2 Attach 5A CTRP Registry Complete Batch Template
The Clinical Trials Reporting Program (CTRP) Database (NCI)
Attachment 5a - CTRP_Registry_Complete_Batch_Template 5.10.22 (3).xlsx
Initial Registration
OMB: 0925-0600
Document [xlsx]
Download: xlsx | pdf
Readme First
Template Instructions
Sample Trial Data
Trial Data Pick List
Trial Data Element Specs
NIH & NCI Values
NCI Code Definitions
3-letter country code
2-letter state_province
Trial Status Date Diagram
Overview
DisclaimerReadme First
Template Instructions
Sample Trial Data
Trial Data Pick List
Trial Data Element Specs
NIH & NCI Values
NCI Code Definitions
3-letter country code
2-letter state_province
Trial Status Date Diagram
Sheet 1: Disclaimer

|
|||||||||||||||

|
|||||||||||||||
Sheet 2: Readme First
| CTRP Trial Registration Batch Upload Specification for Complete Trials | |||||||||
| About this Document | |||||||||
| This document provides you with everything you need to know to upload clinical trial data to the CTRP Trial Registration system, including the following: | |||||||||
| Template Instructions | |||||||||
| The Template Instructions worksheet provides detailed instructions for preparing your data and uploading them to the system. | |||||||||
| Sample Trial Data | |||||||||
| The Sample Trial Data worksheet provides an example of what a typical batch upload file looks like. | |||||||||
| Note: The worksheet that contains your trial data MUST always be the FIRST worksheet (tab) in the file. | |||||||||
| Trial Data Pick List | |||||||||
| The Trial Data Pick List worksheet contains sets of valid values for many of the data elements in the (Sample) Trial Data worksheet. | |||||||||
| The values are displayed in pick lists when you select an appropriate data element cell. | |||||||||
| The pick lists have been provided to assist you in filling out these cells quickly and accurately. | |||||||||
| However, if you prefer, you can type the values instead. | |||||||||
| Note: The drop-down lists will not work if you delete this worksheet. | |||||||||
| Trial Data Element Specifications | |||||||||
| The specifications worksheet includes the following information: | |||||||||
| 1 | Data elements | ||||||||
| 2 | Order in which the data elements must be presented. The element order is set up for you in columns in the Sample Trial Data tab. | ||||||||
| 3 | Data element requirements. Requirements differ for original, updated, and amended submissions. | ||||||||
| 4 | Valid values. The system accepts only those values listed in this document. | ||||||||
| 5 | Comments. Additional information that helps you to ensure successful upload of your data. | ||||||||
| NIH and NCI Values | |||||||||
| The NIH & NCI Values worksheet provides all acceptable values for the following data elements: | |||||||||
| 1 | Funding Mechanisms | ||||||||
| 2 | Institute Codes | ||||||||
| 3 | NCI Division/Program Codes | ||||||||
| 4 | NIH Institute Codes | ||||||||
| NCI Code Definitions | |||||||||
| The NCI Division/Program Code Definitions worksheet lists the long form of each of the division/program acronyms. | |||||||||
| Country Codes | |||||||||
