Form 2 Screenshots of Registration, Update/Amendment, and Accru
The Clinical Trials Reporting Program (CTRP) Database (NCI)
Attach_3_NCI CTRP Screen Shots.DOC
Amendment for the Clinical Trials Reporting Program (CTRP) Database (NCI)
OMB: 0925-0600
NCI CTRP Attachment 3
NCI CTRP Registration, Update, Amendment, Accrual Portal Workflow and Screen Shots
Step 1: User accesses the NCI Clinical Trials Reporting Program website at http://trials.nci.nih.gov – see screenshot, page 2
Step 2: User clicks “Login”
Step 3: User enters “Username” and “Password” – see screenshot, page 3
Step 4: User reviews NCI Clinical Trials Reporting Program burden statement – see screenshot, page 4
Step 5: System displays “Search Submitted Clinical Trials” page – see screenshot, page 19
Step 6: Alternative workflows, a – f, a user may perform any of these actions upon entering the system:
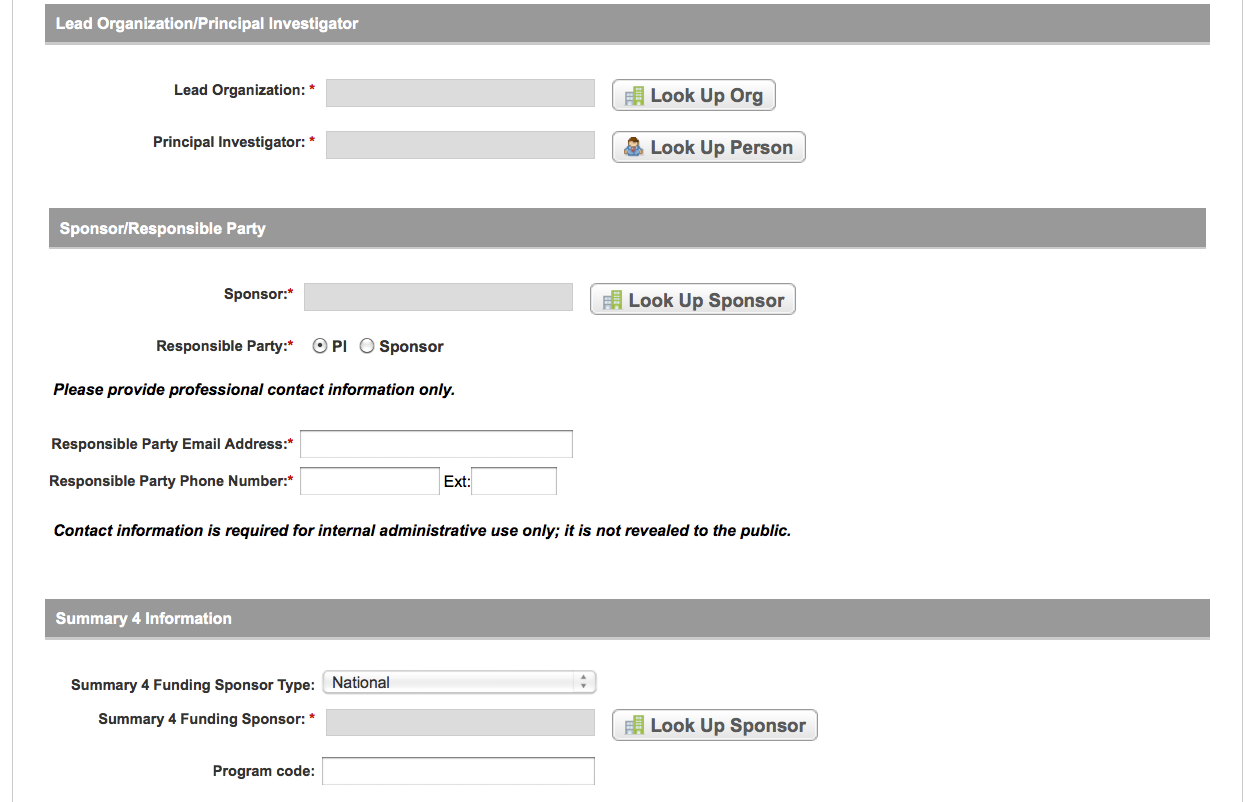
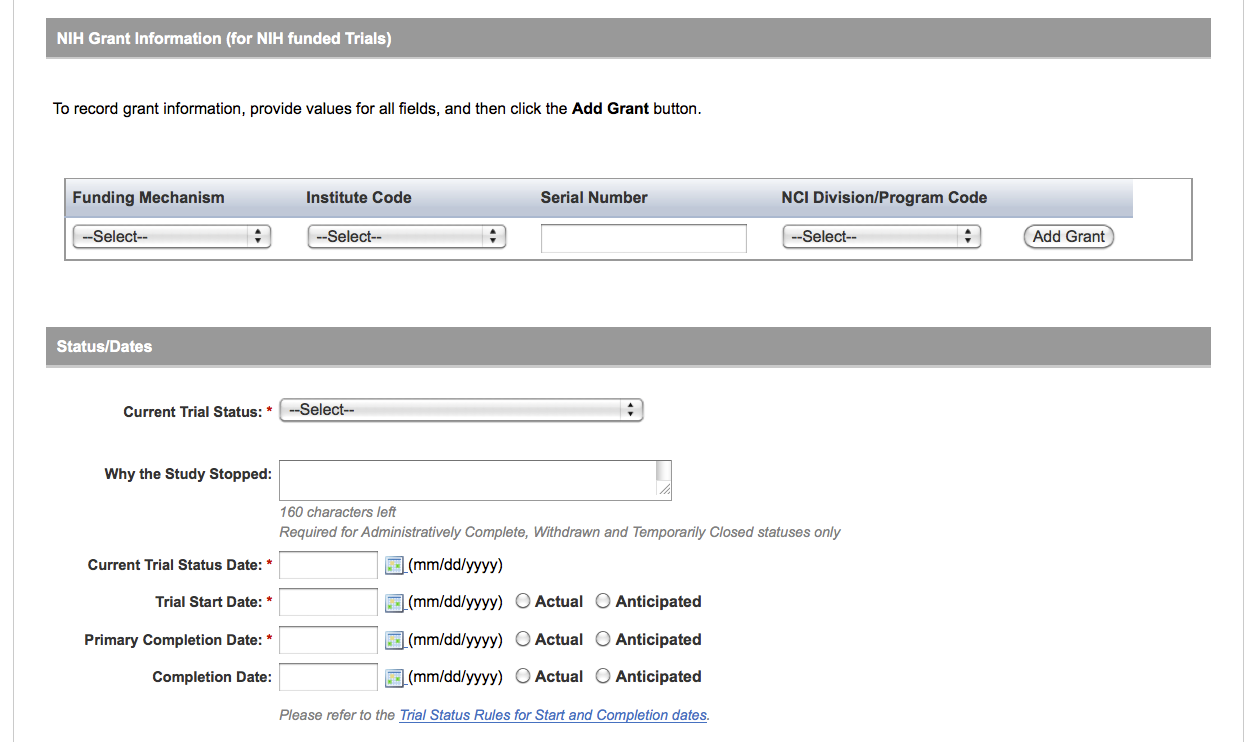
User selects to perform “Initial Trial Registration” and completes initial registration – see screenshots, pages 5 - 8, OR
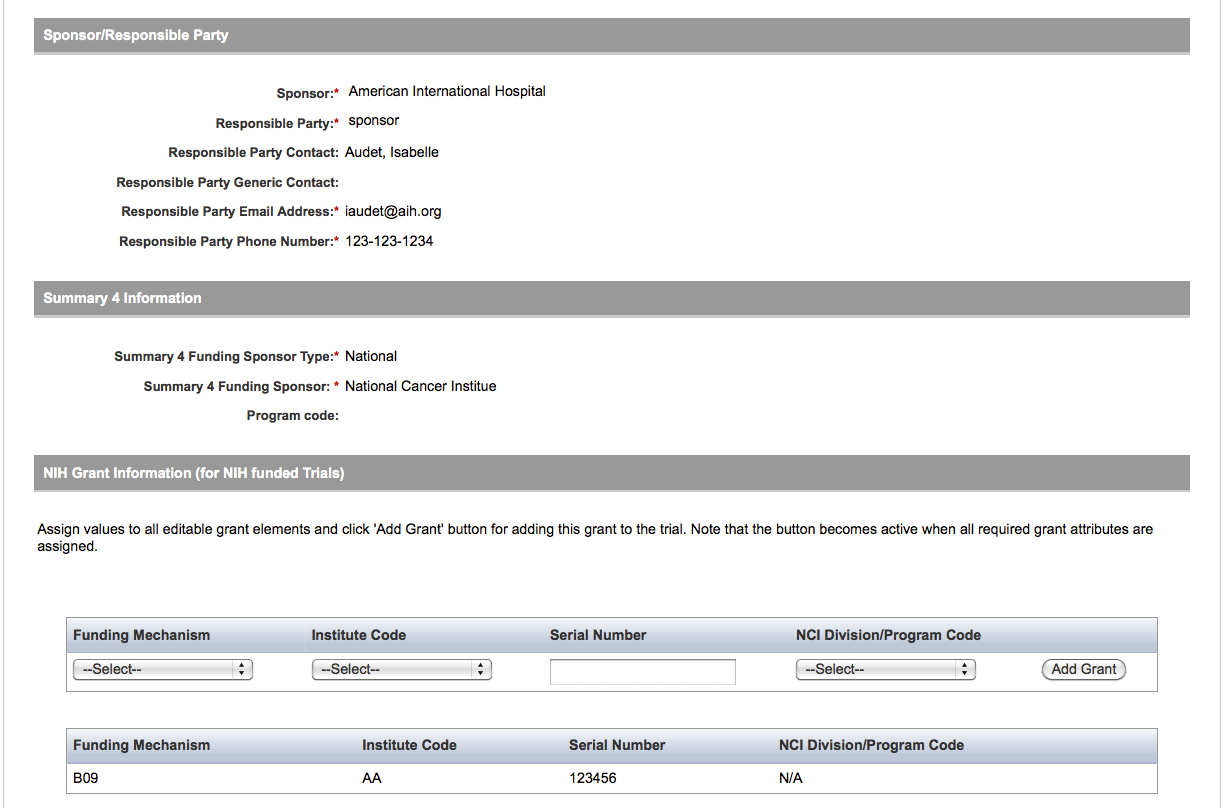
