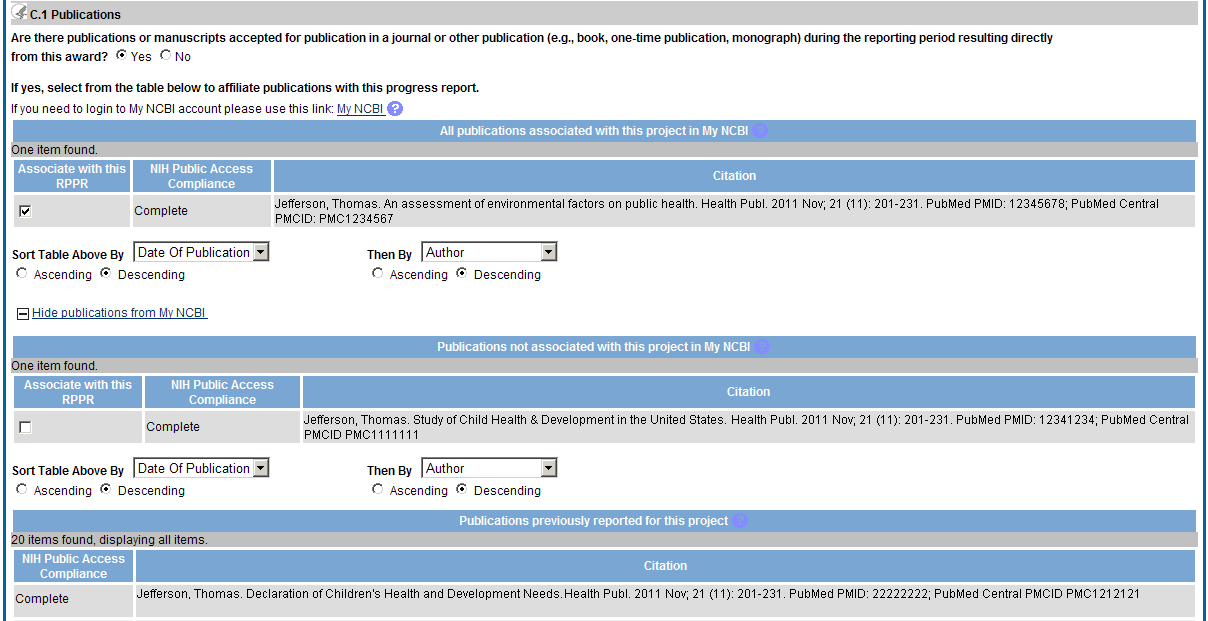
Figure 35: RPPR Section C. Products – Question C1
C.2 Website(s) or other internet site(s).
List the URL for any Internet site(s) that disseminates the results of the research activities. A short description of each site should be provided. It is not necessary to include the publications already specified above.
![]() For
awards not designed to create or maintain one or more websites,
select Nothing to Report. A description is only required for
awards designed to create or maintain one or more websites. Limit
the response to this reporting period.
For
awards not designed to create or maintain one or more websites,
select Nothing to Report. A description is only required for
awards designed to create or maintain one or more websites. Limit
the response to this reporting period.
C.3 Technologies or techniques.
Identify technologies or techniques that have resulted from the research activities. Describe the technologies or techniques and how they are being shared.
![]() Limit
the response to this reporting period.
Limit
the response to this reporting period.

Figure 36: RPPR Section C. Products – Questions C2 & C3
![]() C.4
Inventions, patent applications and/or licenses.
C.4
Inventions, patent applications and/or licenses.
Have inventions, patent applications and/or licenses resulted from the award during this reporting period?
If yes, has this information been previously provided to the PHS or to the official responsible for patent matters at the grantee organization?
Reporting of inventions through iEdison is strongly encouraged.
C.5 Other products and resources.
C.5 .a Other products
Identify any other significant products that were developed under this project.
![]() Describe
the product and how it is available to be shared with the research
community. Do not repeat information provided above. Limit the
response to this reporting period.
Describe
the product and how it is available to be shared with the research
community. Do not repeat information provided above. Limit the
response to this reporting period.
Examples of other products are: audio or video products; data and research material (e.g., cell lines, DNA probes, animal models); databases; educational aids or curricula; instruments or equipment; models; protocols; and software or netware.
![]() C.5.b
Resource Sharing
C.5.b
Resource Sharing
PD/PIs and grantee organizations are expected to make the results and accomplishments of their activities available to the research community and to the public at large. For additional information on NIH Sharing Policies and Related Guidance on NIH-Funded Research Resources see http://grants.nih.gov/grants/sharing.htm.
If the initial research plan addressed, or the terms of award require, a formal plan for sharing final research data, model organisms, Genome Wide Association Studies data, or other such project-specific data, describe the progress in implementing that plan. For sharing model organisms, include information on the number of requests received and number of requests fulfilled during this reporting period. If the sharing plan is fully implemented, provide a final statement on data sharing.

Figure 37: RPPR Section C. Products – Questions C4 & C5
The RPPR Section D. allows the agency to know who has worked on the project to gauge and report performance in promoting partnerships and collaborations.
D.1 What individuals have worked on the project?
Provide or update the information for: (1) program director(s)/principal investigator(s) (PDs/PIs); and (2) each person who has worked at least one person month per year on the project during the reporting period, regardless of the source of compensation (a person month equals approximately 160 hours or 8.3% of annualized effort).
Provide the name and identify the role the person played in the project. Indicate the nearest whole person month (Calendar, Academic, Summer) that the individual worked on the project. Show the most senior role in which the person has worked on the project for any significant length of time. For example, if an undergraduate student graduates, enters graduate school, and continues to work on the project, show that person as a graduate student.
![]() NIH
Instructions:
NIH
Instructions:
An individual's Commons user ID may be used to partially populate his or her information
A Commons ID is required for all individuals with a postdoctoral role
Individuals with a postdoctoral-like role should be identified as Postdoctoral (scholar, fellow, or other postdoctoral position)
Do not include Other Significant Contributors who are not committing any specified measurable effort to this project
Do not report personnel for whom a PHS 2271 Appointment form has been submitted through xTrain
Required fields are marked with an *
eRA Commons User ID: Entering the User ID allows selection of “Populate from Profile” which will partially populate the individual’s information. Those with an Administrator role in the eRA Commons may search for user IDs by following the instructions at:
http://era.nih.gov/commons/commons-help/1001.htm
Senior/key personnel are defined as the PD/PI and other individuals who contribute to the scientific development or execution of a project in a substantive, measurable way, whether or not they receive salaries or compensation under the grant. Typically these individuals have doctoral or other professional degrees, although individuals at the masters or baccalaureate level may be considered senior/key personnel if their involvement meets this definition. Consultants and those with a postdoctoral role also may be considered senior/key personnel if they meet this definition.
Last 4 digits of SS# and Month/Year of birth: The provision of the partial Social Security number and month/year of birth are voluntary, and the information is used only for program management purposes.
Project Role: PD/PI names and information from their Commons Profile(s) will be prepopulated. To update the PD/PI information as displayed, go to the Commons Profile and save the changes there. For all other personnel, select from a dropdown menu of the following options:
Co-Investigator
Faculty
Postdoctoral (scholar, fellow or other postdoctoral position)
Technician
Staff Scientist (doctoral level)
Statistician
Graduate Student (research assistant)
Non-Student Research Assistant
Undergraduate Student
High School Student
Consultant
Other (specify)
Supplement Support: If personnel are supported by a Reentry or Diversity Supplement indicate type of supplement in this field.
Person Months: The metric for expressing the effort (amount of time) devoted to a specific project. The effort is based on the type of appointment of the individual with the organization; e.g., calendar year, academic year, and/or summer term; and the organization's definition of such. For instance, some institutions define the academic year as a 9-month appointment while others define it as a 10-month appointment.
Include (1) the PD/PI regardless of effort devoted to the project and (2) each person who has worked at least one person month per year on the project during the reporting period, regardless of the source of compensation.
Round to the nearest whole person month that the individual worked on the project. For example, if the individual worked 2.25 person months, indicate 2 person months. If the individual worked 4.7 person months, indicate 5 person months. If the PD/PI worked 0.5 to 1 person month, round up to 1 person month. If the PD/PI worked 0.1 to 0.4 person month, round down to 0 (zero).
To calculate person months, multiply the percentage of effort associated with the project by the number of months of the appointment. For example:
25% of a 9 month academic year appointment equals 2.25 (academic year) person months (.25 x 9 = 2.25). Round down to 2.
