3 CTRP Accrual Batch File Tool
The Clinical Trials Reporting Program (CTRP) Database (NCI)
Attach_5E_CTRP_Accrual Batch File Tool.xlsm
NCI CTRP Accrual Portal Workflow and Screen Shots
OMB: 0925-0600
⚠️ Notice: This form may be outdated. More recent filings and information on OMB 0925-0600 can be found here:
Document [xlsx]
Download: xlsx | pdf
Instructions
Input
Collections
Patients
Races
Accrual Count
Export
Definitions



Overview
DisclaimerInstructions
Input
Collections
Patients
Races
Accrual Count
Export
Definitions
Sheet 1: Disclaimer
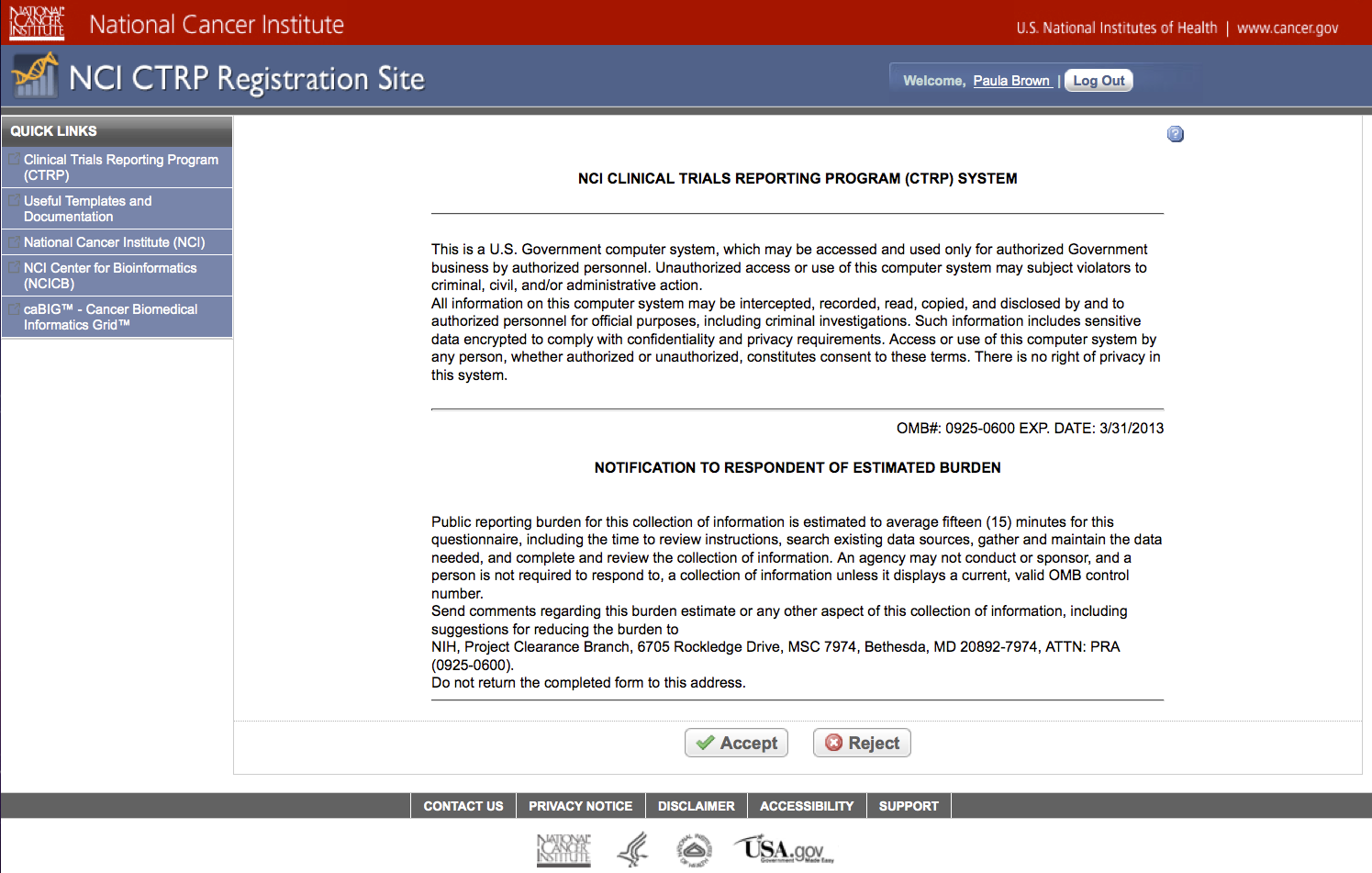
|
||||||||||||||
Sheet 2: Instructions
| CTRP Accruals Spreadsheet | |||
| Revision | Purpose | Date | Author |
| 1.0 | Initial draft | 9/23/2012 | Patrick McConnell |
| 1.1 | Changed language | 10/10/2012 | Patrick McConnell |
| 1.2 | Changed language | 10/17/2012 | Patrick McConnell |
| Purpose | |||
| The purpose of this Workbook is to provide an mechanism to capture accruals data to be imported into the CTRP accruals application using the Batch Import functionality. This is an alternative to entering data directly into the CTRP Accruals application using the website, generating a batch upload file directly, or using the CTRP accruals APIs. The ultimate goal of using this Workbook is to export data for import into CTRP Accruals using the Batch Import function on the CTRP Accruals website. Use of that website is outside the scope of these instructions | |||
| Entering Data | |||
| Data can be entered either through the Input Worksheet or directly into the Collections, Patients, Races, and Accrual Count Worksheets. The Input Worksheet requires that you first enter study details into the first section and then click either the Complete Trials button or Abbreviated Trials button to view the rest of the fields that are available. Then, click the Add Subject or Add Accrual button respectively. If the data is valid and entered correctly, it will be moved into the correct worksheet. If you enter data into the Collections, Patients, Races, and Accrual Count Worksheets manually, you must insure that identifiers are correctly maintained across the spreadsheets and that the data is appropriately formatted. You can click the field names on the Input Worksheet to view the definition of the field. | |||
| Exporting Data | |||
| Data is exported to a CSV file by navigating to the Export Worksheet and clicking the Export button. You will be prompted for a file name, and any existing file will be overwritten. You can only export data for Complete Trials and Abbreviated trials separately by clicking the appropriate Export button. Clicking the Clear All Data button will erase data from the Collections, Patients, Races, and Accrual Count worksheets. | |||
Sheet 3: Input
| Study Details | ||||||
| *Study Id | Change Code | |||||
Add Accrual
|
||||||
| Add Accrual | ||||||