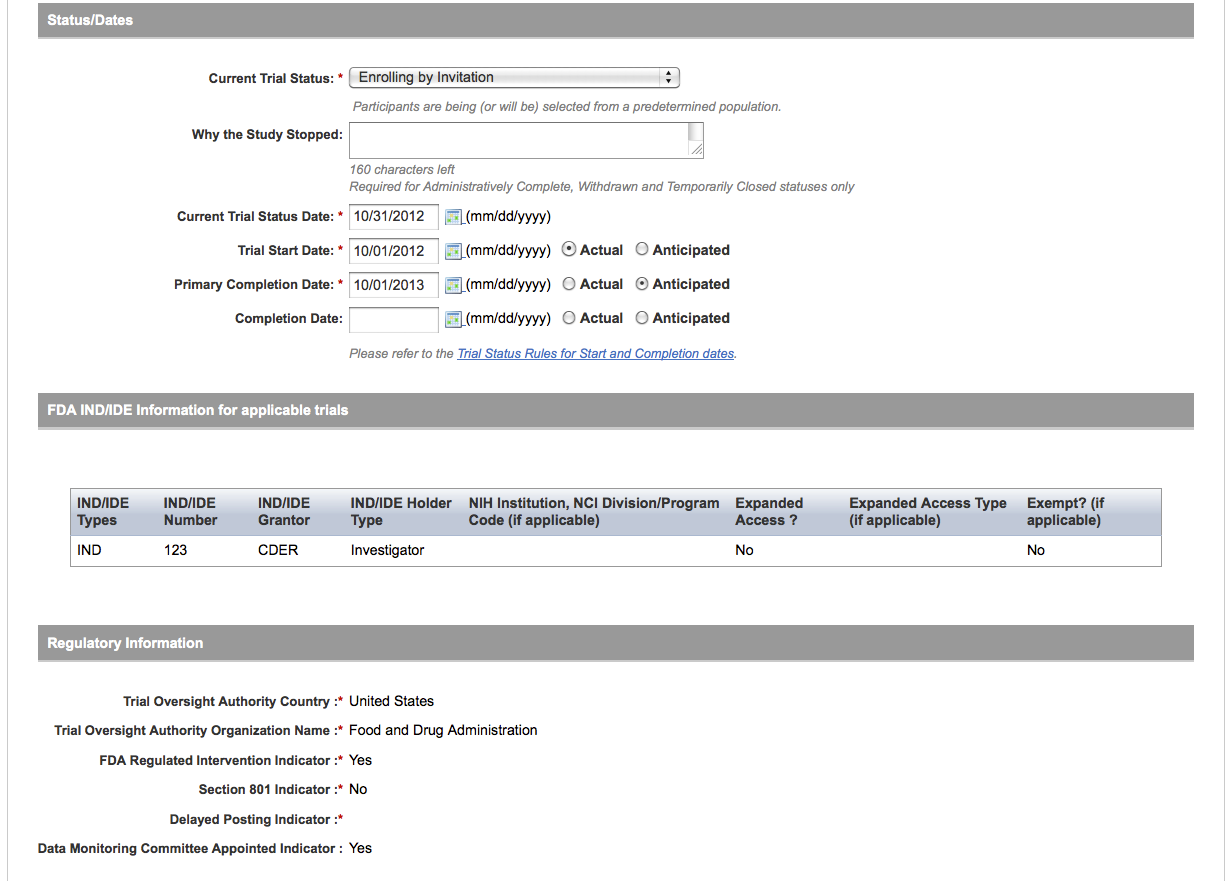
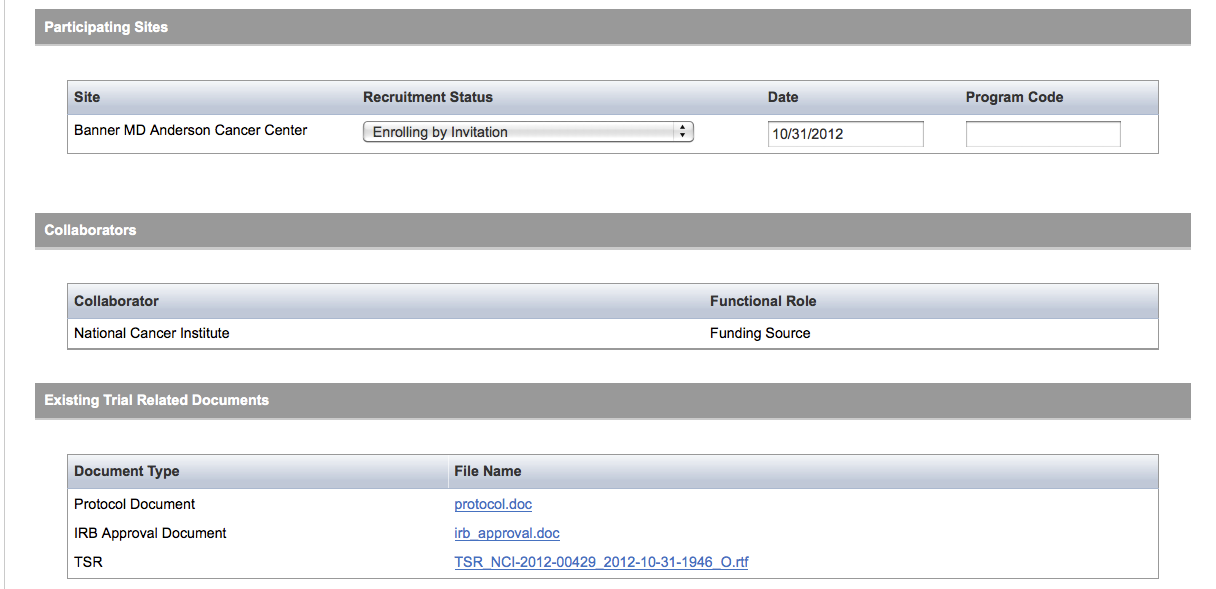
User selects to “Update Trial” and updates an existing trial record – see screenshots, pages 9 – 13, OR
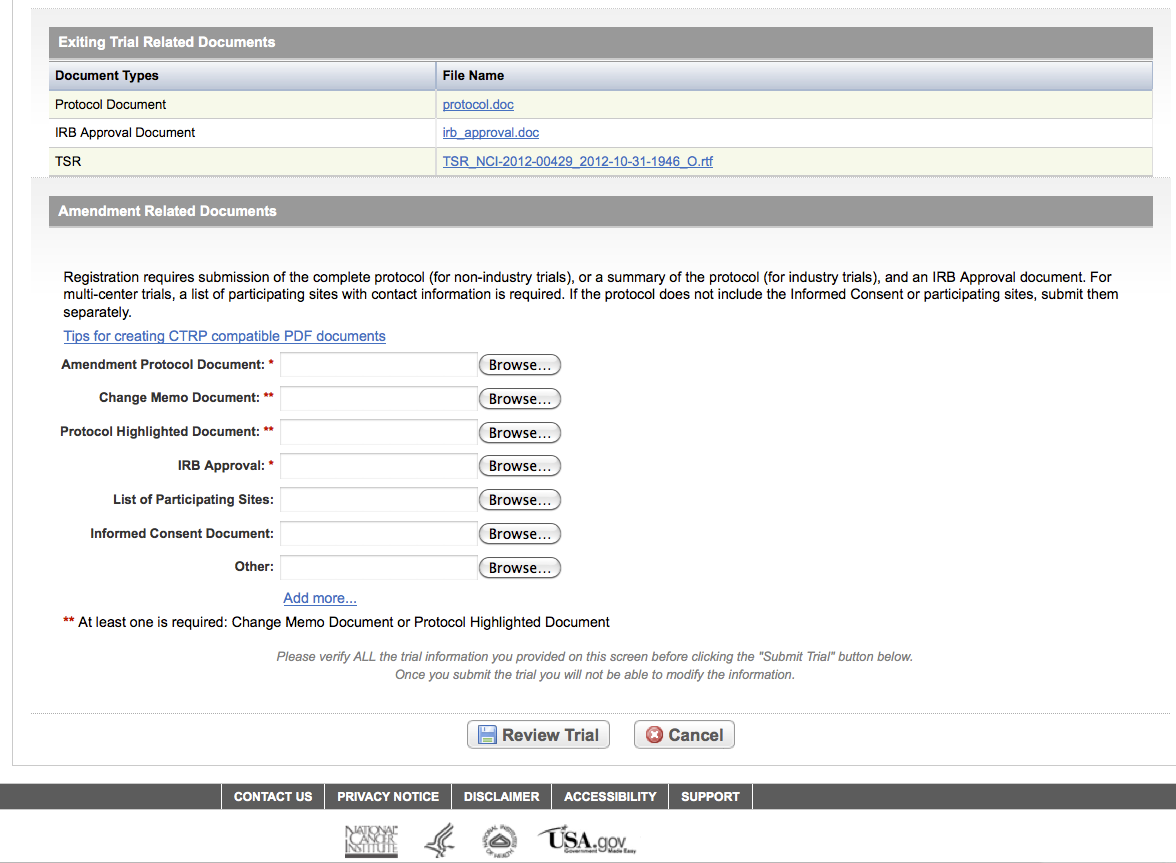
User selects to “Submit Trial Amendment” and amends an existing trial record – see screenshots, pages 14 – 19, OR
