Form Section B: PDO Section B: PDO Section B: PDO
Division of State Programs—Management Reporting Tool (DSP-MRT)
DSP_MRT_Attachment # 3 - rev_PDO_FR_CARA_06.01.2018
Section B: PDO
OMB: 0930-0354
Division
of State Programs–Management Reporting Tool |
DSP-MRT Supplement for PDO, FR-CARA, and Related Grants |
OMB No: 0930-0354
Expiration Date: 7/31/2020
Contents
Accomplishments and Barriers/Challenges 4
Advisory Council and Other Workgroup Meetings 5
Training and Technical Assistance (TA) 7
Accomplishments and Barriers/Challenges 8
Behavioral Health Disparities 9
Disparities Impact Statement (DIS) 9
Population(s) Experiencing the Disparity 9
Access to Prevention Efforts 9
Use and Reach of Prevention Efforts 9
Outcomes of Prevention Efforts 9
Accomplishments and Barriers/Challenges 9
Promising Approaches and Innovations 10
High-Need Community Policies/Protocols 12
Naloxone Education and Other Opioid-Related Trainings 13
High-Need Community-Level Trainings 13
Training Data Collection Information 17
Kits Distributed to Partner Organizations 19
Naloxone Administration by Partner Organization 21
Accomplishments and Barriers/Challenges 25
Accomplishments and Barriers/Challenges 26
Accomplishments and Barriers/Challenges 27
Grantee-Level Overdose Data 28
High-Need Community-Level Overdose Data 30
Note: This document is intended as a supplement to the Division of State Programs–Management Reporting Tool (DSP-MRT). Please refer to the DSP-MRT document where applicable. Also for grantees reporting for a grant other than the PDO/Naloxone Distribution Grant, all references to “naloxone” should be considered a drug or device approved or cleared under the Federal Food, Drug, and Cosmetic Act for emergency treatment of known or suspected opioid overdose.
Administration
Partner organization is used to indicate any of the selected high-need community’s partners (e.g., law enforcement agencies, syringe exchange programs) that receive opioid overdose reversal kits or training, or distribute opioid reversal drugs or devices to laypersons through the grant. Note that the subrecipient may also be considered a partner organization if it will be providing these activities (e.g., distributing to laypersons) rather than simply engaging and coordinating with the other partner organizations. Exhibit 1 illustrates the involved levels and provides an example at each level.
Exhibit 1. Levels of Data Reporting
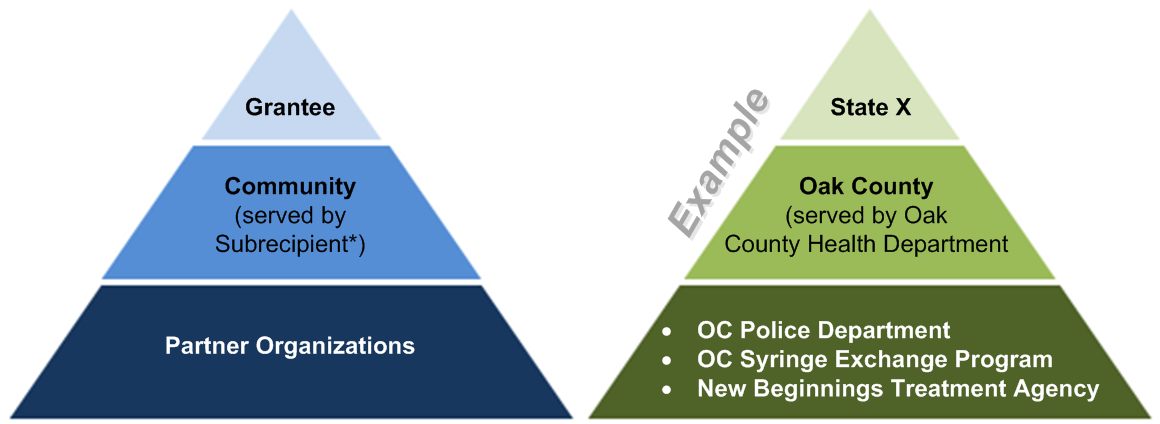
* Some grantees may not have subrecipients. Also, some subrecipients may serve more than one selected high-need community.
Grantee Information
See DSP-MRT.
Subrecipients
See DSP-MRT.
High-Need Communities
See DSP-MRT.