90% of a 12 month calendar appointment equals 10.8 (calendar year) person months (.90 x 12 = 10.8). Round up to 11.
35% of a 3 month summer term appointment equals 1.05 (summer) person months (.35 x 3= 1.05). Round down to 1.
If the regular pay schedule of an institution is a 9 month academic year and the PD/PI will devote 9 academic months at 30% time/effort and 3 months summer term at 30% time/effort , then 3 academic months (.30% x 9 = 2.7, round up to 3), and 1 summer month (.30 x 3 = .9, round up to 1) should be reported
Person months reported on the RPPR are intentionally rounded to the nearest whole number to provide for generalized reporting consistent across federal agencies that support research activities. Although it is possible to report 0 (zero) person month for the PD/PI on the RPPR if the PD/PI worked .1 to .4 person month, a PD/PI must have measurable effort. Change in Level of Effort for the PD/PI(s) and other senior key/personnel designated in the NoA is reported under D.2.a below.
![]() Is
the individual’s primary affiliation with a foreign
organization?
Is
the individual’s primary affiliation with a foreign
organization?
Check No if the individual’s primary affiliation is with a foreign organization but the individual is working on this award solely while in the U.S.
If Yes, provide the name of the organization and country.

Figure 38: RPPR Section D. Participants – Question D1
![]() D.2
Personnel Updates.
D.2
Personnel Updates.
D.2.a Level of effort.
Will there be, in the next budget period, either (1) a reduction of 25% or more in the level of effort from what was approved by the agency for the PD/PI(s) or other senior/key personnel designated in the Notice of Award, or (2) a reduction in level of effort below the minimum amount of effort required by the Notice of Award?
Reductions are cumulative, i.e., the 25% threshold may be reached by two or more successive reductions that total 25% or more. Once agency approval has been given for a significant change in the level of effort, then all subsequent reductions are measured against the approved adjusted level. Selecting Yes constitutes a prior approval request to the agency and the issuance of a subsequent year of funding constitutes agency approval of the request.
D.2.b New senior/key personnel.
Are there, or will there be, new senior/key personnel?
Senior/key personnel are those identified by the grantee institution as individuals who contribute in a substantive measurable way to the scientific development or execution of the project, whether or not salaries are requested. Typically these individuals have doctoral or other professional degrees, although individuals at the masters or baccalaureate level may be considered senior/key personnel if the involvement meets this definition. Consultants may be considered senior/key personnel if they meet this definition.
If yes, upload biosketches and other support for all new senior/key personnel.
Follow the biosketch instructions in the competing application guide and provide active other support for all new senior/key personnel. Combine all biosketches and other support into a single PDF.
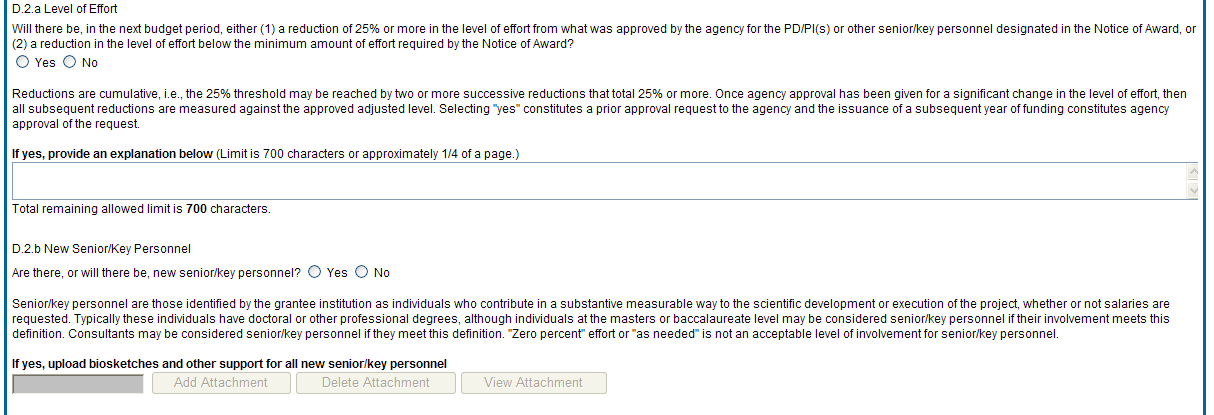
Figure 39: RPPR Section D. Participants – Questions D2a & D2b
D.2.c Changes in other support.
Has there been a change in the active other support of senior/key personnel since the last reporting period?
If yes, upload active other support for senior/key personnel whose support has changed and indicate what the change has been.
Select Yes only if active support has changed for the PD/PI(s) or senior/key personnel.
If a previously active grant has terminated and/or if a previously pending grant is now active, submit complete Other Support information using the suggested format and instructions found at http://grants.nih.gov/grants/funding/2590/2590othersupport.doc. Annotate this information so it is clear what has changed from the previous submission.
Submission of other support information is not necessary if support is pending or for changes in the level of effort for active support reported previously.
Other support information should be submitted only for the PD/PI and for those individuals considered by the grantee to be key to the project for whom there has been a change in other support. Senior/key personnel are defined as individuals who contribute in a substantive measurable way to the scientific development or execution of the project, whether or not a salary is requested. Do not include other support information for Other Significant Contributors; e.g., those that may contribute to the scientific development or execution of the project, but are not committing any specified measurable effort to the project.
D.2.d New other significant contributors.
Are there, or will there be, new other significant contributors?
Other significant contributors are individuals who have committed to contribute to the scientific development or execution of the project, but are not committing any specified measurable effort (i.e., person months) to the project.
If yes, upload biosketches for all new other significant contributors.
D.2.e Will there a change in the MPI Leadership Plan for the next budget period?
Change in status of PD/PI requires prior approval of the agency (e.g., NIH Grants Policy Statement, 8.1.2.6).
If yes, upload a revised MPI Leadership Plan that includes a description of the change(s).
All multiple PD/PI awards have a Leadership Plan that describes the roles and areas of responsibility of the named PD/PIs, the process for making decisions concerning scientific directions, allocation of resources, disputes that may arise, and other information related to the management of the proposed team science project. If there has been any change in the governance and/or organizational structure of the Leadership Plan, provide a description, including communication plans and procedures for resolving conflicts, and any changes to the administrative, technical, and scientific responsibilities of the PD/PIs. If the progress report includes a change in the contact PD/PI (Cover Page, A.1) address this change and the impact, if any, the change has on the administrative, technical, and scientific responsibilities of the PD/PIs. A request to change from a multiple PD/PI model to a single PD/PI model, or a change in the number or makeup of the PD/PIs on a multiple PD/PI award, requires the prior approval of the GMO. The progress report is not the appropriate vehicle to request such a change.
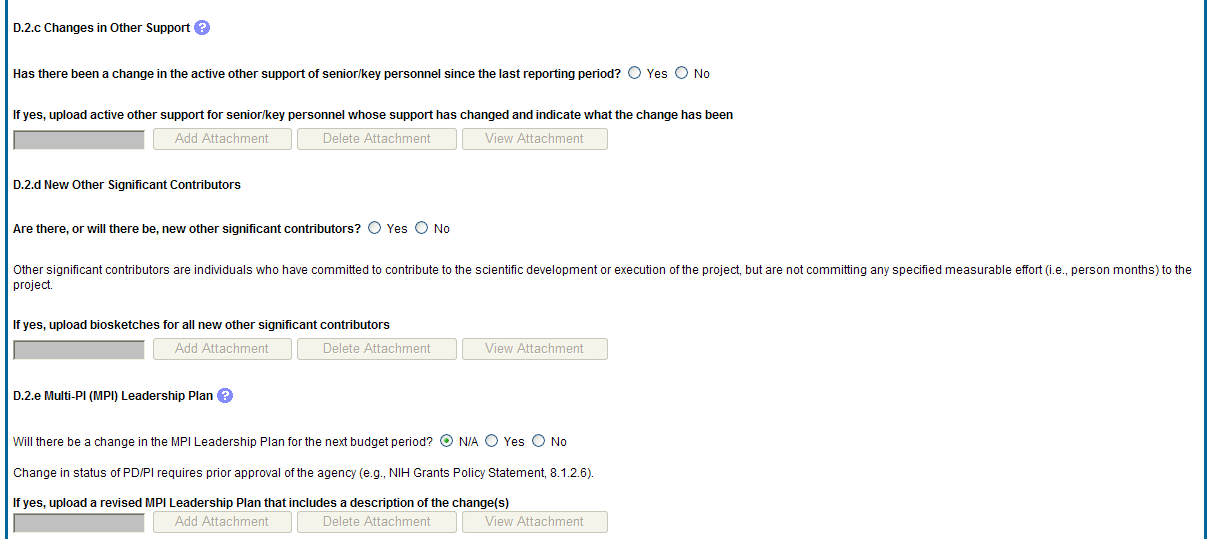
Figure 40: RPPR Section D. Participants – Questions D2c – D2e
The RPPR Section E Impact will be used to describe ways in which the work, findings, and specific products of the project have had an impact during this reporting period.
