Scripts Forms and Instructions for Home Urine Collection
The NHANES Longitudinal Study – Feasibility Component
Att 3c_Living SP Forms_HUC_170209
Field Feasibility Test Home Urine Collection
OMB: 0920-1176
Attachment 3c
Suggested Scripts and Data Collection Forms
for Presumed Living Participants –
Home Urine Collection
Home Urine Collection
Objectives:
Home urine collection is conducted to obtain laboratory results that provide objective measures of biomarker data to assess various health conditions and nutritional status. Inclusion of home urine collection in the present study will help us assess participants’ willingness in providing a urine sample in a home environment for a longitudinal study.
Collected urine samples will be tested for urinary albumin and creatinine values. These values will be used to calculate the urine albumin/creatinine ratio which is recommended by the National Kidney Disease Education Program as the diagnostic test to ascertain early chronic kidney disease. Reporting albumin/creatinine ratio back to the participants will provide added benefit for their participation to the study.
Protocol:
A urine collection kit will be given to participants in their home. A Health Representative will review instructions on how to collect and mail the urine specimen back to the testing laboratory. See instructions in Appendix 3c-1. Participants are asked to send the sample back via US Postal Service in a pre-addressed, pre-paid box within 5 days. A reminder (Appendix 3c-2) will be sent to the participant if the sample has not been recorded in the USPS tracking system as being received after 7 days. This protocol is similar to what was used in NHANES from 2009 to 2014.0
Eligibility:
All participants in the feasibility study.
Risks:
There is no known risk to the participants.
Respondent Burden:
It is estimated that the home urine collection will take approximately 15 minutes to complete.
Justification for Using Vulnerable Populations:
There is no reason to exclude pregnant women, mentally impaired individuals, or persons with functional difficulties because there is no contraindication if they can understand and follow the exam instructions.
Report of Findings:
Laboratory Urine Test |
Result |
Units |
Flag |
Reference Range |
Albumin Creatinine Ratio |
|
mg/g |
High/Blank |
< 30 |
The reference range for albumin creatinine ratio is < 30 mg/g. The Flag field will display “High” if the values equal to or greater than 30 mg/g. The field will be blank if the value is below 30 mg/g.
In addition to the flag, for persons with an abnormal albumin creatinine ratio value (i.e., 30 mg/g), the sentence “We reviewed your test results from your examination on <insert date>, and found that some values were abnormal and require your immediate attention” will also be included in the cover letter of their final report of findings (See Appendix 7-2 in Attachment 7).
Appendix 3c-1. “What to Do” Urine Collection and Shipping Instructions
Form Approved
OMB No. 0920-xxxx
Exp. Date xx/xx/20xx
Assurance of Confidentiality – We take your privacy very seriously. All information that relates to or describes identifiable characteristics of individuals, a practice, or an establishment will be used only for statistical purposes. NCHS staff, contractors and agents will not disclose or release responses in identifiable form without the consent of the individual or establishment in accordance with section 308(d) of the Public Health Service Act (42 USC 242m(d)) and the Confidential Information Protection and Statistical Efficiency Act of 2002 (CIPSEA, Title 5 of Public Law 107-347). In accordance with CIPSEA every NCHS employee, contractor, and agent has taken an oath and is subject to a jail term of up to five years, a fine of up to $250,000, or both if he or she willfully discloses ANY identifiable information about you. In addition, NCHS complies with the Cybersecurity Enhancement Act of 2015. This law requires the Federal government to protect its information by using computer security programs to identify cybersecurity risks against federal computer networks.
NOTICE - CDC estimates the average public reporting burden for this collection of information as 15 minutes per response, including the time for reviewing instructions, searching existing data/information sources, gathering and maintaining the data/information needed, and completing and reviewing the collection of information. An agency may not conduct or sponsor, and a person is not required to respond to a collection of information unless it displays a currently valid OMB control number. Send comments regarding this burden estimate or any other aspect of this collection of information, including suggestions for reducing this burden to CDC/ATSDR Information Collection Review Office, 1600 Clifton Road, NE, MS D-74, Atlanta, Georgia, 30333; ATTN: PRA (0920-xxxx).