Partner Organizations
Use this section to add or update partner organization information for each selected high-need community. Partner organizations are the entities receiving naloxone drugs or naloxone training (e.g., law enforcement agencies) or distributing to and training laypersons (e.g., syringe exchange programs). Please note: if you are reporting for a grant other than the PDO/Naloxone Distribution Grant, all references to “naloxone” should be considered a drug or device approved or cleared under the Federal Food, Drug, and Cosmetic Act for emergency treatment of known or suspected opioid overdose.
In a later section of the progress report, you will be asked to report on naloxone drugs distributed to these partner organizations, and the naloxone administration events reported by these partner organizations for this grant. Note that if the subrecipient for the selected high-need community will also be providing these activities (e.g., distributing to laypersons) rather than simply engaging and assisting the other partner organizations, you will need to enter the subrecipient as a partner organization here. Partner organization information will be carried over from one reporting period to the next.
Item |
Response Options |
In the SPARS data collection system, there will be an “Add” button for this section. Grantees will be able to click that button to add additional records as needed. |
|
Partner organization name |
Free text |
High-Need Community |
|
Sector |
|
Target ZIP codes of the partner organization’s service area |
You will see an add button and a USPS ZIP code look-up link. |
Target County or Counties (Alternative)
(If this partner organization targets an entire county (or counties), indicate the county name(s) here.) |
Free text |
Needs Assessment
Needs Assessment
See DSP-MRT.
Accomplishments and Barriers/Challenges
See DSP-MRT.
Capacity
Membership
See DSP-MRT.
Advisory Council and Other Workgroup Meetings
See DSP-MRT.
Grantee Funding Resources
See DSP-MRT.
Other Resources
Leveraged Resources
Use this section to enter information regarding leveraging resources, including grantee-level opioid workgroups and grantee-level funding resources. Grantee is used to indicate the state/tribal entity/jurisdiction receiving the award.
Unless the information changes from one reporting period to another, this information only needs to be entered once per fiscal year. Please note: if you are reporting for a grant other than the PDO/Naloxone Distribution Grant, all references to “naloxone” should be considered a drug or device approved or cleared under the Federal Food, Drug, and Cosmetic Act for emergency treatment of known or suspected opioid overdose.
Item |
Response Options |
Does a grantee-level workgroup exist in your state/tribal entity/jurisdiction addressing opioid issues (prescribing, misuse, treatment, overdose)? |
|
Does the opioid workgroup serve as your PDO/Naloxone Advisory Council? (This item will only appear if YES is selected for the previous item) |
|
Does a grantee-wide strategic plan exist addressing opioid issues, including prevention of misuse, treatment, and overdose prevention? |
|
How are opioid prevention efforts integrated into the state-wide agenda for opioids? |
Free text |
In what ways have you coordinated opioid funding streams in your state/tribal entity/jurisdiction? |
Free text |
In what ways is your training curriculum informed by or congruent with the SAMHSA Opioid Overdose Prevention Toolkit? |
Free text |
Data Infrastructure
Use this section to enter information regarding data infrastructure and activities. Data infrastructure refers to a system or systems for collecting and disseminating data related to naloxone education trainings, distribution, and administration; and opioid overdose. Please note: if you are reporting for a grant other than the PDO/Naloxone Distribution Grant, all references to “naloxone” should be considered a drug or device approved or cleared under the Federal Food, Drug, and Cosmetic Act for emergency treatment of known or suspected opioid overdose.
Item |
Response Options |
Do you have systems in place for collecting data on naloxone administrations? |
|
Which sectors report data into the system(s)? (This item will only appear if YES is selected for the previous item)
|
|
During this reporting period, have you engaged in efforts to:
…Enhance data infrastructure to track naloxone education trainings? |
|
…Enhance data infrastructure to track naloxone distribution or administration? |
|
… Enhance opioid overdose data infrastructure? |
|
… Enhance access to existing opioid overdose data sources? |
|
Did you provide naloxone or opioid-related data to local community stakeholders during this reporting period? |
|
Training and Technical Assistance (TA)
See DSP-MRT.
Please note that this section only includes trainings and technical assistance (TA) to enhance grantee/partner capacity, such as training around using project data collection systems, building community partnerships, and implementing media campaigns. This section does not include naloxone administration trainings or other types of trainings that are intended to influence outcomes (e.g., trainings related to opioid prescribing or medication-assisted treatment), as such trainings are recorded in the Implementation section of this progress report.