E.1 Not Applicable for most awards. See chapter 7 Supplemental Instructions.
E.2 What is the impact on physical, institutional, or information resources that form infrastructure?
Describe ways, if any, in which the project made an impact, or is likely to make an impact, on physical, institutional, and information resources that form infrastructure, including:
physical resources (such as facilities, laboratories, or instruments);
institutional resources (such as establishment or sustenance of societies or organizations); or
information resources, electronic means for accessing such resources or for scientific communication, or the like.
![]() If
the award or award component(s) is not intended to support physical,
institutional, or information resources that form infrastructure,
select Nothing to Report.
If
the award or award component(s) is not intended to support physical,
institutional, or information resources that form infrastructure,
select Nothing to Report.
E.3 Not Applicable for most awards. See chapter 7 Supplemental Instructions.
E. 4 What dollar amount of the award’s budget is being spent in foreign country(ies)?
![]() For
domestic awardees provide the dollar amount obligated to first-tier
subawards to foreign entities for this reporting period. For foreign
awardees provide the dollar amount of the award, excluding all
first-tier subawards to U.S. entities, for this reporting period.
Dollars provided should reflect total costs.
For
domestic awardees provide the dollar amount obligated to first-tier
subawards to foreign entities for this reporting period. For foreign
awardees provide the dollar amount of the award, excluding all
first-tier subawards to U.S. entities, for this reporting period.
Dollars provided should reflect total costs.
If more than one foreign country identify the distribution between the foreign countries.
Report only cumulative first-tier subawards dollars by country. Do not report foreign travel, purchases, etc., unless part of a first-tier subaward to a foreign country.
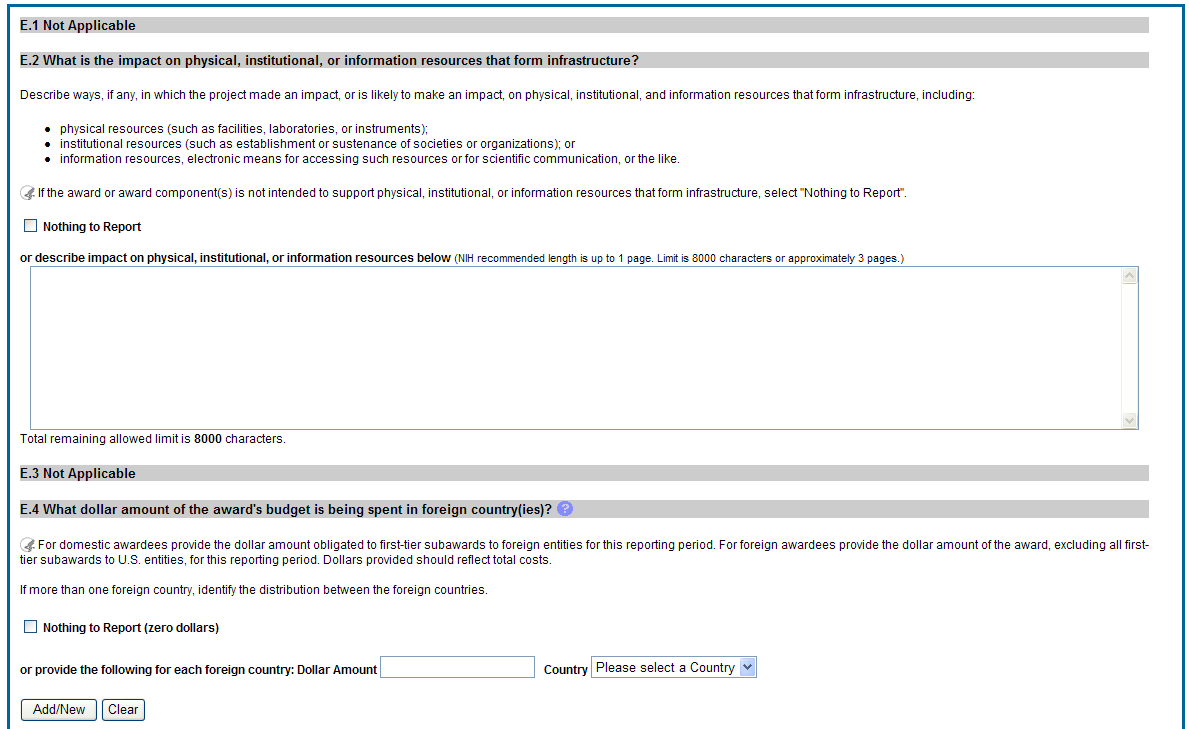
Figure 41: RPPR Section E. Impact – Questions E1 through E4
The RPPR Section F addresses Changes. Grantees are reminded that significant changes in objectives and scope require prior approval of the agency.
F.1 Not Applicable to most awards. See chapter 7 Supplemental Instructions.
F.2 Actual or anticipated challenges or delays and actions or plans to resolve them.
Describe challenges or delays encountered
during the reporting period and actions or plans to resolve them. ![]()
Describe only significant challenges that may impede the research (e.g., accrual of patients, hiring of personnel, need for resources or research tools) and emphasize their resolution.

Figure 42: RPPR Section F. Changes – Questions F1 & F2
![]() F.3
Significant changes to human subjects, vertebrate animals,
biohazards, and/or select agents.
F.3
Significant changes to human subjects, vertebrate animals,
biohazards, and/or select agents.
Describe significant deviations, unexpected outcomes, or changes in approved protocols for human subjects, vertebrate animals, biohazards and/or select agents during this reporting period.
Remember that significant changes in objectives and scope require prior approval of the agency (e.g., NIH Grants Policy Statement, 8.1.2.). If there are changes in any of the following areas, check the appropriate box and provide a description of the changes.
F.3.a Human Subjects
If human subject protocols are or will be different from the previous submission, include a description and explanation of how the protocols differ and provide a new or revised Protection of Human Subjects Section as described in the competing application instructions.
F.3.b Vertebrate Animals
If there are or will be significant changes to the uses of vertebrate animals from the previous submission, provide a description of the changes. Examples of changes considered to be significant include, but are not limited to, changing animal species, changing from noninvasive to invasive procedures, new project/performance site(s) where animals will be used, etc. If studies involving live vertebrate animals are planned and were not part of the originally proposed research design, provide a new or revised Vertebrate Animal Section as described in the competing application instructions.
F.3.c Biohazards
If the use of biohazards is or will be different from that in the previous submission, provide a description and explanation of the difference(s).
F.3 d Select Agents
If the possession, use, or transfer of Select Agents is or will be different from that proposed in the previous submission, including any change in the select agent research location and/or the required level of biocontainment, provide a description and explanation of the differences. If the use of Select Agents was proposed in the previous submission but has not been approved by regulatory authorities, provide an explanation. If studies involving Select Agents are planned and were not part of the originally proposed research design, provide a description of the proposed use, possession, transfer, and research location as described in the competing application instructions.
U.S. Select Agent Registry information: http://www.selectagents.gov/Select%20Agents%20and%20Toxins.html

Figure 43: RPPR Section F. Changes – Question F3
![]() The
RPPR Section G Special Reporting Requirements address agency-specific
award terms and conditions, as well as any award specific reporting
requirements.
The
RPPR Section G Special Reporting Requirements address agency-specific
award terms and conditions, as well as any award specific reporting
requirements.
G.1 Special Notice of Award and Funding Opportunity Announcement Reporting Requirements
Address any special reporting requirements specified in the award terms and conditions in the Notice of Award (NoA) or Funding Opportunity Announcement (FOA).
G.2 Not Applicable to most awards. See chapter 7 Supplemental Instructions.
G.3 Not Applicable to most awards. See chapter 7 Supplemental Instructions.

Figure 44: RPPR Section G. Special Reporting Requirements – Questions G1 through G3
G.4. Human Subjects
G.4.a Does the project involve human subjects?
If activities involving human subjects are planned at any time during the next budget period at the grantee organization or at any other project/performance site or collaborating institution, select Yes. Select Yes even if the project is exempt from the Regulations for the Protection of Human Subjects. Select No if activities involving human subjects are not planned at any time during the next budget period.