User selects to “Search Submitted Clinical Trials” and searches for an existing trial – see screenshot, page 20
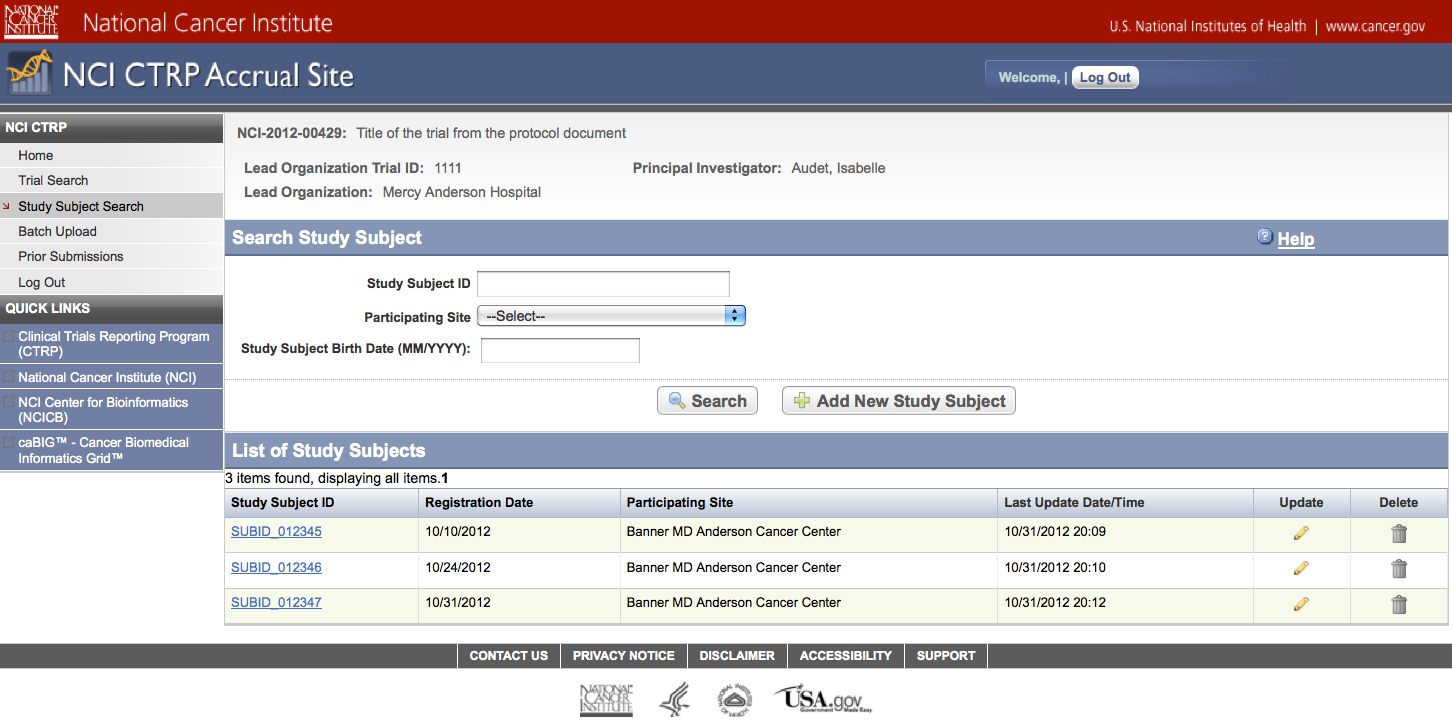
User selects to “Submit Study Subject Accrual Information” and submits subject level accrual information on a registered trial – see screenshots, pages 21 – 24
User selects to “Update Study Subject Accrual Information” and updates subject level accrual information on a registered trial – see screenshot, page 25