Sheet 4: Collections
| Study Id | Change Code |
Sheet 5: Patients
| Study Id | Study Subject Identifier | Zip Code (if US) | Country of Residence | Patient’s Date of Birth | Gender of a Person | Ethnicity | Payment Method | Subject Registration Date | Registering Group Identifier | Study Site Identifier | Subject Disease Code |
Sheet 6: Races
| Study Id | Study Subject Identifier | Race |
Sheet 7: Accrual Count
| Study Id | Study Site Accrual Count | Study Site Identifier |
Sheet 8: Export

|

|
||||

|
|||||
Sheet 9: Definitions
| Data Element | Definition |
| *Study Id | This is the unique identifier assigned to the study. |
| Change Code | Whether the data has not changed since the last report |
| *Study Subject Identifier | Unique identifier (PO ID) assigned to the institution accruing the patient to the study. |
| *Zip Code (if US) | The string of characters used to identify the five-digit Zone Improvement Plan (ZIP) code that represents the geographic segment that is a subunit of the ZIPcode, assigned by the U.S. Postal Service to a geographic location to facilitate mail delivery. |
| *Country of Residence | The name of a country from which a person or their biological family had previous residence or past ancestors. Condition: either Zip code (if U.S resident) or country (if not U.S resident) is mandatory. |
| *Patient’s Date of Birth | The month and year on which the person was born |
| *Gender of a Person | Text designations that identify gender. Gender is described as the assemblage of properties that distinguish people on the basis of their societal roles. |
| *Ethnicity | The text for reporting information about ethnicity based on the Office of Management and Budget (OMB) categories. |
| Payment Method | Text term for an entity, organization, government, corporation, health plan sponsor, or any other financial agent who pays a healthcare provider for the healthcare service rendered to a person or reimburses the cost of the healthcare service.<y. |
| *Subject Registration Date | Date the subject was registered for the study.<y. |
| Registering Group Identifier | Unique identifier (PO ID) assigned to the group that originally registered the patient for the study |
| *Study Site Identifier | Unique identifier (numeric or alphanumeric) assigned to the study site |
| *Subject Disease Code | Code that identifies a disease.< study.<y. |
| *Race | The text for reporting information about race based on the Office of Management and Budget (OMB) categories.<y. |
| *Study Site Accrual Count | Numeric count of subjects accrued at a study site to date |
| File Type | application/vnd.openxmlformats-officedocument.spreadsheetml.sheet |
| File Modified | 0000-00-00 |
| File Created | 0000-00-00 |
© 2026 OMB.report | Privacy Policy