Policy on research involving human subjects, including definitions, can be found in the NIH Grants Policy Statement or in the competing application instructions.
Is the research exempt from federal regulations? Not applicable unless the answer to G.4.a. is Yes. If all of the proposed human subjects research meet the criteria for one or more of the exemptions from the requirements in the DHHS regulations (45 CFR 46.101(b)), Yes should be selected, and the appropriate exemption number(s) checked. The six categories of research exempt from the DHHS human subject regulations appear in Part III of the competing application instructions, under Definitions, Human Subjects.
If in doubt, consult with the Office for Human Research Protections (OHRP), Department of Health and Human Services, or the NIH Office of Extramural Research, Office of Extramural Programs at [email protected].
Note that if the proposed research involves only the use of human data or biological specimens, first determine whether the research involves human subjects. The exemptions do not apply if the research does not involve human subjects. For help determining whether research that involves the use of human data or biological specimens is human subjects research, refer to the NIH Research Involving Human Subjects website.
Does this project involve a clinical trial? Not applicable unless the answer to G.4.a. is Yes. The NIH defines a clinical trial as a prospective biomedical or behavioral research study of human subjects that is designed to answer specific questions about biomedical or behavioral interventions (drugs, treatments, devices, or new ways of using known drugs, treatments, or devices). Clinical trials are used to determine whether new biomedical or behavioral interventions are safe, efficacious, and effective. Behavioral human subjects research involving an intervention to modify behavior (diet, physical activity, cognitive therapy, etc.) fits this definition of a clinical trial.
Human subjects research to develop or evaluate clinical laboratory tests (e.g. imaging or molecular diagnostic tests) might be considered to be a clinical trial if the test will be used for medical decision making for the subject or the test itself imposes more than minimal risk for subjects.
Biomedical clinical trials of experimental drug, treatment, device or behavioral intervention may proceed through four phases:
Phase I clinical trials test a new biomedical intervention in a small group of people (e.g., 20-80) for the first time to evaluate safety (e.g., to determine a safe dosage range and to identify side effects).
Phase II clinical trials study the biomedical or behavioral intervention in a larger group of people (several hundred) to determine efficacy and to further evaluate its safety.
Phase III studies investigate the efficacy of the biomedical or behavioral intervention in large groups of human subjects (from several hundred to several thousand) by comparing the intervention to other standard or experimental interventions as well as to monitor adverse effects, and to collect information that will allow the intervention to be used safely.
Phase IV studies are conducted after the intervention has been marketed. These studies are designed to monitor effectiveness of the approved intervention in the general population and to collect information about any adverse effects associated with widespread use.
If yes, is this an NIH defined Phase III Clinical Trial?
An NIH-defined Phase III clinical trial is a broadly based prospective Phase III clinical investigation, usually involving several hundred or more human subjects, for the purpose of evaluating an experimental intervention in comparison with a standard or controlled intervention or comparing two or more existing treatments. Often the aim of such investigation is to provide evidence leading to a scientific basis for consideration of a change in health policy or standard of care. The definition includes pharmacologic, non-pharmacologic, and behavioral interventions given for disease prevention, prophylaxis, diagnosis, or therapy. Community trials and other population-based intervention trials are also included.
G4.b Inclusion enrollment data.
Unless otherwise notified by the program official, reporting the cumulative enrollment of subjects and the distribution by sex/gender, race, and ethnicity is required for NIH-defined clinical research as defined in the competing application instructions. If you have inclusion enrollment, update the Inclusion Enrollment Report, with the total cumulative data collected to-date. You may have more than one Inclusion Enrollment Report. If there are details or concerns related to your inclusion enrollment progress or if the enrollment data does not reflect the targeted enrollment by race, ethnicity, and/or sex/gender, the reasons for this should be addressed in the text of the progress report.
In Section F.3.a of the RPPR, please describe details or concerns related to your inclusion enrollment progress.
If the system determines that the RPPR does not require inclusion monitoring or if the inclusion enrollment data records have a Closed status, the Inclusion Enrollment section of the form displays the following message:
The RPPR does not have any Inclusion Enrollment Reports or the report records have a status of Closed. If you have any questions, please contact your NIH Program Official [PO’s name] at [email address].
If the system determines that the RPPR does require inclusion monitoring, the Inclusion Enrollment section of the form displays the following message:
You are required to complete Inclusion Enrollment Report for the following studies: [list of all protocols]
If it is determined that inclusion monitoring is required and one or more inclusion enrollment data records exist for the previous budget period, inclusion enrollment data records are created in the RPPR Population Tracking tables. If the inclusion enrollment data records from the current budget period are in an Open status, the existing enrollment data is refreshed with the data from the previous year.
If inclusion monitoring is required, but inclusion enrollment data records from the previous support year do not exist, a default inclusion enrollment data record is created for the RPPR. Additionally, an email notification is sent to the NIH Program Official associated with the RPPR.
Below are instructions for how to collect and report data on the basis of sex/gender, race, and ethnicity with additional guidance for handling subpopulations, foreign populations, changes to target data, and NIH-defined Phase III clinical trials.
For questions about the NIH policies for inclusion, please refer to: http://grants.nih.gov/grants/funding/women_min/women_min.htm or contact your program officer.
Standards for Collecting Data from Study Participants: The Office of Management and Budget (OMB) Directive No. 15 defines minimum standards for maintaining, collecting and presenting data on ethnicity and race for all Federal (including NIH) reporting purposes. The categories in this classification are social-political constructs and should not be interpreted as being anthropological in nature. The standards were revised in 1997 and now include two ethnic categories: Hispanic or Latino, and Not Hispanic or Latino. There are five racial categories: American Indian or Alaska Native, Asian, Black or African American, Native Hawaiian or Other Pacific Islander, and White. Reports of data on ethnicity and race should use these categories. The definitions below apply for the ethnic and racial categories.
Ethnic Categories:
Hispanic or Latino: A person of Cuban, Mexican, Puerto Rican, South or Central American, or other Spanish culture or origin, regardless of race. The term, “Spanish origin,” can be used in addition to “Hispanic or Latino”.
Not Hispanic or Latino
Racial Categories:
American Indian or Alaska Native: A person having origins in any of the original peoples of North, Central, or South America and maintains tribal affiliation or community.
Asian: A person having origins in any if the original peoples of the Far East, Southern Asia, or the Indian subcontinent including, for example, Cambodia, China, India, Japan, Korea, Malaysia, Pakistan, the Philippine Islands, Thailand, and Vietnam.
Black or African American: A person having origins in any of the black racial groups of Africa. Terms such as “Haitian” or “Negro” can be used in addition to “Black or African American.”
Native Hawaiian or Other Pacific Islander: A person having origins in any of the original peoples of Hawaii, Guam, Samoa, or other Pacific Islands.
White: A person having origins in any of the original peoples of Europe, North Africa, or the Middle East.
Reporting Data on Race and Ethnicity: NIH is required to use the above standards and definitions for race and ethnicity to allow comparisons to other federal databases, especially the census and national health databases. Federal agencies shall not present data on detailed categories if doing so would compromise data quality or confidentiality standards.
When collecting data on ethnicity and race, as well as sex/gender, use the categories listed to obtain the data from individuals on the basis of self-identification. Participants should be asked to identify their ethnicity and their race. The OMB recommends collecting this information using two separate questions, with ethnicity information collected first followed by race, with the option to select more than one racial designation (http://www.whitehouse.gov/omb/fedreg_directive_15). The Inclusion Enrollment Report format is not designed for use as a data collection instrument. Collect the data using instruments prepared for the study and use the information from the study database to fill out the Inclusion Enrollment Report. Study participants who self-identify with more than one race should be reported in the aggregate in the "More Than One Race" category.
When reporting these data to NIH, include the following items:
Part A of the Inclusion Enrollment Report:
the total number of subjects in each ethnic category or who did not self-identify with an ethnic category (unknown or not reported);
the total number of Hispanic or Latino and Not Hispanic or Latino subjects who selected only one category from each of the five racial categories;
the total number of Hispanic or Latino and Not Hispanic or Latino subjects who selected more than one racial category reported as the number selecting “more than one race”;
the total number of Hispanic or Latino and Not Hispanic or Latino subjects who did not self-identify with any racial category (unknown or not reported); and,
Part B of the Inclusion Enrollment Report:
the total number of Hispanic or Latino subjects who selected only one of the five racial categories as well as Hispanic or Latino subjects who selected more than one racial category or who did not self-identity with a racial category (unknown or not reported).