| The Country Codes worksheet lists the 3-letter country codes for all countries that submit clinical trial data to the CTRP system. | |||||||||
| State and Province Codes | |||||||||
| The State and Province Codes worksheet lists the 2- or 3-letter state/province/territory codes for the United States, Canada, and Australia. | |||||||||
| Oversight Authorities | |||||||||
| The Oversight Authorities worksheet lists the names of oversight authorities for all countries that submit clinical trial data to the CTRP system. | |||||||||
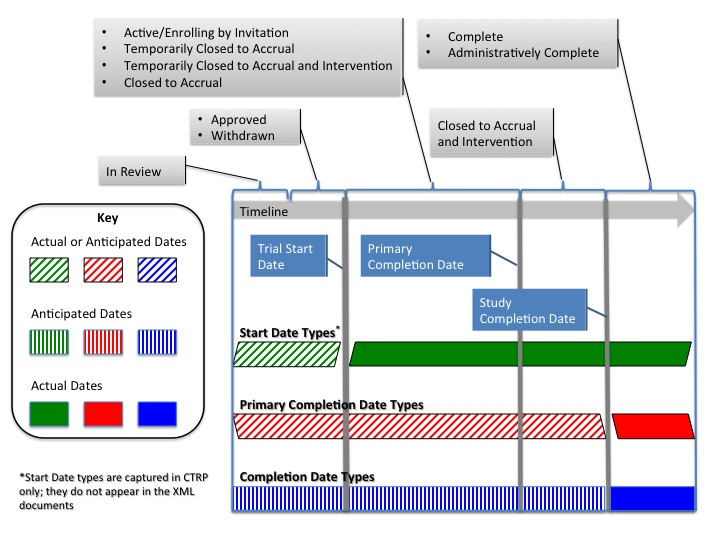
| Trial Status Date Diagram | |||||||||
| The Trial Status Date diagram illustrates the relationships between trial start dates, primary completion dates, and completion dates. |
Sheet 3: Template Instructions
| How to Upload Clinical Trial Data to the CTRP Trial Registration System | |||
| Before You Begin | |||
| Contact the CTRO at [email protected] to request approval for sending batch files to CTRP. Include your login name, first and last names, and email address stored in your CTRP profile. | |||
| Note: Once you have received approval, you do not have to request approval for subsequent uploads. | |||
| Main Steps for Uploading Your Data | |||
| 1 | Prepare the trial data file. | ||
| 2 | Prepare the trial documents Zip file. | ||
| 3 | Upload your files to the CTRP system via the NCI Trial Registration application batch upload web page at https://trials.nci.nih.gov/registry/admin/batchUpload.action. | ||
| Preparing Trial Data Files | |||
| 1 | Ensure that your trial conforms to the supported criteria. This template supports the following: | ||
| * | Interventional trials | ||
| * | Complete (Data Table 4 Funding Sponsor Category is 'National', 'Externally Peer-Reviewed' or 'Institutional') trials submission | ||
| * | Amendments to complete CTRP trials with "Abstraction Verified Response" or "Abstraction Verified No Response" processing statuses | ||
| * | Updates to complete CTRP trials with the processing status "Accepted" and beyond | ||
| * | 100 trials per data file | ||
| * | Multiple grants per submitted trial | ||
| * | Multiple IND/IDE per submitted trial | ||
| * | Generic contacts for Responsible Party or Sponsor | ||
| * | Multiple "Other" trial identifiers | ||
| Tip: You can add NCT IDs when updating or amending registered trials. | |||
| Note: You can request a list of CTRP persons and organizations along with PO-IDs from the CTRO at [email protected]. Or, you can use the search organization/person feature in the CTRP Trial Registration application to ascertain PO-IDs. |
|||
| 2 | Create a new Excel spreadsheet (.xls) that will contain the mandatory and optional data for the trial(s) as specified in this document. | ||
| 3 | Copy the Sample Trial Data and Trial Data Pick List tabs to your new spreadsheet. | ||
| Most cells on the Sample Trial Data tab for which there are a defined set of valid values have drop-down lists. These allow you to select valid values from a list. The sets of valid values for these drop-down lists are derived from Trial Data Pick List worksheet. | |||
| 4 | Delete the sample data from the Sample Trial Data tab in your new spreadsheet. Optionally, you can rename the tab. | ||