Accomplishments and Barriers/Challenges
See DSP-MRT.
Planning
Needs Assessment
See DSP-MRT.
Accomplishments and Barriers/Challenges
See DSP-MRT.
Behavioral Health Disparities
Disparities Impact Statement (DIS)
See DSP-MRT.
Population(s) Experiencing the Disparity
See DSP-MRT.
Focus and Data Gaps
Access to Prevention Efforts
See DSP-MRT.
Use and Reach of Prevention Efforts
See DSP-MRT.
Outcomes of Prevention Efforts
See DSP-MRT.
Accomplishments and Barriers/Challenges
See DSP-MRT.
Implementation
Promising Approaches and Innovations
See DSP-MRT.
Policy
Use this section to report information about state-level policies related to naloxone or similar drugs. Most of the information will be prepopulated based on publicly available, state-level information at the time of the grant award. Please review for accuracy (to the best of your knowledge), and update this section when naloxone policies change in your state. Grantee is used to indicate the state/tribal entity/jurisdiction receiving the award from SAMHSA/CDC. Please note: if you are reporting for a grant other than the PDO/Naloxone Distribution Grant, all references to “naloxone” should be considered a drug or device approved or cleared under the Federal Food, Drug, and Cosmetic Act for emergency treatment of known or suspected opioid overdose.
Naloxone Access Laws
Item |
Response Options |
Does the state/tribal entity/jurisdiction have a naloxone access law (legislation designed to improve access to naloxone?) (If YES is checked, the items below will appear.) |
|
Prescribing and Dispensing Policies |
|
Do prescribers have CIVIL immunity for prescribing, dispensing, or distributing naloxone to a layperson? |
|
Do prescribers have CRIMINAL immunity for prescribing, dispensing, or distributing naloxone to a layperson? |
|
Do prescribers have DISCIPLINARY immunity for prescribing, dispensing, or distributing naloxone to a layperson? |
|
Do dispensers (pharmacists) have CIVIL immunity for prescribing, dispensing, or distributing naloxone to a layperson? |
|
Do dispensers (pharmacists) have CRIMINAL immunity for prescribing, dispensing, or distributing naloxone to a layperson? |
|
Do dispensers (pharmacists) have DISCIPLINARY immunity for prescribing, dispensing, or distributing naloxone to a layperson? |
|
Are prescriptions to third parties (e.g., family members, friends) authorized? |
|
Are insurers required to pay for naloxone drugs dispensed to third parties? |
|
Are insurers restricted from having a prior authorization policy for naloxone drugs prescriptions? |
|
Is prescription by a standing order authorized? |
|
Is a statewide standing order in place? (Will only appear if YES is selected for the previous item.) |
|
Do pharmacists have authority to initiate prescriptions for naloxone (prescriptive authority)? |
|
Layperson Administration/Possession Policies |
|
Is a layperson immune from CIVIL liability when administering naloxone drugs? |
|
Is a layperson immune from CRIMINAL liability when administering naloxone drugs? |
|
Is participation in a naloxone education program required as a condition of immunity? |
|
Good Samaritan Laws
Item |
Response Options |
Does the state have an overdose Good Samaritan law (legislation designed to reduce criminal concerns when a layperson summons aid during an overdose)? (If YES is checked, the items below will appear.) |
|
What protection, if any, does the Good Samaritan law provide from controlled substance possession laws? Protection from…
|
(For each type of protection) |
What protection, if any, does the Good Samaritan law provide from drug paraphernalia laws?
|
(For each type of protection) |
Does the Good Samaritan law provide protection from parole or probation violations? |
|
Is reporting an overdose considered a mitigating factor in sentencing? |
|
Does the Good Samaritan law provide protection from outstanding warrants? |
|
High-Need Community Policies/Protocols
Use this section to provide information about whether local naloxone standing orders, collaborative practice agreements, or other policies exist within each of your selected high-need communities. High-Need Community is used to indicate the grantee’s selected high-need communities. Please note: if you are reporting for a grant other than the PDO/Naloxone Distribution Grant, all references to “naloxone” should be considered a drug or device approved or cleared under the Federal Food, Drug, and Cosmetic Act for emergency treatment of known or suspected opioid overdose.