In completing the race sections of the Inclusion Enrollment Report, individuals who identify as Hispanic or Latino should be included in both race tables: the table where all participants’ races are reported (Part A) and the table where only the race of individuals identifying as Hispanic or Latino is reported (Part B).
Collecting and Reporting Data on Subpopulations: Each ethnic/racial group contains subpopulations that are delimited by geographic origins, national origins, and/or cultural differences. It is recognized that there are different ways of defining and reporting racial and ethnic subpopulation data. The subpopulation to which an individual is assigned depends on self-reporting of specific origins and/or cultural heritage. Attention to subpopulations also applies to individuals who self identify with more than one ethnicity or race. These ethnic/racial combinations may have biomedical, behavioral, and/or social-cultural implications related to the scientific question under study. The collection of greater detail is encouraged, e.g., on ethnic/racial subpopulations; however, any collection that uses more detail needs to be organized in such a way that the additional categories can be aggregated into the OMB categories for reporting data on ethnicity, race, and more than one race. Investigators who have data on subpopulations are encouraged to provide that information in the Comments field of the Inclusion Enrollment Report and/or in the text of their progress report.
Collecting and Reporting Data on Foreign Populations: If conducting clinical research outside of the United States, design culturally sensitive and appropriate data collection instruments that allow participants to self-identify their ethnic and/or racial affiliation. These items, however, should be designed in a way that allows the information to be aggregated into the OMB minimally required ethnic and racial categories and which will allow you to complete the inclusion enrollment report(s). Enrollment of foreign participants should be reported to NIH in an Inclusion Enrollment Report separate from that for reporting domestic participants.
Changes to Targeted/Planned Enrollment: If there are changes from the Targeted/Planned Enrollment Table originally approved for funding, contact your Program Officer to discuss updating/revising your Targeted/Planned Enrollment Table and address the change in Section F.3.a of the RPPR.
Reporting Data on NIH-defined Phase III Clinical Trials: If conducting an NIH-defined Phase III Clinical Trial, report on the cumulative enrollment (as described above) and indicate if data analysis has begun for the trial. If analysis has begun, report on progress made in conducting valid analyses for sex/gender, racial, and/or ethnic differences.
G.4.c ClinicalTrials.gov.
Does this project include one or more applicable clinical trials that must be registered in ClinicalTrials.gov under FDAAA?
If yes, provide the ClinicalTrials.gov identifier, NCT number (e.g., NCT00654321) for those trials.
See What NIH Grantees Need to Know About FADAA , and FAQ When must an applicable clinical trial be registered? If the grant number was entered into ClinicalTrials.gov, the ClinicalTrials.gov identifier (NCT number) may be readily identified by using the ClinicalTrials.gov Advanced Search and entering the grant number in the Study IDs field.
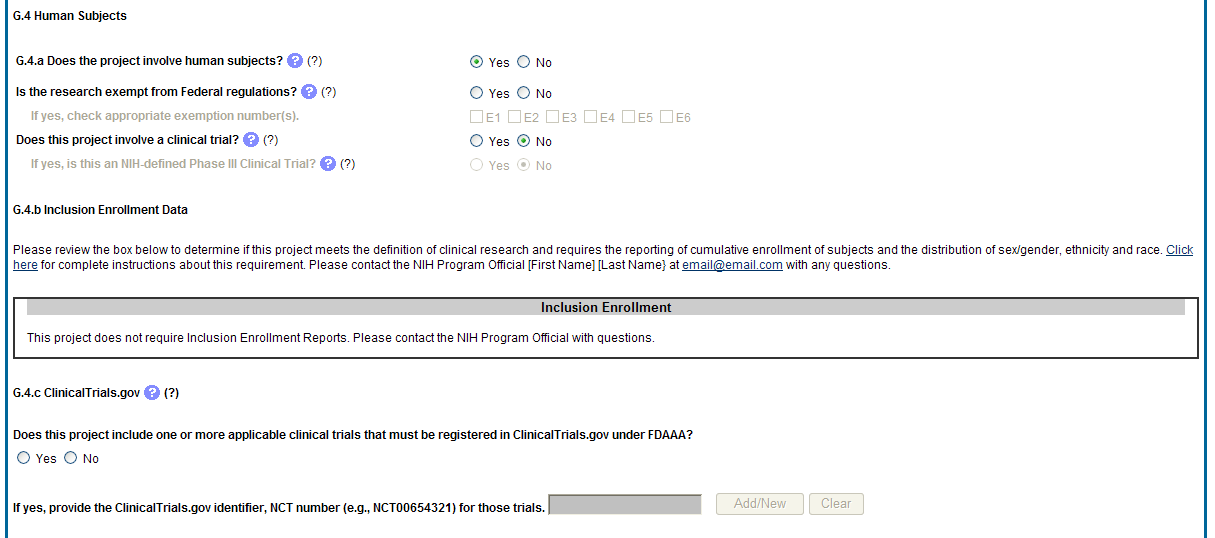
Figure 45: RPPR Section G. Special Reporting Requirements – Question G4
G.5 Human Subjects Education Requirement.
Are there personnel on this project who are or will be newly involved in the design or conduct of human subjects research?
If yes, provide the following:
names of individuals,
title of the human subjects education program completed by each individual, and
a one-sentence description of the program.
G.6 Human Embryonic Stem Cell(s).
Does this project involve human embryonic stem cells?
Only hESC lines listed as approved in the NIH Registry may be used in NIH funded research.
If yes, identify the hESC Registration number(s) from the NIH Registry.
If there is a change in the use of hESCs provide an explanation.
G.7 Vertebrate Animals
Does this project involve vertebrate animals?
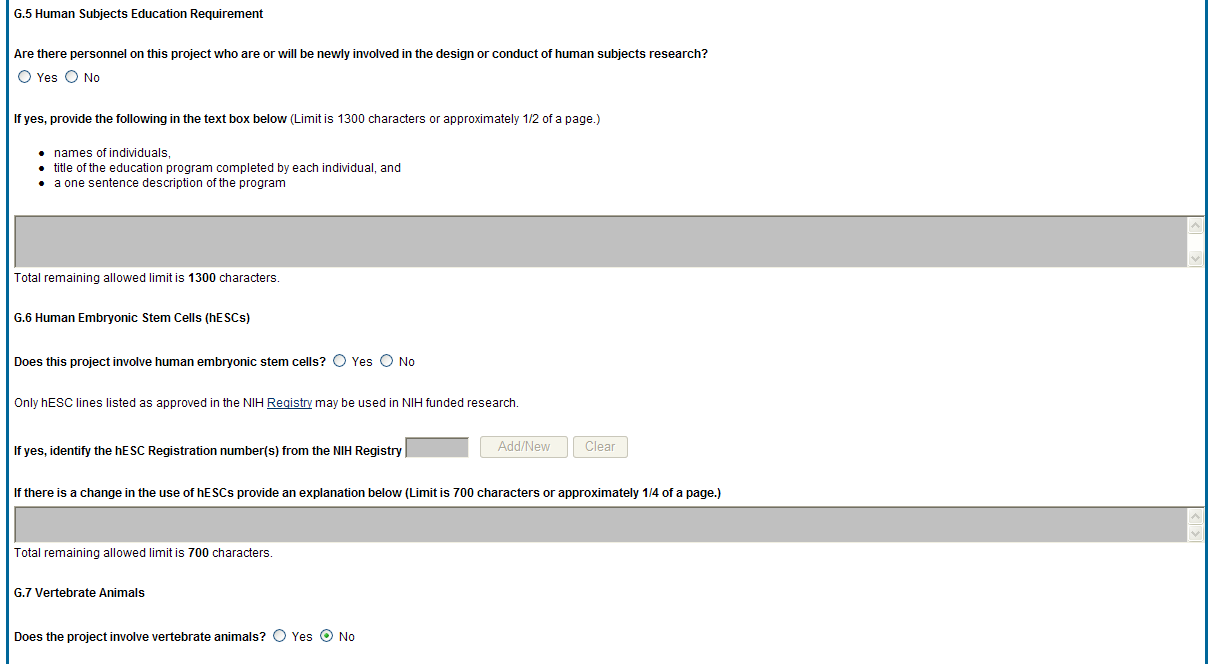
Figure 46: RPPR Section G. Special Reporting Requirements – Questions G5 through G7
G.8 Project/Performance Sites.
If there are changes to the project/performance site(s) displayed, edit as appropriate.