“WHAT TO DO”
Urine Collection and Shipping Instructions
Collect a urine sample and ship it to the laboratory using these instructions. |
Collect your urine sample as soon as possible - ideally within the next 5 days. However, if you cannot collect it within 5 days then please collect and ship it at your earliest convenience. |
An additional $20.00 will be added to your debit card as soon as you ship the package. |
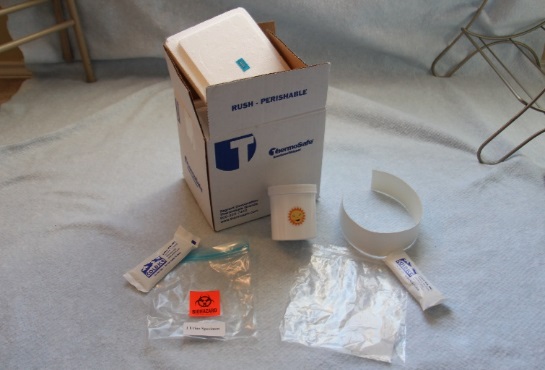
The urine kit includes:
|
|
|
|
|
Home Urine Collection Kit Contents:
|
|
|
|
|
|
|
|
|
|
|
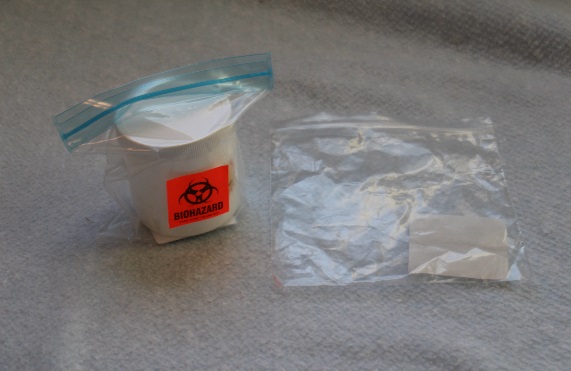
Your Personal Picture Sticker:
Your picture sticker is unique to you and it is found on:
The picture sticker identifies your kits from kits assigned to other people in your household. When collecting and shipping your urine, make sure you use the collection cups and shipping boxes marked with your unique picture sticker. |
|
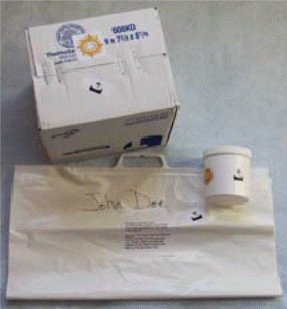
Kit Preparation:
1. Remove the ice packs from your large tote bag and place them in the freezer compartment of your home freezer for at least 6 hours before you collect your samples. Freeze the ice packs flat. |
|
2. When you are ready to collect your sample, remove the kit from the large tote bag. |
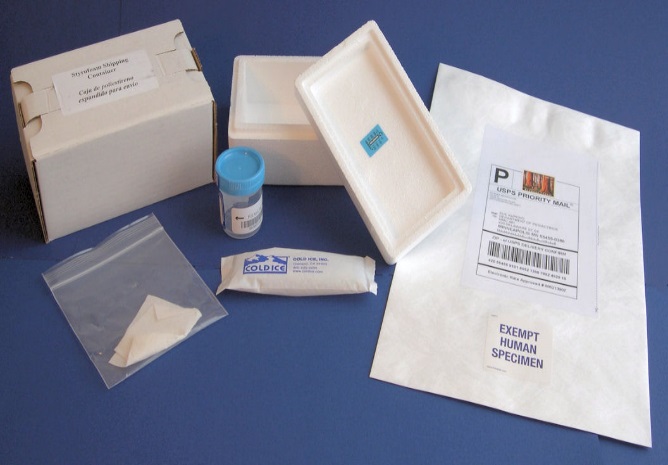
|
3. Open the white cardboard box. |
|
4. Remove the Styrofoam lid. |
|
5. Remove the collection cup from the box. |
Collect the Urine Sample:
Whenever possible, mail the sample on the same day you collect the sample. |
|
|
|
|
|
|
Pack the Urine Samples:
Pack the sample in its shipping container with two ice packs immediately after collection, even if you do not mail the package until later in the day. |
||
Discard these instructions and the large tote bag that the kit came in. Do not add additional packaging. |
||
|
|
|
|
|
|
|
|
|
|
|
|
Ship the Urine Sample:
Use the US Postal Service (USPS) to send the package. |
|
Take the packages inside the Post Office and hand it to a clerk. |
|
Or, take the packages inside the Post Office and put it in the large priority mail drop box. (The large priority drop box is usually located near the self-service kiosk).
If you have any questions call 1-8XX-XXX-XXX. |
|
Appendix 3c-2. Reminder Card
Reminder0
NHANES Urine Collection Kit
|
You recently completed a home health examination as part of the National Health and Nutrition Examination Survey (NHANES). You received the urine collection kit during this home visit.
Please remember to mail the urine samples to the laboratory using the kit that were given to you. |
Once you mail the urine samples, we will add $20.00 to your debit card, as promised. |
|
We appreciate your participation and interest in NHANES. |
|
If you have any questions, please call the toll free number 1-8XX-XXX-XXXX.
If you have already mailed your kit, please disregard this notice.
0 Starting in 2009, NHANES MEC examined participants were asked to collect a home urine specimen one week after the examination and to mail their specimen to the laboratory. Of all participants who were given a home urine collection kit in 2009-2013, 89 - 95% mailed the home-collected urine sample back to the laboratory. In 2014, the home urine collection was only offered to survey participants who were successfully compliant with the 24-Hour Urine Collection project. Of all participants who were given a home urine collection kit during this time, 83% mailed the home-collected urine sample back to the laboratory.
0 The reminder will be sent in an envelope via first-class mail
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| File Title | CDC INSTITUTIONAL REVIEW BOARD (IRB) |
| Author | vlt0 |
| File Modified | 0000-00-00 |
| File Created | 2021-01-22 |
© 2026 OMB.report | Privacy Policy