| 5 | Click the cell in which you want to enter data. | ||
| Arrows are displayed whenever a drop-down list is available. | |||
| 5a. If arrows are displayed next to the cell, click the arrow and select the appropriate value from the drop-down list. | |||
| 5b. If no arrows are displayed, enter the appropriate information using the valid values in this template. | |||
| You must adhere to the following requirements: | |||
| * List trial elements required for registration in the order specified in the Trial Data Element Spec tab in this spreadsheet. | |||
| * Do not change the spelling of data elements or valid values. Changes to spelling or to the order of the trial elements will cause the upload to fail. Similarly, the addition of new/extra trial elements will also cause a failure. | |||
| * Conform to the valid values guidelines when entering trial data. Valid values for each of the trial elements, where applicable, are listed in the Trial Data Element Spec or other valid value tabs in this spreadsheet. | |||
| * Identify each trial uniquely. For example, append your cancer center unique trial identifier to the file name. | |||
| 6 | Delete all empty columns that may appear after the last data element column. | ||
| 7 | Delete the Trial Data Pick List worksheet from your new file. | ||
| Preparing Trial Document Zip Files | |||
| 1 | Prepare a separate Zip file containing applicable trial documents (e.g. Protocol, IRB approval, Informed Consent, Participating Sites, Change Memo) for the trials in the data file. For trial amendments, you can include either a Change Memo document or Protocol Highlight document. NOTE: The MacOSX native compression utility is not supported. | ||
| 2 | To avoid overwriting existing files when the system extracts your latest upload, rename the document files if they are not unique. For detailed instructions and best practices for file preparation, refer to the CTRP Registration Site User's Guide at https://wiki.nci.nih.gov/x/SwnCBg. | ||
| For example, prefix files with a unique trial identifier such as XXXX_document name.doc. | |||
| If using trial identification prefixes, ensure that each of a given trial's document file names is unique. | |||
| 3 | Provide the document names (including their extensions) in the file containing the trial data. Up to seven (7) files can be specified in one single trial record. | ||
| 4 | Zip all trial-related documents. Do not include pathnames in the Zip files. | ||
| The trial document Zip file that you intend to upload MUST NOT include folders or other Zip files. All trial-related documents must be Word documents ( .doc) or Adobe PDFs (.pdf). No other file types are currently accepted. Zip files created with the MAC OS native compression utility may fail. | |||
| Note: Some elements will be ignored when updating existing CTRP trials via batch upload. | |||
| Uploading Your Files | |||
| 1 | Open your browser and navigate to the NCI Trial Registration application batch upload web page at https://trials.nci.nih.gov/registry/admin/batchUpload.action. | ||
| 2 | Follow the instructions provided on the Batch Trial Upload web page. For more comprehensive information, see the CTRP Registration User's Guide at: | ||