One of the sites indicated must be the identified as the Primary Performance Site. If including a new Project/Performance Site where either human subjects or vertebrate animals will be involved, address the change under F.3.a or F.3.b. If a Project/Performance Site is engaged in research involving human subjects, the grantee organization is responsible for ensuring that the Project/Performance Site operates under an appropriate Federal Wide Assurance for the protection of human subjects and complies with 45 CFR Part 46 and other NIH human subject related policies described in Part II of the competing application instructions and the NIH Grants Policy Statement.
For research involving live vertebrate animals, the grantee organization must ensure that all Project/Performance Sites hold OLAW-approved Assurances. If the grantee organization does not have an animal program or facilities and the animal work will be conducted at an institution with an Assurance, the grantee must obtain an Assurance from OLAW prior to the involvement of vertebrate animals.
Figure 47: RPPR Section G. Special Reporting Requirements – Question G8
G.9 Foreign component.
Provide the organization name, country, and description of each foreign component.
Foreign component is defined as significant scientific activity that was performed outside of the United States, either by the grantee or by a researcher employed by a foreign organization, whether or not grant funds were expended. The following grant-related activities are significant and must be reported:
involvement of human subjects or research with live vertebrate animals;
extensive foreign travel by grantee project staff to collect data, or conduct surveys or sampling activities; or
any grantee activity that may have an impact on U.S. foreign policy.
Examples of other grant-related activities that may be significant are:
collaborations with investigators at a foreign site anticipated to result in co-authorship;
use of facilities or instrumentation at a foreign site; or
receipt of financial support or resources from a foreign entity.
Foreign travel for consultation does not meet the definition of foreign component.

Figure 48: RPPR Section G. Special Reporting Requirements – Question G9
G.10 Estimated unobligated balance.
G.10.a Is it anticipated that an estimated unobligated balance (including prior year carryover) will be greater than 25% of the current year’s total approved budget?
The total approved budget equals the current fiscal year award authorization plus any approved carryover of funds from a prior year(s). The numerator equals the total amount available for carryover and the denominator equals the current year’s total approved budget.
If yes, provide the estimated unobligated balance.
G.10.b Provide an explanation for unobligated balance.
G.10.c If authorized to carryover the balance, provide a general description of how it is anticipated that the funds will be spent. To determine carryover authorization, see the Notice of Award.
Grantees not authorized to carryover unobligated balances automatically must submit a prior approval request to the awarding IC. See instructions in NIH Grants Policy Statement Section 8.1.2.4 Carryover of Unobligated Balances.
G.11 Program Income.
Is program income anticipated during the next budget period?
If yes, provide the amount and source(s).
Program Income is defined as gross income earned by the grantee organization, a consortium participant, or a contractor under the grant that is directly generated by the grant-supported project or activity or earned as a result of the award. Program income includes, but is not limited to, income from fees for services performed; charges for the use or rental of real property, equipment or supplies acquired under the grant; the sale of commodities or items fabricated under an award; charges for research resources; registration fees for grant-supported conferences, and license fees and royalties on patents and copyrights. Program income from license fees and royalties from copyrighted material, patents, and inventions is exempt from reporting requirements unless otherwise specified in the terms and conditions of award
G.12 F&A Costs [applicable to SNAP awards only]
Is there a change in performance sites that will affect F&A costs?
If yes, provide an explanation.

Figure 49: RPPR Section G. Special Reporting Requirements – Questions G10 through G12
Follow the instructions in the SF424(R&R) Application Guide for NIH and Other PHS Agencies, Section I, 4.7 Budget Component, to complete the R&R budget, sections A-K, and the R&R Cumulative Budget, for the remainder of the project period. The budget justification should be uploaded and must include detailed justification for those line items and amounts that represent a significant change from previously recommended levels (e.g., total rebudgeting greater than 25 percent of the total award amount for this budget period). If you have subaward/consortiums, follow the SF424(R&R) instructions in Section I, 4.8 Special Instructions for Preparing Applications with a Subaward/Consortium.
The RPPR Component Instructions in chapter 6 apply to the following awards: D71, DP1, DP5, G08, G11, G13, P40, R00, R01, R03, R18, R21, R33, R34, R36, R37, R56, RC1, RC2, RL1, S10, S21, S22, SC1, SC2, SC3, UB1, UC2, UH1, UH2, UH3, UP5. For all other awards, see Table 1 below for supplemental instructions that either replace or are in addition to the RPPR Component Instructions.
Table 1: Applicable Supplemental Instructions
Award |
Applicable Supplemental Instructions |
K01, K02, K05, K06, K08, K18, K22, K23, K24, K25, K26, K99, KL1 |
7.1 Individual Career Development (K) Awards |
F05, F30, F31, F32, F33, F34, F37 |
7.2 Fellowship Awards |
R41, R42, R43, R44, U43, U44 |
7.3 SBIR/STTR Awards |
D43, K12, KL2, R90, RL9, T15, T32, T34, T35, T37, T90, TL1, TU2, U2R |
7.4 Training Awards |
D43, K30, R13, R25, RL5, T14, T36, U13, U2R |
7.5 Educational Awards |
G12 M01, P01, P20, P30, P41, P42, P50, P51, P60, PL1, PN1, PN2, R24, S06, S07, S11, U01, U10, U19, U24, U34, U41, U42, U45, U54, U56, UC7, UL1, UM1 |
7.6 Complex Awards |
The RPPR Component Instructions in chapter 6 are applicable to individual Career Development Awards (K01, K02, K05, K06, K08, K18, K22, K23, K24, K25, K26, K99, KL1) with the following exceptions:
B.4 What opportunities for training and professional development has the project provided?
Describe activities such as teaching, clinical care, professional consultation, service on advisory groups, and administrative activities. Indicate percent of time spent in each of these activities and the relationship to the awardee's research career development. For awards that include a requirement to mentor others (e.g., K05 and K24), indicate the percent of time devoted to mentoring activities, individuals mentored during the reporting period, the frequency and kinds of mentoring, financial and other support provided to mentees, and the productivity of the mentoring relationship.
B. 6. What do you plan to do for the next reporting period to accomplish the goals?
Provide a timeline for the activities planned for the next year, including plans to apply for subsequent grant support. Recipients of transition awards (e.g., K22, K99) should report on progress in identifying an independent research position. Additionally, awardees charged with mentoring others (e.g., K05, K24) should provide information describing planned mentoring activities and proposed mentees (e.g., backgrounds, interests, professional levels, etc.) sufficient to evaluate the quality of the mentoring.
C.2. Not Applicable.
C.3. Not Applicable.
D.2.e Not Applicable.
E.1 Not Applicable.
E.2 Not Applicable.
E.3 Not Applicable.
F.1 Not Applicable.
G.2 Responsible Conduct of Research
Describe the responsible conduct of research instruction received (or instruction given as a course director, discussion leader, etc., in the case of senior fellows or senior career awardees) by formal and/or informal means, during this reporting period. If instruction or participation as a course director/discussion leader occurred in a prior budget period, note the dates of occurrence. Any activities undertaken to individualize instruction appropriate to career stage should be discussed. Address the five components: Format, Subject Matter, Faculty Participation, Duration, and Frequency. Additional detailed guidance on this requirement is found in the competing application instructions.
G.3 Mentor’s Report
For mentored K awards, provide a letter signed by the mentor, in PDF format, assessing the awardee's progress and performance during this reporting period, both in research and in terms of development into an independent investigator in the area of the award. Include information on the availability of support for the candidate’s research project during the next budget segment. For applicable career transition awards (e.g., K22, K99), the mentor should describe the awardee’s efforts to transition into a permanent research position and the sponsor’s contributions to that process. If required to submit letters from more than one mentor, letters should be assembled in one PDF file. For non-mentored K awards, select “Not Applicable.”
G.11 Not Applicable.
G.12 Not Applicable.
H. Budget. [Applicable to non-SNAP awards only.]
Follow the instructions for SF 424 (R&R) for K awards in SF424 Part I, section 7.6.4. Base the awardee's salary and fringe benefits request on a full-time, 12-month appointment following the guidelines in the appropriate career award instructions. Support for other personnel and amounts in other budget categories may be requested in accordance with applicable CDA guidelines.
The RPPR Component Instructions in chapter 6 are applicable to Fellowship Awards (F05, F30, F31, F32, F33, F34, F37) with the following exceptions:
B.6 What do you plan to do during the next reporting period to accomplish the goals?
Include any course work and any important modifications to the original plans. Provide a scientific justification for any changes involving research with human subjects or vertebrate animals. A detailed description of such changes must be provided under Changes.
C.2 Not Applicable.
C.3 Not Applicable.
C.4 Not Applicable.
D.1 Not Applicable.