Item |
Response Options |
Do any of your high-need communities have local naloxone standing orders, collaborative practice agreements, or other naloxone policies/ protocols? |
|
High-Need Community (Will only appear if YES is selected for the previous item.) |
|
Please provide a brief description of the local policies/protocols in this community. (Will only appear if YES is selected for the first item in this section.) |
Free text |
Naloxone Education and Other Opioid-Related Trainings
High-Need Community-Level Trainings
Use this section to report information on the naloxone education and other opioid-related trainings offered in each selected high-need community during the reporting period. These trainings can include group or individual trainings. Please note: if you are reporting for a grant other than the PDO/Naloxone Distribution Grant, all references to “naloxone” should be considered a drug or device approved or cleared under the Federal Food, Drug, and Cosmetic Act for emergency treatment of known or suspected opioid overdose.
Item |
Response Options |
High-Need Community |
|
Item |
Response Options |
Number of requests for training services related to opioid and heroin overdose.
(this should include training requests on how to administer naloxone or a drug or device approved or cleared under the Federal Food, Drug, and Cosmetic Act for emergency treatment of known or suspected opioid overdose.) |
Numerical |
Type of community-level training(s) provided |
|
Naloxone Administration Trainings
(If Naloxone Administration is checked for the previous item, the items in this section of the table will appear) |
|
Which of the following audience(s) received naloxone trainings as part of the grant during this reporting period? |
|
If you selected professional first responders as one of your audiences, then the following questions will appear. |
|
What is the approximate duration of the professional first responder training? |
Numerical |
Number of professional first responder trainings provided during this reporting period |
Numerical |
Total number of professional first responders who participated in trainings during this reporting period |
Numerical |
Number of professional first responders who completed a post-survey |
Numerical |
Number of professional first responders completing post-surveys who reported feeling confident administering naloxone in case of an overdose |
Numerical |
Number of professional first responders completing post-surveys who reported perceiving they had learned new information or skills as a result of the training |
Numerical |
If layperson and community organization/agency staff is one of the selected audiences, then the following questions will appear. |
|
What is the approximate duration of the layperson and community organization staff training? |
Numerical |
Number of layperson and community staff trainings provided during this reporting period |
Numerical |
Total number of layperson and community staff who participated in trainings during this reporting period |
Numerical |
Number of layperson and community staff who completed a post-survey |
Numerical |
Number of layperson and community staff completing post-surveys who reported feeling confident administering naloxone drugs in case of an overdose |
Numerical |
Number of layperson and community staff completing post-surveys who reported perceiving they had learned new information or skills as a result of the training |
Numerical |
If you selected other individuals as one of your audiences, then the following questions will appear. |
|
Please specify the other individuals. |
Free text |
What is the approximate duration of the trainings for other individuals? |
Free text (in case more than one “other” training audience is offered) |
Number of other individuals’ trainings provided during this reporting period |
Numerical |
Total number of other individuals who participated in trainings during this reporting period |
Numerical |
Number of other individuals who completed a post-survey |
Numerical |
Number of other individuals completing post-surveys who reported feeling confident administering naloxone drugs in case of an overdose |
Numerical |
Number of other individuals completing post-surveys who reported perceiving they had learned new information or skills as a result of the training |
Numerical |
Other Opioid-Related Trainings
(If Other Opioid-Related trainings is checked in the first item of this table, the items in this section of the table will appear) |
|
Audience of training(s) |
|
Please specify the other audience type. (if Other is selected in previous question) |
Free text |
If medical professionals (excluding pharmacists) is one of the selected audiences, then the following questions will appear. |
|
Focus/Topic(s) of training(s) for medical professionals (excluding pharmacists) |
Free text |
Number of trainings |
Numerical |
Total number of trainees |
Numerical |
If you selected pharmacists as one of your audiences, then the following questions will appear. |
|
Focus/Topic(s) of training(s) for pharmacists |
Free text |
Number of trainings |
Numerical |
Total number of trainees |
Numerical |
If other is one of the selected audiences, then the following questions will appear. |
|
Focus/Topic(s) of training(s) for other audiences |
Free text |
Number of trainings |
Numerical |
Total number of trainees |
Numerical |
Grantee-Level Trainings
If you provided any grantee-level naloxone administration or other opioid-related trainings, use this section to report the grantee-level trainings you provided during the reporting period. Examples of grantee-level trainings include a training delivered to all pharmacists attending a state pharmacy conference or a naloxone administration training provided to all state police officers at a statewide training. Remember that trainings provided to enhance community partner capacity to implement the grant are reported under Implementation. Please note: if you are reporting for a grant other than the PDO/Naloxone Distribution Grant, all references to “naloxone” should be considered a drug or device approved or cleared under the Federal Food, Drug, and Cosmetic Act for emergency treatment of known or suspected opioid overdose.