| https://wiki.nci.nih.gov/x/Ey0ZCQ |
Sheet 4: Sample Trial Data
| Unique Trial Identifier | Submission Type | NCI Trial Identifier | Amendment Number | Amendment Date | Lead Organization Trial Identifier | NCT | Other Trial Identifier | Title | Trial Type | Primary Purpose | [Primary Purpose] Additional Qualifier | [Primary Purpose] Other Text | Phase | Pilot Trial? | [Sponsor] Organization PO-ID | Responsible Party | [Responsible Party] Investigator Person PO-ID | [Responsible Party] Title | [Responsible Party] Affiliation Organization PO-ID | [Lead Organization] Organization PO-ID | [Principal Investigator] Person PO-ID | Data Table 4 Funding Category | [Data Table 4 Funding Sponsor/Source] Organization PO-ID | Program Code | [NIH Grant] Funding Mechanism | [NIH Grant] Institute Code | [NIH Grant] Serial Number | [NIH Grant] NCI Division/Program Code | Current Trial Status | Why Study Stopped? | Current Trial Status Date | Study Start Date | Study Start Date Type | Primary Completion Date | Primary Completion Date Type | Study Completion Date | Study Completion Date Type | IND/IDE Type | IND/IDE Number | IND/IDE Grantor | IND/IDE Holder Type | [IND/IDE] NIH Institution | [IND/IDE] NCI Division /Program | [IND/IDE] Availability of Expanded Access? | [IND/IDE] Expanded Access Record | Studies a US FDA regulated Drug Product | Studies a US FDA regulated Device Product | Unapproved/Uncleared Device | Pediatric Post-Market Survelliance | Product Exported from the US | FDA Regulatory Information Indicator | Section 801 Indicator | Data Monitoring Committee Appointed Indicator | Protocol Document File Name | IRB Approval Document File Name | Participating Sites Document File Name | Informed Consent Document File Name | Other Trial Related Document File Name | Change Memo Document Name | Protocol Highlight Document Name |
| 10 | O | 53112 | NCT000123 | 123;123-A | A Phase I study of Taxol in refractory leukemia in children | Interventional | Treatment | I | Principal Investigator | 1234 | Principal Investigator | 123 | 1234 | Institutional | F34 | AG | 72345 | CTEP | Complete | 8/1/2010 | 2/1/2009 | Actual | 08/01/10 | Actual | Yes | No | Yes | protocol_document_T10.doc | IRB_Approval.doc | Participating_Sites_T10.xls | 10_Informed_Consent.PDF | 10_Other_document.doc | ||||||||||||||||||||||||||||
| 1000 | A | NCI-2009-00001 | A1 | 39938 | 1234 | NCT00045 | Phase III Study of Zoladex Adjuvant to Radiotherapy in Unfavorable Prognosis Carcinoma of the Prostate | Interventional | Treatment | III | Sponsor | Institutional | Temporarily Closed to Accrual | Accrual target was reached for this phase of the study | 8/2/2009 | 1/2/2009 | Actual | 10/02/11 | Anticipated | Yes | No | Yes | protocol_document_T1000.doc | IRB_Approval_06082007.doc | Participating_Sites_T1000_new.xls | Change_memo_doc.doc | ||||||||||||||||||||||||||||||||||
| 2001 | O | 12345 | A Phase I trial of Ifosfamide and Taxol in refractory Pelvic Malignancies | Interventional | Treatment | I | 654512 | Sponsor | 87456 | Institutional | IM | K08;CO6 | HV;AO | 97521;012345 | CTEP;CTEP | In Review | 8/3/2009 | 12/3/2010 | Anticipated | 10/3/2011 | Anticipated | Yes | Yes | Yes | Yes | protocol_document_T2001.doc | IRB_Approval_T2001.doc | Participating_Sites_T2001.xls | Informed_Consent_T2001.PDF | Other_document_T2001.doc | ||||||||||||||||||||||||||||||