User selects to “Submit Aggregate Study Subject Accrual Information” and submits aggregate accrual information on a registered trial – see screenshot, page 26
CTRP Home page
CTRP Login Screen
CTRP Burden Statement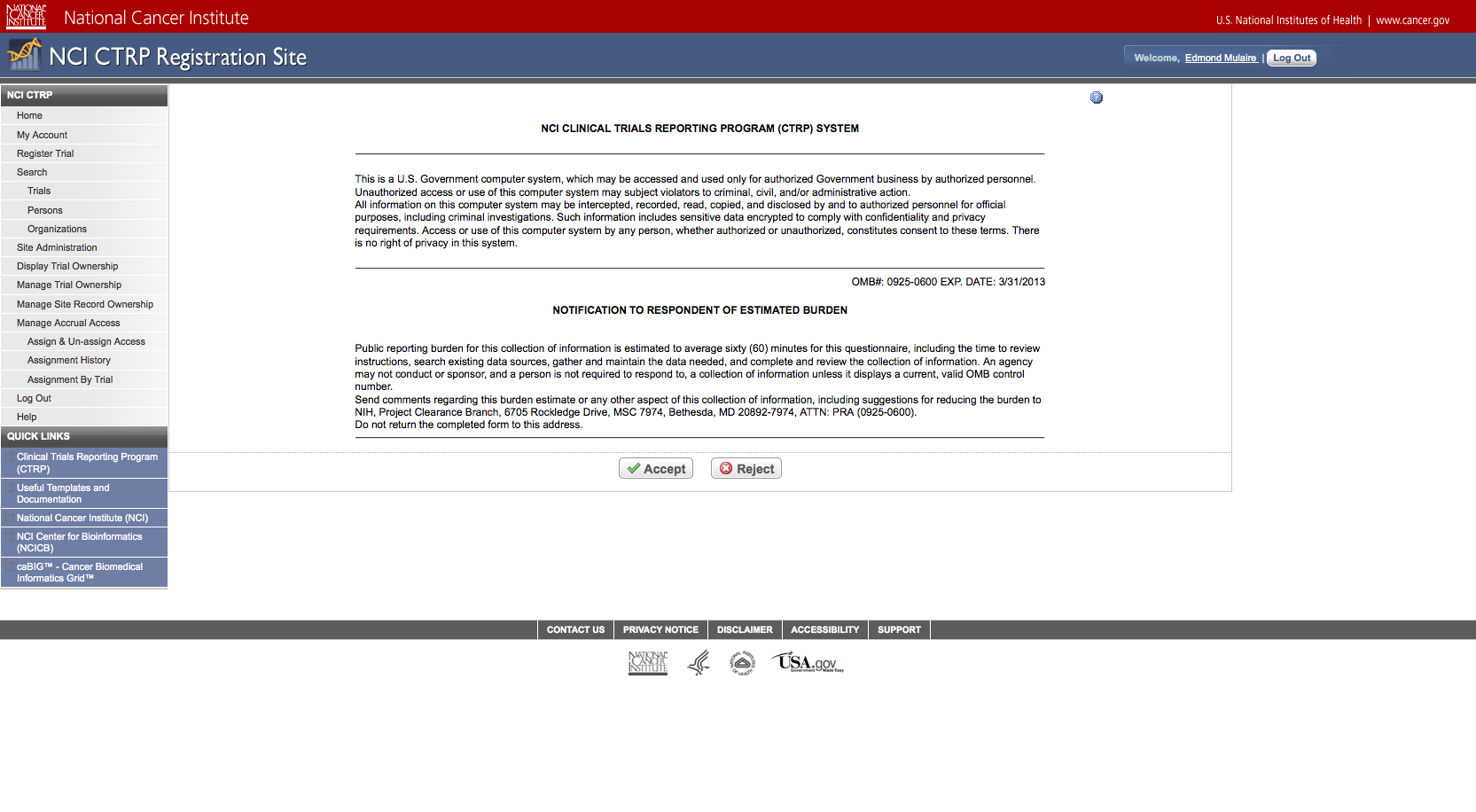
Initial Trial Registration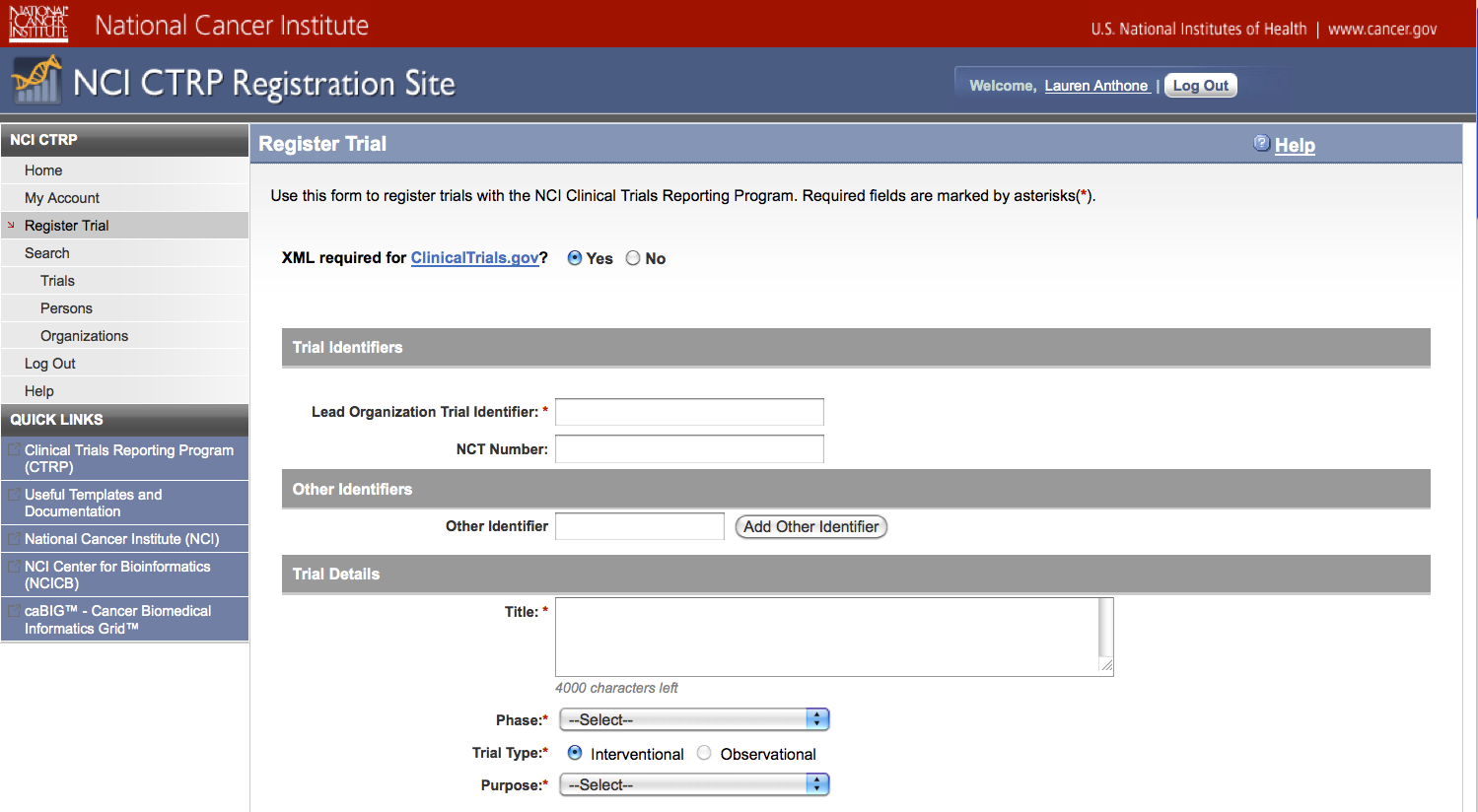