Item |
Response Options |
Number of requests for training services related to opioid and heroin overdose.
(this should include training requests on how to administer naloxone or a drug or device approved or cleared under the Federal Food, Drug, and Cosmetic Act for emergency treatment of known or suspected opioid overdose.) |
Numerical |
Did you provide any grantee-level naloxone administration or opioid-related trainings during this reporting period? |
|
Type of grantee-level training(s) provided (Will only appear if YES is selected for the previous item.)
(If Naloxone Administration is checked, the items in the “Naloxone Administration Trainings” section of the previous table will appear.
|
|
Training Data Collection Information
Please provide information about the survey items you used to report trainee results.
Item |
Response Options |
Did your post-training surveys include a question related to respondents’ confidence? |
|
Please provide the exact wording, including response options, of the survey question(s), as well as any information that would be helpful in understanding the data (e.g., which response option(s) were included in the reported percentage). (If YES is selected for the previous item) |
Free text |
Did your post-training surveys include a question related to whether respondents learned new information and skills? |
|
Please provide the exact wording, including response options, of the survey question(s), as well as any information that would be helpful in understanding the data (e.g., which response option(s) were included in the reported percentage). (If YES is selected for the previous item) |
Free text |
Please provide information about the data collection/management tool(s) you are using to track training data (such as a web-based data entry system) and any additional information that would be useful in understanding the training data you have provided.
Item |
Response Options |
Information about your training data collection/management tool and any additional information. |
Free text |
Naloxone Distribution Plan
Upload and provide a brief description of your document. Use the Browse button to select a file from your computer, use the upload button to add your document, enter a description, then click the Save button. If your document has not changed since your previous upload, then you do not need to upload a new document.
Item |
Response Options |
Upload Document. |
Browse button |
Provide a brief description of your document and, if relevant, any changes made to your document between the previous version and this one. |
Free text |
Naloxone Distribution
Costs
Use this section to report grant funds used to purchase naloxone during the reporting period. Please note: if you are reporting for a grant other than the PDO/Naloxone Distribution Grant, all references to “naloxone” should be considered a drug or device approved or cleared under the Federal Food, Drug, and Cosmetic Act for emergency treatment of known or suspected opioid overdose.
Item |
Response Options |
Total amount of funds from this grant spent on the purchase of naloxone products during this reporting period. |
Currency |
Type of kit purchased. Of the total grant funds spent to purchase kits, what amount was spent on and how many of each type of kit were purchased?
(The currency fields in these items must total the amount reported in the total grant funds spent on purchasing kits.) |
|
Nasal spray kits, 2 mg (Adapt/Narcan) |
Funds Spent and Number of Kits |
Nasal spray kits, 4 mg (Adapt/Narcan) |
Funds Spent and Number of Kits |
Injectable (intramuscular), .4 mg/10 ml vial kits (Hospira) |
Funds Spent and Number of Kits |
Injectable (intramuscular), .4 mg/1 ml vial kits (Mylan or West-Ward) |
Funds Spent and Number of Kits |
Injectable (intramuscular), 1 mg/2 ml vial kits (Aurum) |
Funds Spent and Number of Kits |
Auto-injector kits (Kaleo/Evzio) |
Funds Spent and Number of Kits |
Other Specify |
Checkbox |
Specify name of kit |
Free text, Funds Spent and Number of Kits |
Other Specify |
Checkbox |
Specify name of kit |
Free text, Funds Spent and Number of Kits |
Item |
Response Options |
Total amount spent on the purchase of naloxone products during this reporting period using funds from other sources (if known). |
Funds Spent and Number of Kits – include “Don’t Know” checkbox |
Comments |
Free text |
Kits Distributed to Partner Organizations
Use this section to report information regarding the distribution of naloxone kits to the selected high-need communities’ partner organizations. This includes distribution to partner organizations whose staff will be responsible for administering naloxone drugs (as in the case of law enforcement) and to partner organizations whose staff then distribute the naloxone drugs to family/friends/at-risk individuals (as may be the case with syringe exchange programs).