| 3000 | O | 65432 | Phase III study of priming with granulocyte-macrophage colony stimulating factor (rhu-gm-csf)and of three induction regimens in adult patients (over 55) with acute non-lymphocytic leukemia | Interventional | Treatment | III | Principal Investigator | 1234 | Principal Investigator | 12345 | Institutional | Approved | 8/4/2009 | 12/4/2010 | Anticipated | 9/4/2012 | Anticipated | Yes | 3000_protocol_document.doc | 3000_IRB_Approval.doc | 3000_Participating_Sites.xls | 3000_Informed_Consent.PDF | 3000_Other_document.doc | |||||||||||||||||||||||||||||||||||||
| 4000 | O | 1233 | Phase III Comparison of Methotrexate, Vinblastine, Doxorubicin, and Cisplatin (MVAC) vs. Doxorubicin and Cisplatin (AC) in Women with Advanced Primary or Recurrent Metastatic Carcinoma of the Uterine Endometrium | Interventional | Other | Other | Laboratory | NA | Yes | 87654 | Sponsor | 45689 | Institutional | Administratively Complete | Closed prematurely | 8/5/2009 | 1/5/2009 | Actual | 8/5/2009 | Actual | IND;IND | 67899;10,264 | CDER;CDER | NIH;NCI | NIA;NA | NA;DCP | Yes; | NCT01234567; | Yes | No | Yes | 4000_protocol_document.doc | 4000_IRB_Approval.doc | 4000_Participating_Sites.xls | 4000_Informed_Consent.PDF | 4000_Other_document.doc | ||||||||||||||||||||||||
| 5000 | U | NCI-2009-00001 | 12308 | NCT009876 | 321-12 | An Open-Labeled, Non-Randomized Phase I Study of Alvocidib (Flavopiridol) Administered with Oxaliplatin and Fluorouracil/Leucovorin in Patients with Advanced Solid Tumors | Interventional | Treatment | I | Sponsor | Institutional | BR | Approved | 8/1/2009 | 12/1/2010 | Anticipated | 12/1/2011 | Anticipated | Yes | No | Yes |
Sheet 5: Trial Data Pick List
| Submission Type | Yes_No | Trial Type | Primary Purpose | Primary Purpose Additional Qualifier | Phase | Country Code | State Code | Responsible Party | Sponsor Contact Type | Data Table 4 Funding Category | NIH Grant Funding Mechanism | NIH Grant Institute Code | NCI Division/Program Code | Current Trial Status | Date Type | IND/IDE Type | IND/IDE Grantor | IND/IDE Holder Type | NIH Institution | IND/IDE Expanded Access Status |
| A | No | Interventional | Basic Science | Other | Early Phase I | ABW | AK | Principal Investigator | Personal | National | B01 | AA | CCR | In Review | Actual | IND | CDER | Investigator | NEI-National Eye Institute | Available |
| O | Yes | Observational | Diagnostic | I | AFG | AL | Sponsor | Generic | Externally Peer-Reviewed | B08 | AE | CCT/CTB | Approved | Anticipated | IDE | CBER | Organization | NHLBI-National Heart, Lung, and Blood Institute | No longer available | |
| U | Health Services Research | I/II | AGO | AR | Sponsor Investigator | Institutional | B09 | AF | CTEP | Active | CDRH | Industry | NHGRI-National Human Genome Research Institute | Temporarily not available | ||||||
| Other | II | AIA | AZ | C06 | AG | DCB | Closed to Accrual | NIH | NIA-National Institute on Aging | Approved for marketing | ||||||||||