Update Trial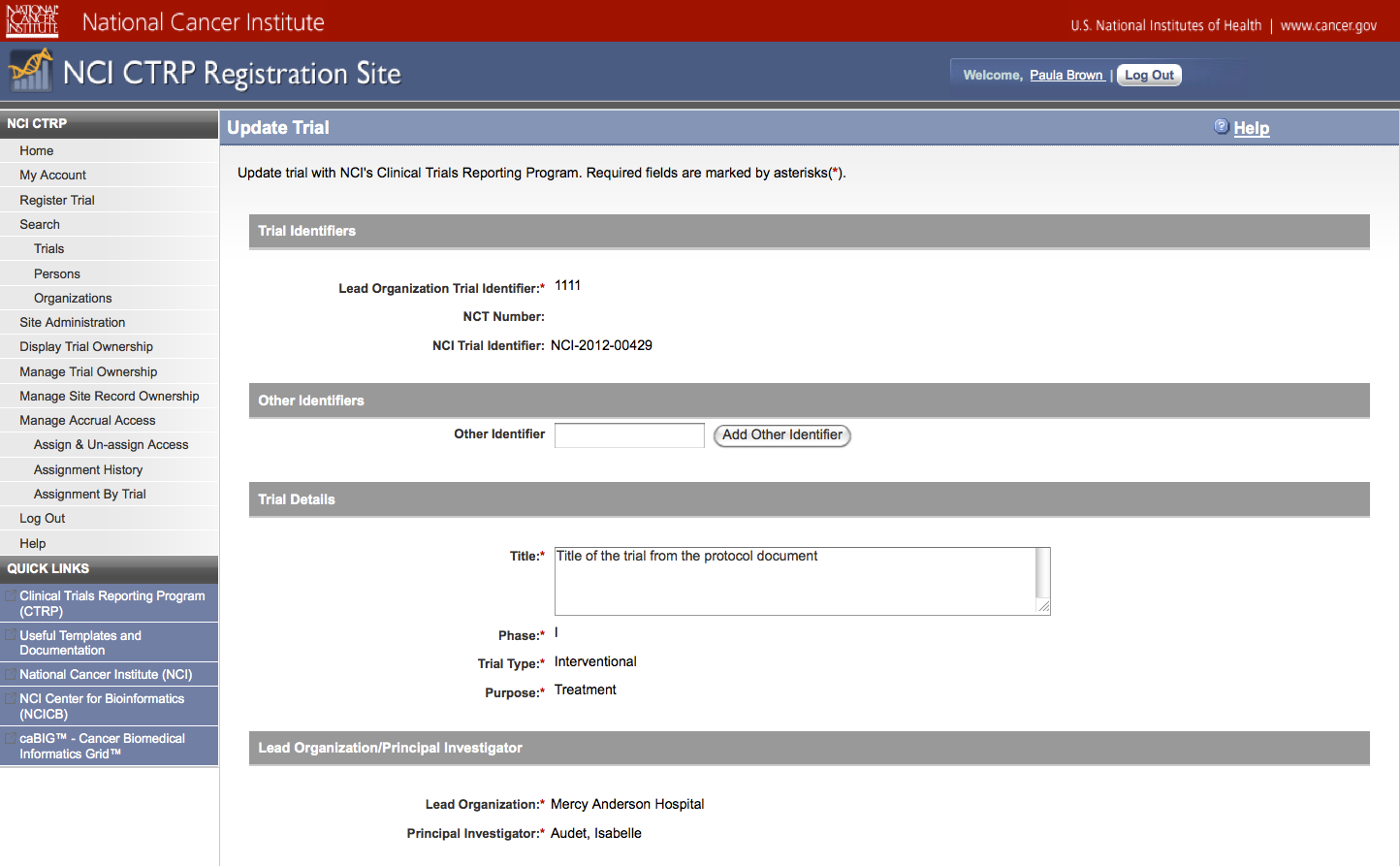
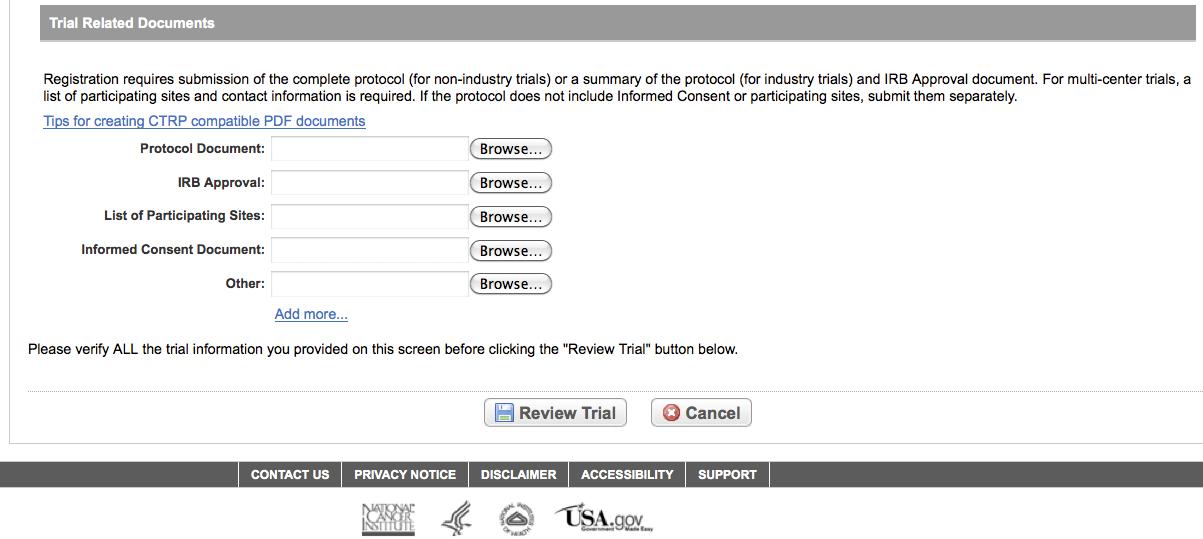
Submit Trial Amendment
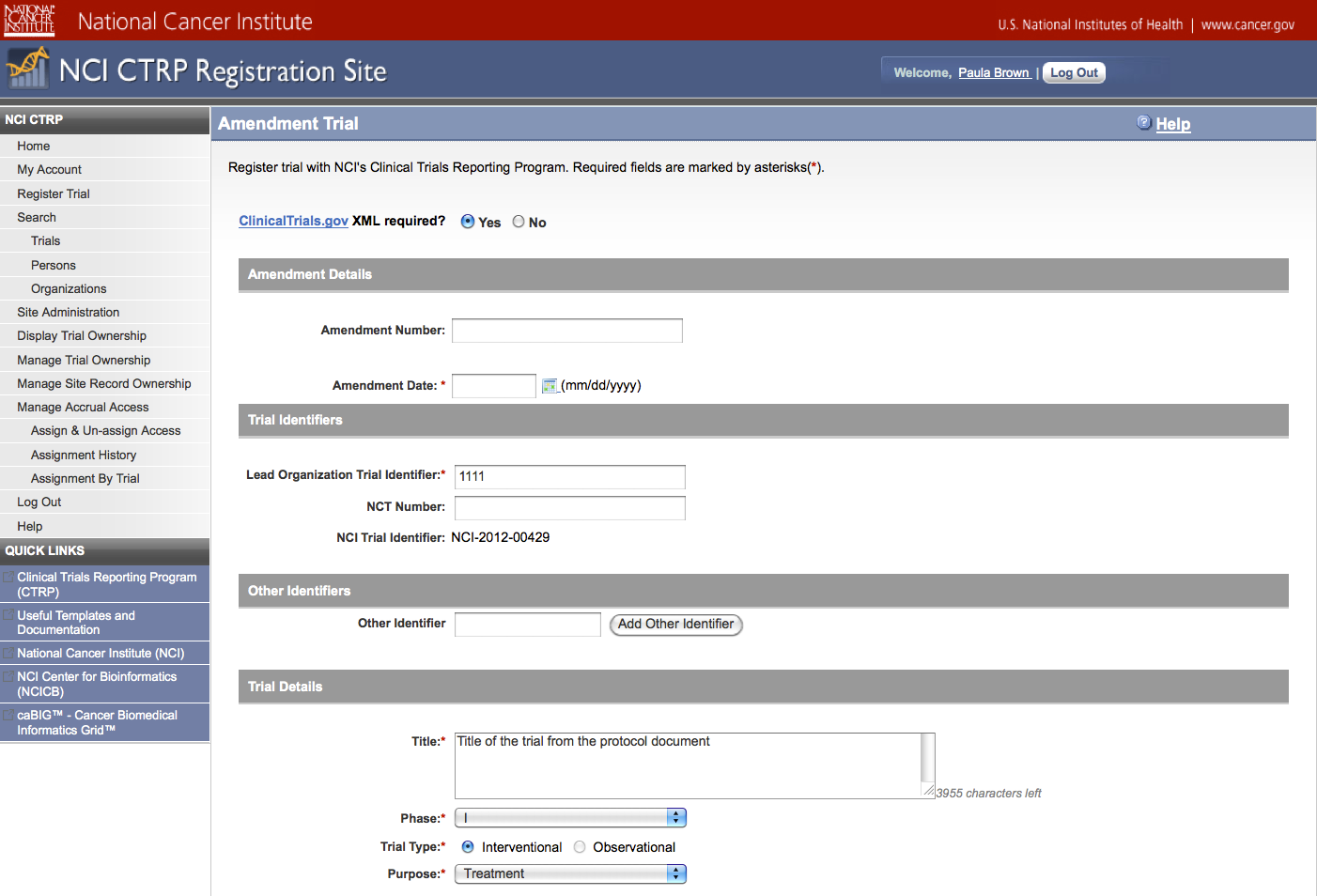
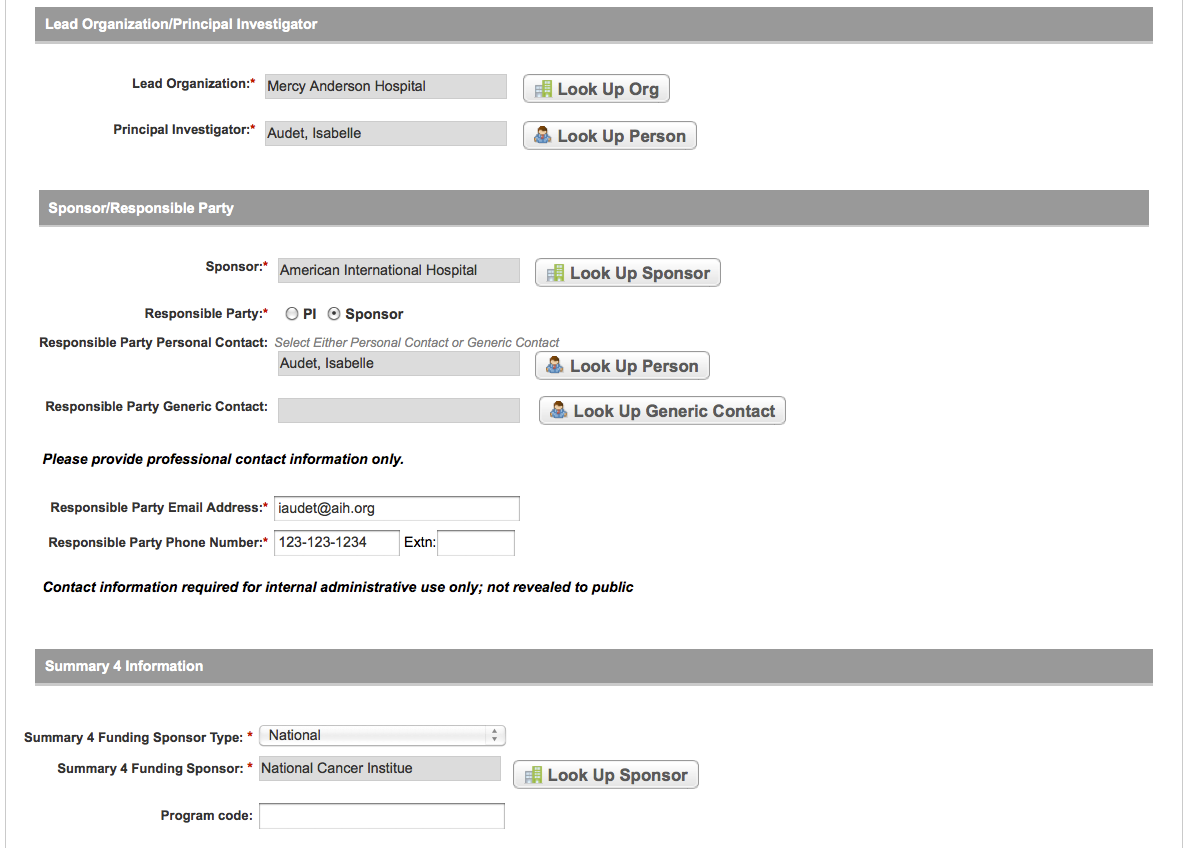

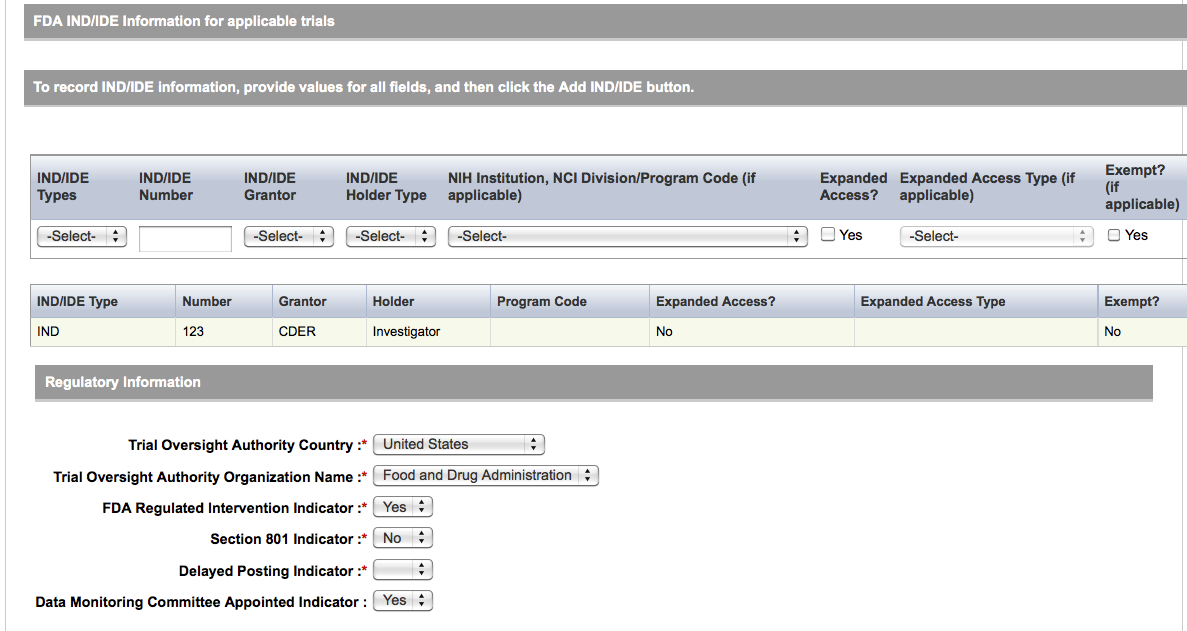
Search Submitted Clinical
Trials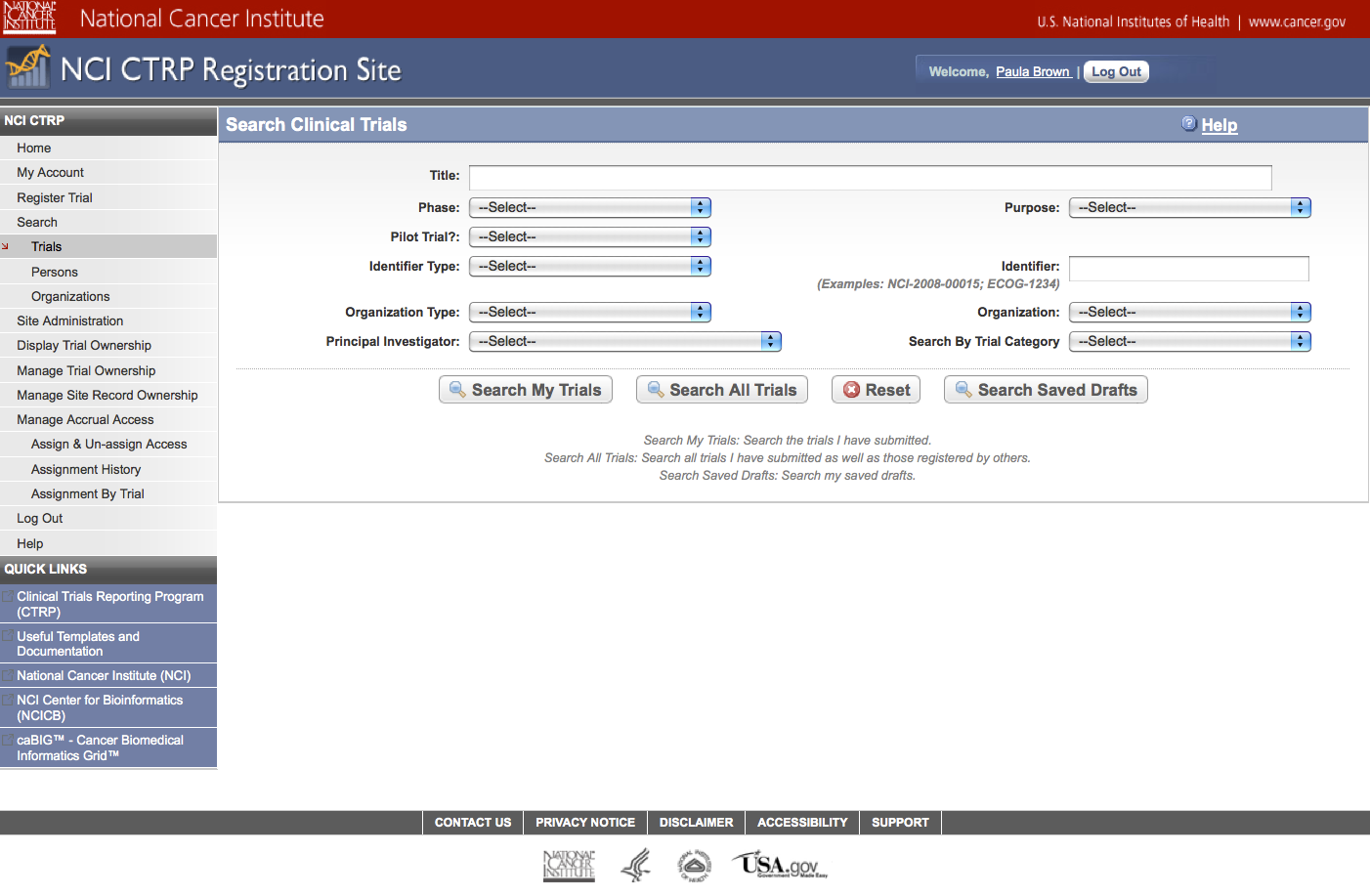
CTRP Accrual Burden
Statement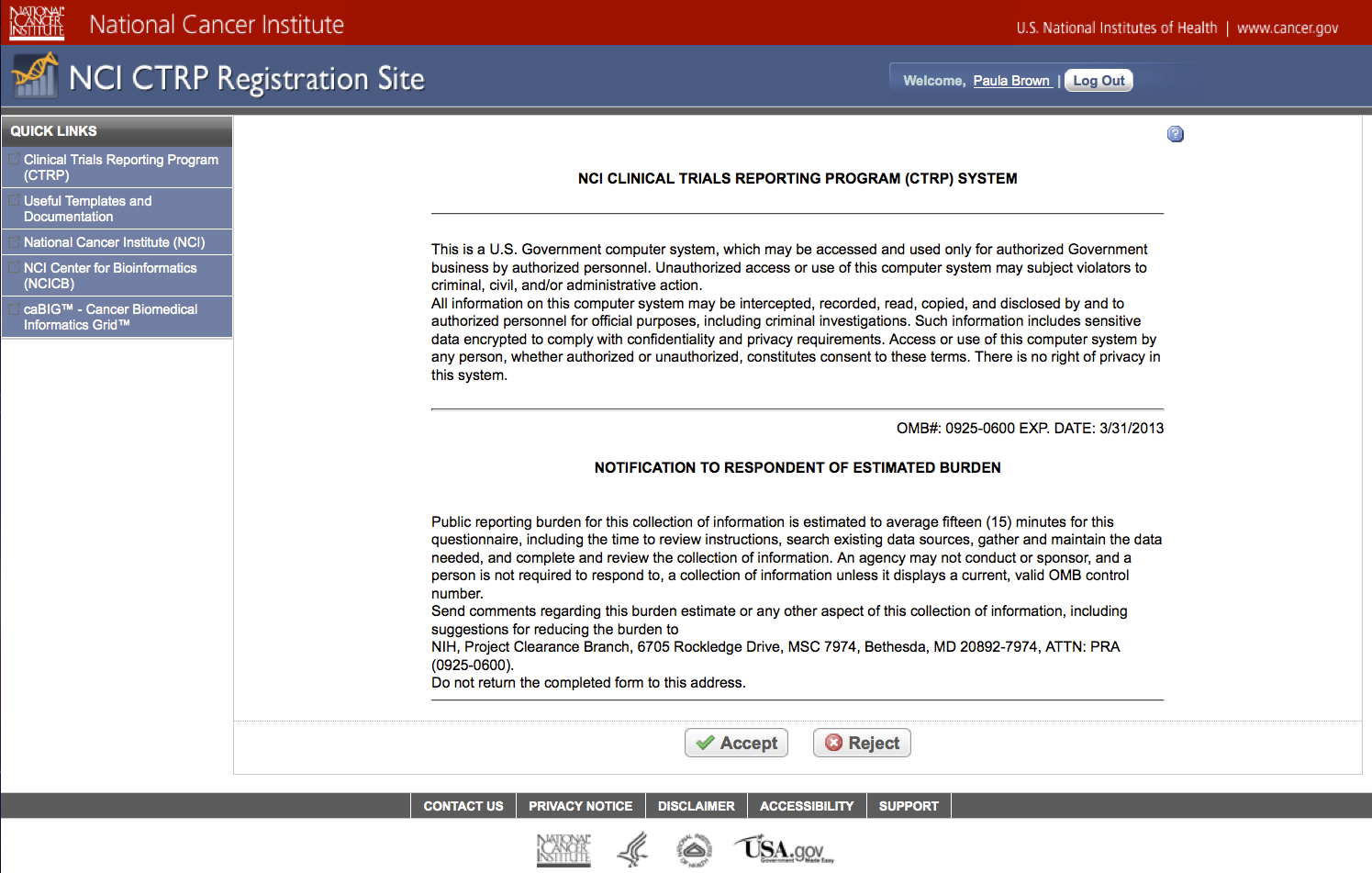