You will first select the high-need community for which you are reporting; once you select a community, the partner organizations specific to that community (entered in the Administration > Partner Organizations section) will appear in a dropdown list, and you will report distribution to each relevant partner organization.
Item |
Response Options |
High-Need Community |
|
Partner Organization |
|
Item |
Response Options |
In the SPARS data collection system, the grantee must enter the data in this section for each partner organization reported in the Administration Section by clicking on each community and partner organization above. |
|
Total number of kits distributed to this organization using funds from this grant |
Numerical |
Type of kit distributed. Of the total kits distributed using funds from this grant, how many were:
(These items must total the number reported in the total number of kits distributed.) |
|
Nasal spray kits, 2 mg (Adapt/Narcan) |
Numerical |
Nasal spray kits, 4 mg (Adapt/Narcan) |
Numerical |
Injectable (intramuscular), .4 mg/10 ml vial kits (Hospira) |
Numerical |
Injectable (intramuscular), .4mg/1ml vial kits (Mylan or West-Ward) |
Numerical |
Injectable (intramuscular), 1 mg/2 ml vial kits (Aurum) |
Numerical |
Auto-injector kits (Kaleo/Evzio) |
Numerical |
Other kits |
Numerical |
Other kits (specify) |
Free text |
Other kits |
Numerical |
Other kits (specify) |
Free text |
Item |
Response Options |
Total number of kits distributed to or procured by this organization using funds from other sources (if known) |
Numerical and “Don’t Know” checkbox |
Please provide information about the data collection/management tool(s) or system(s) you are using to track distribution and any additional information that would be useful in understanding the data you have provided.
Item |
Response Options |
Information about your distribution data collection/management tool and any additional information |
Free text |
Naloxone Administration by Partner Organization
Use this section to report information on the naloxone administrations reported during this reporting period by each of the partner organizations receiving naloxone or naloxone training from this grant. Please note: if you are reporting for a grant other than the PDO/Naloxone Distribution Grant, all references to “naloxone” should be considered a drug or device approved or cleared under the Federal Food, Drug, and Cosmetic Act for emergency treatment of known or suspected opioid overdose.
Ideally, you will report all administration events reported by partner organizations including those using kits paid for by this grant and those using kits paid for by other funding sources. However, if you are not able to report events using kits paid for by other sources, you will be able to report just those using kits paid for with grant funds.
You will first select the high-need community for which you are reporting; once you select a community, the partner organizations specific to that community (entered in the Administration > Partner Organizations section) will appear in a dropdown list, and you will report naloxone administration data provided to you by each relevant partner organization.
|
Item |
Response Options |
|
|
In the SPARS data collection system, the grantee must enter the data in this section for each high-need community and partner organization reported in the Administration Section by clicking on each community and partner organization above. |
||
|
High-Need Community |
|
|
|
Partner Organization |
|
|
|
Are you reporting all administration events reported by this organization or only events using a kit paid for by this grant? |
|
|
|
Approximately what percentage of this organization’s kits were paid for using funds from this grant? (This item only appears if “All events” is selected.) |
Percentage |
|
|
Total number of administration events |
Numerical |
|
|
Type of kit administered. Of the total administration events, how many were:
(These items must total the number reported in the total number of administration events.) |
||
|
Nasal spray kits, 2 mg (Adapt/Narcan) |
Numerical |
|
|
Nasal spray kits, 4 mg (Adapt/Narcan) |
Numerical |
|
|
Injectable (intramuscular), .4 mg/10 ml vial kits (Hospira) |
Numerical |
|
|
Injectable (intramuscular), .4 mg/1 ml vial kits (Mylan or West-Ward) |
Numerical |
|
|
Injectable (intramuscular), 1 mg/2 ml vial kits (Aurum) |
Numerical |
|
|
Auto-injector kits (Kaleo/Evzio) |
Numerical |
|
|
Other kits |
Numerical |
|
|
Other types of kits (specify) |
Numerical |
|
|
Other kits |
Numerical |
|
|
Other types of kits (specify) |
Numerical |
|
|
Single or Multiple Dose. Of the total administration events, how many consisted of:
(These items must total the number reported in the total number of administration events.) |
||
A single dose/unit administered |
Numerical |
||
Multiple doses/units administered |
Numerical |
||
Unknown |
Numerical |
||
Location of administration. Of the total administration events, how many were administered…
(These items must total the number reported in the total number of administration events.) |
|||
At a private residence |
Numerical |
||
In a public outdoor location (e.g., street, park), car, camp, or shelter |
Numerical |
||
At an indoor public place/business (including hotel/motel) |
Numerical |
||
Unknown |
Numerical |
||
Other |
Numerical |
||
Other (specify) |
Free text |
||
Outcome of administration event. Of the total administration events, how many had the following outcome: Please record the acute outcome (at the scene, at time of event); there is not an expectation that grantee will monitor outcome after patient has been transported to the ED.