D.2.a Not Applicable
D.2.b Not Applicable.
D.2.e Not Applicable.
E.1 Not Applicable.
E.2 Not Applicable.
E.3 Not Applicable.
F.1 Not Applicable.
G.2 Responsible Conduct of Research
Describe the responsible conduct of research instruction received (or instruction given as a course director, discussion leader, etc., in the case of senior fellows or senior career awardees) by formal and/or informal means, during this reporting period. If instruction or participation as a course director/discussion leader occurred in a prior budget period, note the dates of occurrence. Any activities undertaken to individualize instruction appropriate to career stage should be discussed. Address the five components: Format, Subject Matter, Faculty Participation, Duration, and Frequency. Additional detailed guidance on this requirement is found in the competing application instructions.
G.3 Sponsor Comments
Provide a letter signed by the sponsor, in PDF format, assessing the quality of the research training (including academic work) and research progress made by the Fellow during this reporting period.
G. 10 Not Applicable.
G.11 Not Applicable.
G.12 Not Applicable.
H. Not Applicable.
The RPPR Component Instructions in chapter 6 are applicable to SBIR/STTR awards (R41, R42, R43, R44, U43, U44) with the following exceptions:
B.2 What was accomplished under these goals?
Goals is equivalent to specific aims and/or milestones.
B.3 Competitive Revisions/Administrative Supplements
For this reporting period, is there one or more Revision/Supplement associated with this award for which reporting is required?
If yes, identify the Revision(s) by grant number (e.g., 3R01CA098765-01S1) or title and describe the specific aims and/or milestones for each Revision. Include any supplements to promote or enhance diversity and re-entry, or other similar supplements to support addition of an individual or a discrete project.
B.6 What do you plan to do during the next reporting period to accomplish the goals?
For FastTrack and Phase II progress reports include a one-page abstract describing the research plan for Phase II and, as necessary, an updated commercialization plan.
C.5.a Other products
For SBIR/STTR awards commercial technologies will be addressed under Impact.
E.1 Not Applicable.
E.3 What is the impact on technology transfer?
Describe ways in which the project made an impact, or is likely to make an impact, on commercial technology or public use, including:
transfer of results to entities in government or industry;
instances where the research has led to the initiation of a start-up company; or
adoption of new practices.
E.3.a Commercialization Activities.
Report on the status of commercialization activities resulting from the award:
Nothing to report or select one or more of the following:
Sales = $_______
Licensing Revenues = $_______
3rd Party Investment since award start (Non-federal) = $_______
Sale of company
Sale of technology rights
Company merger related to product
Joint Venture agreement
Marketing/Distribution agreement(s)
Manufacturing agreement(s)
R&D agreements
Customer alliance(s)
Other ________________________ [60 character limit]
E.3.b FDA Interactions.
Report on interactions with the Food and Drug Administration during the reporting period related to the technology that is the subject of the award:
Not applicable to this technology or select one or more of the following:
Discussion with FDA not initiated
Discussion with the FDA initiated
Applied for approval
Approval Granted: Type ________________________ [60 character limit]
Review ongoing
Not approved
In human clinical trials
Other________________________ [60 character limit]
F.1 Not Applicable.
G.2 Not Applicable.
G.3 Not Applicable.
G.12 F & A Costs. [Applicable to SNAP awards only.]
H. Budget [Applicable to non-SNAP awards only.]
Follow the instructions in the SF424(R&R) SBIR/STTR User Guide, Part I, Section 4.6 R&R Budget Component.
The RPPR Component Instructions in chapter 6 are applicable to Training awards (K12, KL2, R90, RL9, T15, T32, T34, T35, T37, T90, TL1, TU2) with the following exceptions:
B.1. What are the major goals of the project?
Provide a description of the training objectives and goals. List the major goals of the project as stated in the approved application or as approved by the agency. If the application lists milestones/target dates for important activities or phases of the project, identify these dates and show actual completion dates or the percentage of completion.
B.2 What was accomplished under these goals?
Since the last report or application, describe implementation of training and other specific programmatic objectives, and the recruitment and retention of trainees from diverse groups.
B.4 What opportunities for training and professional development has the project provided?
For all awards provide a PDF that includes the following items: (1) completed Trainee Diversity Report format page to report on the diversity of the trainees supported by the award during the reporting period (generally not applicable for FIC awards); (2) a paragraph for each trainee/scholar supported by the award in the reporting period that identifies mentor, research project, and course work of each trainee/scholar. Include conference presentations, honors, fellowships, workshops and related activities. This description should be sufficient to allow evaluation of the trainees’ progress towards the goals of the training grant.
For T awards, include updated data on trainees supported by the training grant in Table 12A and/or 12B, from the competing application instructions as applicable, to reflect trainees supported by the grant in the reporting period.
For D43, TU2, T15, T32, T37, T90, U2R, U90, and TL1 awards, include program statistics for doctoral training in Table 12A.
The Trainee Diversity Report format page is available at: http://grants.nih.gov/grants/funding/2590/2590.htm.
B.6 What do you plan to do during the next reporting period to accomplish the goals?
Include plans for any modification based on the findings of your internal evaluations.
C.1 Publications
It will be necessary for the Program Director to add trainee publications to his/her MyBib. They may be placed in the section entitled Other Publications.
C.2 Not Applicable.
C.3 Not Applicable.
C.4 Not Applicable.
C.5.b Not Applicable.
D.1 What individuals have worked on the project?
Provide or update the following information only for K12 and KL2 progress reports: (1) program director(s)/principal investigators(s) (PDs/PIs); and (2) each person who has worked at least one person month per year on the project during the reporting period, regardless of the source of compensation (a person month equals approximately 160 hours or 8.3% of annualized effort). Do not report personnel for whom a PHS 2271 Appointment form has been submitted through xTRAIN. If not reporting on a K12 or KL2 award, disregard this section.
D.2.b New senior/key personnel.
Are there new training faculty?
If yes, provide biosketches and other support for all new training faculty.
E.1 Not Applicable.
E.2 Not Applicable.
E.3 Not Applicable.
F. 1 Changes in approach and reasons for change
Describe changes in the program for the next budget period, including changes in training faculty. Include, as appropriate, the role of external advisory committees, significant new training content, procedures or experiences, and indicate how these aid in strengthening and realizing the objectives and goals of the program.
F.2 Not Applicable.
F.3 Significant changes to Human Subjects, Vertebrate Animals, Biohazards, and/or Select Agents
Complete this section only if the use or care of human subjects, vertebrate animals, biohazards and/or select agents is not reported under another NIH award.
G.2 Responsible Conduct of Research
Describe the nature of the responsible conduct of research instruction and the extent of trainee (or scholar, in the case of the Institutional Career Development Programs) and faculty participation. Include a description of any enhancements and/or modifications to the five instructional components (Format, Subject Matter, Faculty Participation, Duration, and Frequency) from the plan described in the competing application. Faculty members who were contributors to formal instruction in responsible conduct of research during the last budget period must be named. Additional detailed guidance on this requirement is found in the competing application instructions.
G.3 Not Applicable
G.6 Human Embryonic Stem Cells (hESCs)
Complete this section only if the use of hESCs is not reported under another NIH award.
G.11 Not Applicable.
G.12 Not Applicable.
H. Budget
Submit the PHS 398 Training Budget form pages following the instructions in the SF424(R&R) competing application guide Part I, section 8.5 PHS 398 Training Budget.
The RPPR Component Instructions in chapter 6 are applicable to Education awards (D42, K30, R13, R25, RL5, T14, T36, U13, U2R) with the following exceptions:
B.4 What opportunities for training and professional development has the project provided?
Describe opportunities for training and professional development provided to anyone who worked on the project or anyone who was involved in the activities supported by the project. Training activities are those in which individuals with advanced professional skills and experience assist others in attaining greater proficiency. Training activities may include, for example, courses or one-on-one work with a mentor. Professional development activities result in increased knowledge or skill in one’s area of expertise and may include workshops, conferences, seminars, study groups, and individual study. Include participation in conferences, workshops, and seminars not listed under major activities. Grantees with NIH institutional training grant awards with the following specified activity codes are required to provide program statistics for doctoral training in Table 12A: D43, TU2, T15, T32, T37, T90, U2R, U90, and U54/TL1.
For T, F, K, R25, R13, D43 and other awards or award components designed to provide training and professional development opportunities, a response is required. Do not reiterate what is reported under Accomplishments. Limit the response to this reporting period.
C.3. Not Applicable.
C.4 Not Applicable.
C.5.b Not Applicable.
E.1 What is the impact on the development of human resources?