Submit Study Subject
Accrual Information

Update Study Subject
Accrual Information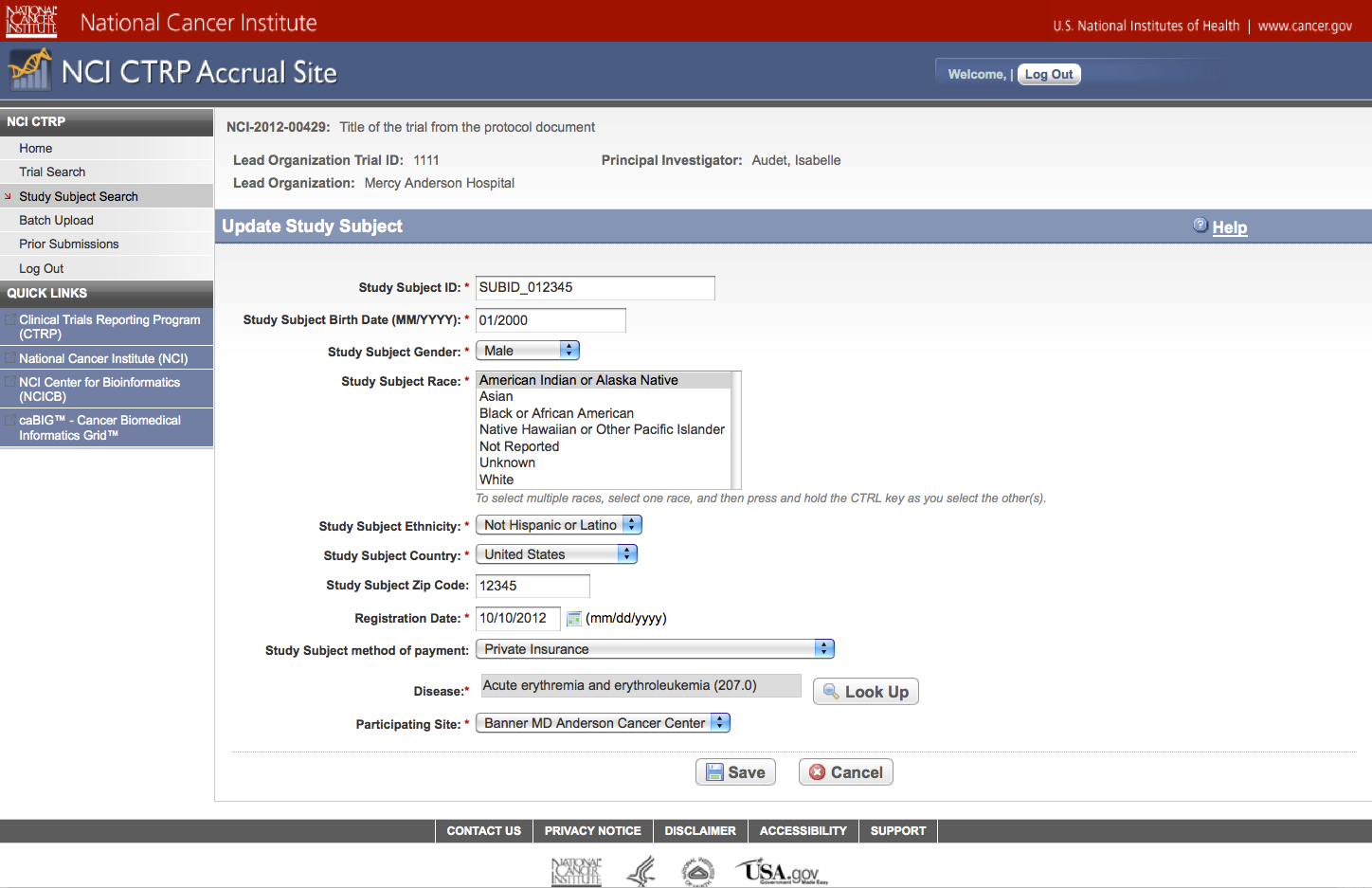
Submit Aggregate Study
Subject Accrual Information

| File Type | application/msword |
| Author | David Loose |
| Last Modified By | David Loose |
| File Modified | 2013-01-24 |
| File Created | 2012-11-19 |
© 2026 OMB.report | Privacy Policy