(These items must total the number reported in the total number of kits administered.) |
|||
Overdose reversal |
Numerical |
||
Death |
Numerical |
||
Event was likely not an opioid overdose |
Numerical |
||
Unknown outcome |
Numerical |
||
Please provide information about the data collection/management tool(s) or system(s) you are using to track administration and any additional information that would be useful in understanding the data you have provided.
Item |
Response Options |
Information about your administration data collection/management tool and any additional information |
Free text |
Other Interventions
Use this section to report any other interventions you or your selected high-need communities implemented as part of this grant initiative during the reporting period. To respond at the grantee level, click the edit button to the right of the “Grantee” record below. To respond for each of your high-need communities, click the “Add Community Interventions” button. After your communities are added, you can use the edit button to modify the records, as needed. Please note: if you are reporting for a grant other than the PDO/Naloxone Distribution Grant, all references to “naloxone” should be considered a drug or device approved or cleared under the Federal Food, Drug, and Cosmetic Act for emergency treatment of known or suspected opioid overdose.
Item |
Response Options |
Public Policy Interventions |
|
Naloxone policy change effort |
|
Pharmacy benefit strategy change (e.g., institute drug utilization reviews for high-dose opioids, add nasal naloxone to Medicaid formulary, remove prior authorization for naloxone) |
|
Other policy intervention. If checked, a free text “specify” field will appear. |
|
Community/Organizational Interventions |
|
Collaboration with prescribers to obtain standing orders |
|
Collaboration with pharmacies to distribute naloxone drugs |
|
Solidifying partnerships with community entities experienced in naloxone distribution to laypeople |
|
Solidifying partnerships with first responder agencies experienced in naloxone administration |
|
Efforts to expand naloxone distribution to new community partners that have not received or distributed naloxone or related drugs previously |
|
Enhancement of state or local cross-agency coordination of naloxone efforts |
|
Other community/organizational intervention. If checked, a free text “specify” field will appear. |
|
Information Dissemination for Prescribers/Pharmacists. Information dissemination includes dissemination of print and electronic materials, speaking engagements targeting prescribers/pharmacists, etc. This does not include naloxone education, which is captured in the Naloxone Education Trainings section. |
|
Information dissemination to prescribers on naloxone co-prescribing and opioid overdose risk |
|
Information dissemination to pharmacists on naloxone dispensing |
|
Other effort related to information dissemination to prescribers/pharmacists. If checked, a free text “specify” field will appear. |
|
Information Dissemination to Community Members |
|
Media campaigns and community information dissemination about overdose, naloxone drugs, Good Samaritan laws |
|
Messaging to pharmacy patients |
|
Other effort related to information dissemination to community members. If checked, a free text “specify” field will appear. |
|
Treatment and Recovery Access |
|
Efforts or services to facilitate access to treatment and recovery |
|
System changes for post-overdose or high-risk treatment/referral |
|
Other effort related to treatment and recovery access. If checked, a free text “specify” field will appear. |
|
Number of strategies developed to refer overdose victims and families to treatment services |
Numerical |
Number of overdose victims and families receiving information about treatment services |
Numerical |
Number of overdose victims receiving treatment |
Numerical |
Of those receiving treatment listed above, how many received:
|
Numerical |
Accomplishments and Barriers/Challenges
See DSP-MRT.