| Prevention | II/III | ALA | CA | D43 | AI | DCCPS | Closed to Accrual and Intervention | NCI | NIAAA-National Institute on Alcohol Abuse and Alcoholism | |||||||||||
| Screening | III | ALB | CO | D71 | AM | DCEG | Temporarily Closed to Accrual | NIAID-National Institute of Allergy and Infectious Diseases | ||||||||||||
| Supportive Care | IV | AND | CT | DP1 | AO | DTP | Temporarily Closed to Accrual and Intervention | NIAMS-National Institute of Arthritis and Musculoskeletal and Skin Diseases | ||||||||||||
| Treatment | NA | ANT | DE | DP2 | AR | DCP | Complete | NIBIB-National Institute of Biomedical Imaging and Bioengineering | ||||||||||||
| ARE | FL | DP3 | AT | DEA | Administratively Complete | NICHD-Eunice Kennedy Shriver National Institute of Child Health and Human Development | ||||||||||||||
| ARG | GA | E11 | BC | OD | Withdrawn | NIDCD-National Institute on Deafness and Other Communication Disorders | ||||||||||||||
| ARM | HI | F05 | BX | OSB/SPOREs | NIDCR-National Institute of Dental and Craniofacial Research | |||||||||||||||
| ASM | IA | F30 | CA | CIP | NIDDK-National Institute of Diabetes and Digestive and Kidney Diseases | |||||||||||||||
| ATA | ID | F31 | CB | CDP | NIDA-National Institute on Drug Abuse | |||||||||||||||
| ATF | IL | F32 | CD | TRP | NIEHS-National Institute of Environmental Health Sciences | |||||||||||||||
| ATG | IN | F33 | CE | RRP | NIGMS-National Institute of General Medical Sciences | |||||||||||||||
| AUS | KS | F34 | CH | N/A | NIMH-National Institute of Mental Health | |||||||||||||||
| AUT | KY | F37 | CI | NINDS-National Institute of Neurological Disorders and Stroke | ||||||||||||||||
| AZE | LA | F38 | CK | NINR-National Institute of Nursing Research | ||||||||||||||||
| BDI | MA | G07 | CL | NLM-National Library of Medicine | ||||||||||||||||
| BEL | MD | G08 | CM | CIT-Center for Information Technology | ||||||||||||||||
| BEN | ME | G11 | CN | CSR-Center for Scientific Review | ||||||||||||||||
| BFA | MI | G12 | CO | FIC-John E. Fogarty International Center for Advanced Study in the Health Sciences | ||||||||||||||||
| BGD | MN | G13 | CP | NCCAM-National Center for Complementary and Alternative Medicine | ||||||||||||||||
| BGR | MO | G20 | CR | NCMHD-National Center on Minority Health and Health Disparities | ||||||||||||||||
| BHR | MS | G94 | CT | NCRR-National Center for Research Resources (NCRR | ||||||||||||||||
| BHS | MT | H13 | CU | CC-NIH Clinical Center | ||||||||||||||||
| BIH | NC | H23 | CX | OD-Office of the Director | ||||||||||||||||
| BLM | ND | H25 | DA | |||||||||||||||||
| BLR | NE | H28 | DC | |||||||||||||||||
| BLZ | NH | H50 | DD | |||||||||||||||||
| BMU | NJ | H57 | DE | |||||||||||||||||
| BOL | NM | H62 | DK | |||||||||||||||||
| BRA | NV | H64 | DP | |||||||||||||||||
| BRB | NY | H75 | EB | |||||||||||||||||
| BRN | OH | H79 | EH | |||||||||||||||||
| BTN | OK | HD4 | EM | |||||||||||||||||
| BVT | OR | HR! | EP | |||||||||||||||||
| BWA | PA | I01 | ES | |||||||||||||||||
| CAF | RI | K01 | EY | |||||||||||||||||
| CAN | SC | K02 | FD | |||||||||||||||||
| CCK | SD | K05 | GD | |||||||||||||||||