Describe how the project made an impact or is likely to make an impact on human resource development in science, engineering, and technology. For example, how has the project: 1) provided opportunities for research and teaching in the relevant fields; 2) improved the performance, skills, or attitudes of members of underrepresented groups that will improve their access to or retention in research, teaching, or other related professions; 3) developed and disseminated new educational materials or provided scholarships; or 4) provided exposure to science and technology for practitioners, teachers, young people, or other members of the public?
E.2 Not Applicable.
E.3 Not Applicable
F.1 Changes in approach and reasons for change
Describe changes for the next budget period. Include, as appropriate, the role of external advisory committees, significant new content, procedures or experiences, and indicate how these aid in strengthening and realizing the objectives and goals of the award.
G.2 Responsible Conduct of Research
If required in the FOA for this award, describe the nature of the responsible conduct of research instruction and the extent of participant and faculty involvement. Include a description of any enhancements and/or modifications to the five instructional components (Format, Subject Matter, Faculty Participation, Duration, and Frequency) from the plan described in the competing application. Faculty members who were contributors to formal instruction in responsible conduct of research during the last budget period must be named. Additional detailed guidance on this requirement is found in the competing application instructions.
G.3 Not Applicable.
G.10 Not Applicable.
G.12 F&A Costs [Applicable to SNAP awards only.]
H. Budget. [Applicable to non-SNAP awards only.]
Follow the instructions in the SF424(R&R) Application Guide for NIH and Other PHS Agencies, Section I, 4.7 Budget Component, to complete the R&R budget, sections A-K, and the R&R Cumulative Budget, for the remainder of the project period. The budget justification should be uploaded and must include detailed justification for those line items and amounts that represent a significant change from previously recommended levels (e.g., total rebudgeting greater than 25 percent of the total award amount for this budget period). If you have subaward/consortiums, follow the SF424(R&R) instructions in Section I, 4.8 Special Instructions for Preparing Applications with a Subaward/Consortium.
For the purposes of the RPPR, the following activity codes are always categorized as complex awards: G12 M01, P01, P20, P30, P41, P42, P50, P51, P60, PL1, PN1, PN2, R24, S06, S07, S11, U01, U10, U19, U24, U34, U41, U42, U45, U54, U56, UC7, UL1, UM1. These awards may or may not include subcomponents (projects, cores), but they all could potentially include subcomponents. For each subcomponent, the grantee will click the Add Project/Core link at the end of this section and follow the instructions under Subcomponent.
The RPPR Component Instructions in chapter 6 are applicable to complex awards (even if the award does not include any subcomponents) with the following exceptions.
B.1 What are the major goals of the project?
Emphasize the synergy, collaboration and integration of major activities of the project. Report the major goals specific to an individual project or core under that project/core.
B.2 What was accomplished under these goals?
For this reporting period describe for the overall award: 1) major activities; 2) significant results, including major findings, developments, or conclusions (both positive and negative), and 3) key outcomes or other achievements. Include a discussion of stated goals not met. Report the accomplishments of individual projects and cores under that project/core.
B.3 Is there one or more Revision associated with this award or a project under this award for which reporting is required?
If the Revision is associated with a specific project or core, identify the project/core.
B.5 How have the results been disseminated to communities of interest?
If there are individual projects/cores designed to disseminate information or conduct outreach activities, report those activities under that project/core.
B.6 What do you plan to do during the next reporting period to accomplish the goals?
Report goals and objectives of individual projects or cores under that project/core.
C.5 a Other products
Identify any other significant products that were developed under the overall project. Report other products and resources resulting from an individual project or core under that project/core.
C.5.b Resource sharing
Report resource sharing for an individual project or core under that project/core.
D.1 Participants
Specify the project/core(s) on which the individual worked in the appropriate text box. This personnel information is for the entire project.
D.2 Personnel Updates
Personnel questions (D.2.a.-e.) are applicable to entire project. For D.2.b, new senior/key personnel, identify the project/core(s) on which the individuals worked or will work. For D.2.e, new other significant contributors, identify the project/core(s) on which the individual worked or will work.
E.1 Not Applicable.
E.3 Not Applicable.
F.1 Not Applicable.
F.3 Significant changes to Human Subjects, Vertebrate Animals, Biohazards, and/or Select Agents.
If there are changes in any of the following areas check the appropriate box and provide a d3escription of the changes. If applicable, report the change under the relevant project/core.
G.2 Not Applicable.
G.3 Not Applicable.
G.4.c ClinicalTrials.gov
Associate the number with the relevant project or core, if applicable.
G.12 F&A Costs [Applicable to SNAP awards only.]
For each subcomponent (project/core), click the Add Project/Core link, and complete the instructions. The RPPR Component Instructions are applicable to each individual project/core with the following exceptions:
A. Provide the title or identifying number of the project/core.
A.1 Provide the name, email, phone number and address of the PI of the project/core.
B. 3 Not Applicable.
B.5 How have the results been disseminated to communities of interest?
Reporting the routine dissemination of information (e.g., websites, press releases) is not required. If the Project/Core is not designed to disseminate information to the public or conduct similar outreach activities, select Nothing to Report. If the Project/Core is designed to disseminate information or conduct outreach activities, report those activities here. Note that scientific publications and the sharing of research resources will be reported under Products.
C.1 Not Applicable.
C.2 Not Applicable.
C.4 Not Applicable.
D. Not Applicable.
E.1 Not Applicable.
E.2 Not Applicable.
E.3 What is the impact on technology transfer?
Describe ways in which the project/core made an impact, or is likely to make an impact, on commercial technology or public use, including:
transfer of results to entities in government or industry;
instances where the research has led to the initiation of a start-up company; or
adoption of new practices.
E.4 Not Applicable.
F.1 Not Applicable.
G.1 Not Applicable.
G.2 Not Applicable.
G.3 Not Applicable.
G.4.c Not Applicable.
G.5 Not Applicable.
G.7 Not Applicable.
G.8 Not Applicable.
G.9 Not Applicable.
G.10 Not Applicable.
G.11 Not Applicable.
G.12 Not Applicable.
H. Not Applicable.
Each progress report to the PHS requires that the following policies, assurances, and/or certifications be verified by the Signing Official (or PD/PI with delegated authority for SNAP awards). These assurances are explained in Part III: Policies, Assurances, Definitions, and Other Information of the competing application instructions. Applicants and grantees must comply with a number of additional public policy requirements. Refer to your institution’s research grant administrative office or the NIH Grants Policy Statement for additional information.
The policies, assurances and certifications listed below may or may not be applicable to your project, program, or type of applicant organization.
Research on Transplantation of Human Fetal Tissue
Research Using Human Embryonic Stem Cells
Women and Minority Inclusion Policy
ClinicalTrials.gov Requirements
Non-Delinquency on Federal Debt
Recombinant DNA Research, including Human Gene Transfer Research
Financial Conflict of Interest (except Phase I SBIR/STTR)
Project Director/Principal Investigator Assurance
Impact of Grant Activities on the Environment and Historic Properties
Institutions Receiving Awards for Training of Graduate Students for Doctoral Degrees
Submission of the RPPR to the agency includes the following certification:
In submitting this RPPR, the SO (or PD/PI with delegated authority), certifies that the grantee organization is in compliance with the terms and conditions specified in the Notice of Award and verifies the accuracy and validity of all administrative, fiscal, and scientific information in the progress report. The SO (or PD/PI with delegated authority) further certifies that the grantee organization will be accountable for the appropriate use of any funds awarded and for the performance of the grant-supported project or activities resulting from the progress report. Deliberate withholding, falsification, or misrepresentation of information could result in administrative actions such as withdrawal of a progress report, suspension and/or termination of an award, debarment of individuals, as well as possible criminal penalties. The grantee institution may be liable for the reimbursement of funds associated with any inappropriate or fraudulent conduct of the project activity.
Privacy Act Statement. The NIH maintains application and grant records as part of a system of records as defined by the Privacy Act: NIH 09-25-0036, Extramural Awards and Chartered Advisory Committees (IMPAC 2), Contract Information (DCIS), and Cooperative Agreement Information, HHS/NIH: http://oma.od.nih.gov/ms/privacy/pa-files/0036.htm.
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| File Title | NIH Research Performance Progress Report (RPPR) Instruction Guide |
| Subject | Guide for completing the RPPR |
| Author | DHHS/NIH/OD/OER/ORIS/eRA/DCSS/USB |
| File Modified | 0000-00-00 |
| File Created | 2021-01-29 |
© 2026 OMB.report | Privacy Policy