Evaluation
Evaluation Plan Upload
See DSP-MRT
Evaluation Report
See DSP-MRT
Other Document Upload
See DSP-MRT
Accomplishments and Barriers/Challenges
See DSP-MRT
Sustainability
Accomplishments and Barriers/Challenges
See DSP-MRT
Overdose Outcomes
Use this section to report annual numbers of opioid-related overdose and overdose deaths. The numbers should be aggregated across all types of opioids, whether opioid pain relievers or illicit opioids (e.g., heroin). This section will only appear on the progress report due following the end of the federal fiscal year. You will report any data/time points that have become available prior to the report deadline.
Grantee is used to indicate the state/tribal entity/jurisdiction receiving the award from SAMHSA/CDC. High-Need Community is used to indicate the grantee’s selected high-need communities.
Grantee-Level Overdose Data
First, you will report grantee-level adult (age 18+) data on deaths related to opioid overdose, and emergency department and other hospital visits involving opioid overdose. Note that grantee-level data refers to the entire state (or tribal area or jurisdiction). It does not refer to the aggregate of the selected high-need communities.
Grantees are asked to report both emergency department and hospitalization data if available, but we are aware that some grantees may not have access to both or either type of data. Grantees may also report opioid overdose events from a different data source if desired, or if emergency department or hospitalizations data are not available.
Item |
Response Options |
2015 |
“Edit” link |
2016 |
“Edit” link |
2017, etc. |
“Edit” link |
Item |
Population (Denominator) |
Opioid Overdose Deaths |
Emergency Department Visits Involving Opioid Overdose |
Hospitalizations Involving Opioid Overdose |
Other Opioid Overdose Events (optional) |
|
Data Source and Comments: |
||||||
Total |
State grantees do not need to provide these data, as they will be pulled from CDC WONDER
(This section may not be visible in the data entry system at launch) |
Numerical |
Numerical |
Numerical |
||
Data source |
Free text |
Free text |
Free text |
|||
Additional Infor-mation |
Free text |
Free text |
Free text |
|||
Age: |
||||||
15-24 yr |
State grantees do not need to provide these data, as they will be pulled from CDC WONDER
(This section may not be visible in the data entry system at launch) |
Numerical |
Numerical |
Numerical |
||
25-34 yr |
Numerical |
Numerical |
Numerical |
|||
35-44 yr |
Numerical |
Numerical |
Numerical |
|||
45-54 yr |
Numerical |
Numerical |
Numerical |
|||
55-64 yr |
Numerical |
Numerical |
Numerical |
|||
65+ yr |
Numerical |
Numerical |
Numerical |
|||
Not Available |
Numerical |
Numerical |
Numerical |
|||
Sex: |
||||||
Males |
State grantees do not need to provide these data, as they will be pulled from CDC WONDER
(This section may not be visible in the data entry system at launch) |
Numerical |
Numerical |
Numerical |
||
Females |
Numerical |
Numerical |
Numerical |
|||
Not Available |
Numerical |
Numerical |
Numerical |
|||
Note: The values entered for the age groups and the sexes must each total the values entered in the total line. Users will get an error message if the totals do not match.
Please provide information about the data source, any additional information that would be useful in understanding the overdose data you have provided, or both.
High-Need Community-Level Overdose Data
Next, you will report any community-level data that are available on opioid-related overdose deaths and events in your selected high-need communities.
Item |
Response Options |
2015
|
“Edit Overdose Data” button |
2016
|
“Edit Overdose Data” button |
2017, etc.
|
“Edit Overdose Data” button |
Item |
Population (Denominator) |
Opioid Overdose Deaths |
Emergency Department Visits Involving Opioid Overdose |
Hospitalizations Involving Opioid Overdose |
Other Opioid Overdose Events (optional) |
Data Source and Comments |
|||||
Total |
Numerical |
Numerical |
Numerical |
Numerical |
Numerical |
Data Source |
Free text |
Free text |
Free text |
Free text |
Free text |
Additional Information |
Free text |
Free text |
Free text |
Free text |
Free text |
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| File Title | Division of State Programs-Management Reporting Tool (DSP-MRT) PDO Unique Items Supplement |
| Subject | Supplement to the Division of State Programs-Management Reporting Tool (DSP-MRT) for the Grants to Prevent Prescription Drug/Opi |
| Author | Substance Abuse and Mental Health Services Administration, Cente |
| File Modified | 0000-00-00 |
| File Created | 2021-01-20 |
© 2026 OMB.report | Privacy Policy