| CHE | TN | K06 | GH | |||||||||||||||||
| CHL | TX | K07 | GM | |||||||||||||||||
| CHN | UT | K08 | GW | |||||||||||||||||
| CIV | VA | K12 | HB | |||||||||||||||||
| CMR | VT | K14 | HC | |||||||||||||||||
| COD | WA | K18 | HD | |||||||||||||||||
| COG | WI | K21 | HG | |||||||||||||||||
| COK | WV | K22 | HI | |||||||||||||||||
| COL | WY | K23 | HK | |||||||||||||||||
| COM | AB | K24 | HL | |||||||||||||||||
| CPV | BC | K25 | HM | |||||||||||||||||
| CRI | MB | K26 | HO | |||||||||||||||||
| CUB | NB | K30 | HP | |||||||||||||||||
| CXR | NL | K99 | HR | |||||||||||||||||
| CYM | NS | KD1 | HS | |||||||||||||||||
| CYP | NT | KL1 | HV | |||||||||||||||||
| CZE | NU | KL2 | HX | |||||||||||||||||
| DEU | ON | L30 | HY | |||||||||||||||||
| DJI | PE | L32 | IP | |||||||||||||||||
| DMA | QC | L40 | JT | |||||||||||||||||
| DNK | SK | L50 | LM | |||||||||||||||||
| DOM | YT | L60 | MD | |||||||||||||||||
| DZA | ACT | M01 | MH | |||||||||||||||||
| ECU | NSW | N01 | MN | |||||||||||||||||
| EGY | NT | N02 | NB | |||||||||||||||||
| ERI | QLD | N03 | NH | |||||||||||||||||
| ESH | SA | N43 | NR | |||||||||||||||||
| ESP | TAS | N44 | NS | |||||||||||||||||
| EST | VIC | P01 | NU | |||||||||||||||||
| ETH | WA | P20 | OA | |||||||||||||||||
| FIN | P30 | OC | ||||||||||||||||||
| FJI | P40 | OD | ||||||||||||||||||
| FLK | P41 | OF | ||||||||||||||||||
| FRA | P42 | OH | ||||||||||||||||||
| FRO | P50 | OL | ||||||||||||||||||
| FSM | P51 | OR | ||||||||||||||||||
| GAB | P60 | PC | ||||||||||||||||||
| GBR | P76 | PH | ||||||||||||||||||
| GEO | PL1 | PR | ||||||||||||||||||
| GGY | PN1 | PS | ||||||||||||||||||
| GHA | PN2 | RC | ||||||||||||||||||
| GIB | R00 | RD | ||||||||||||||||||
| GIN | R01 | RG | ||||||||||||||||||
| GLP | R03 | RM | ||||||||||||||||||
| GMB | R04 | RR | ||||||||||||||||||
| GNB | R06 | RX | ||||||||||||||||||
| GNQ | R08 | SC | ||||||||||||||||||
| GRC | R13 | SF | ||||||||||||||||||
| GRD | R15 | SH | ||||||||||||||||||
| GRL | R17 | SM | ||||||||||||||||||
| GTM | R18 | SP | ||||||||||||||||||
| GUF | R21 | SU | ||||||||||||||||||
| GUM | R24 | TI | ||||||||||||||||||
| GUY | R25 | TP | ||||||||||||||||||
| HKG | R30 | TS | ||||||||||||||||||
| HMD | R33 | TW | ||||||||||||||||||
| HND | R34 | VA | ||||||||||||||||||
| HRV | R36 | WC | ||||||||||||||||||
| HTI | R37 | WH | ||||||||||||||||||
| HUN | R41 | WT | ||||||||||||||||||
| IDN | R42 | |||||||||||||||||||
| IMN | R43 | |||||||||||||||||||
| IND | R44 | |||||||||||||||||||
| IOT | R49 | |||||||||||||||||||
| IRL | R55 | |||||||||||||||||||
| IRN | R56 | |||||||||||||||||||
| IRQ | R90 | |||||||||||||||||||
| ISL | RC1 | |||||||||||||||||||
| ISR | RC2 | |||||||||||||||||||
| ITA | RC3 | |||||||||||||||||||
| JAM | RC4 | |||||||||||||||||||
| JEY | RL1 | |||||||||||||||||||
| JOR | RL2 | |||||||||||||||||||
| JPN | RL5 | |||||||||||||||||||
| KAZ | RL9 | |||||||||||||||||||
| KEN | RS1 | |||||||||||||||||||
| KGZ | S06 | |||||||||||||||||||
| KHM | S10 | |||||||||||||||||||
| KIR | S11 | |||||||||||||||||||
| KNA | S21 | |||||||||||||||||||
| KOR | S22 | |||||||||||||||||||
| KWT | SC1 | |||||||||||||||||||
| LAO | SC2 | |||||||||||||||||||
| LBN | SC3 | |||||||||||||||||||
| LBR | T01 | |||||||||||||||||||
| LBY | T02 | |||||||||||||||||||
| LCA | T03 | |||||||||||||||||||
| LIE | T06 | |||||||||||||||||||
| LKA | T09 | |||||||||||||||||||
| LSO | T14 | |||||||||||||||||||
| LTU | T15 | |||||||||||||||||||
| LUX | T32 | |||||||||||||||||||
| LVA | T34 | |||||||||||||||||||
| MAC | T35 | |||||||||||||||||||
| MAF | T36 | |||||||||||||||||||
| MAR | T37 | |||||||||||||||||||
| MCO | T42 | |||||||||||||||||||
| MDA | T90 | |||||||||||||||||||
| MDG | TL1 | |||||||||||||||||||
| MDV | TU2 | |||||||||||||||||||
| MEX | U01 | |||||||||||||||||||
| MHL | U09 | |||||||||||||||||||
| MKD | U10 | |||||||||||||||||||
| MLI | U11 | |||||||||||||||||||
| MLT | U13 | |||||||||||||||||||
| MMR | U14 | |||||||||||||||||||
| MNE | U17 | |||||||||||||||||||
| MNG | U18 | |||||||||||||||||||
| MNP | U19 | |||||||||||||||||||
| MOZ | U1A | |||||||||||||||||||
| MRT | U1Q | |||||||||||||||||||
| MSR | U1S | |||||||||||||||||||
| MTQ | U1T | |||||||||||||||||||
| MUS | U1V | |||||||||||||||||||
| MWI | U21 | |||||||||||||||||||
| MYS | U22 | |||||||||||||||||||
| MYT | U23 | |||||||||||||||||||
| NAM | U24 | |||||||||||||||||||
| NCL | U27 | |||||||||||||||||||
| NER | U2G | |||||||||||||||||||
| NFK | U2R | |||||||||||||||||||
| NGA | U30 | |||||||||||||||||||
| NIC | U32 | |||||||||||||||||||
| NIU | U34 | |||||||||||||||||||
| NLD | U36 | |||||||||||||||||||
| NOR | U38 | |||||||||||||||||||
| NPL | U41 | |||||||||||||||||||
| NRU | U42 | |||||||||||||||||||
| NZL | U43 | |||||||||||||||||||
| OMN | U44 | |||||||||||||||||||
| PAK | U45 | |||||||||||||||||||
| PAN | U47 | |||||||||||||||||||
| PCN | U48 | |||||||||||||||||||
| PER | U49 | |||||||||||||||||||
| PHL | U50 | |||||||||||||||||||
| PLW | U51 | |||||||||||||||||||
| PNG | U52 | |||||||||||||||||||
| POL | U53 | |||||||||||||||||||
| PRI | U54 | |||||||||||||||||||
| PRK | U55 | |||||||||||||||||||
| PRT | U56 | |||||||||||||||||||
| PRY | U57 | |||||||||||||||||||
| PSE | U58 | |||||||||||||||||||
| PYF | U59 | |||||||||||||||||||
| QAT | U60 | |||||||||||||||||||
| REU | U61 | |||||||||||||||||||
| ROU | U62 | |||||||||||||||||||
| RUS | U65 | |||||||||||||||||||
| RWA | U66 | |||||||||||||||||||
| SAU | U75 | |||||||||||||||||||
| SDN | U79 | |||||||||||||||||||
| SEN | U81 | |||||||||||||||||||
| SGP | U82 | |||||||||||||||||||
| SGS | U83 | |||||||||||||||||||
| SHN | U84 | |||||||||||||||||||
| SJM | U87 | |||||||||||||||||||
| SLB | U88 | |||||||||||||||||||
| SLE | U90 | |||||||||||||||||||
| SLV | UA1 | |||||||||||||||||||
| SMR | UC1 | |||||||||||||||||||
| SOM | UC2 | |||||||||||||||||||
| SPM | UC3 | |||||||||||||||||||
| SRB | UC6 | |||||||||||||||||||
| STP | UC7 | |||||||||||||||||||
| SUR | UD1 | |||||||||||||||||||
| SVK | UE1 | |||||||||||||||||||
| SVN | UE2 | |||||||||||||||||||
| SWE | UH1 | |||||||||||||||||||
| SWZ | UH2 | |||||||||||||||||||
| SYC | UH3 | |||||||||||||||||||
| SYR | UL1 | |||||||||||||||||||
| TCA | UR1 | |||||||||||||||||||
| TCD | UR3 | |||||||||||||||||||
| TGO | UR6 | |||||||||||||||||||
| THA | UR8 | |||||||||||||||||||
| TJK | US3 | |||||||||||||||||||
| TKL | US4 | |||||||||||||||||||
| TKM | UT1 | |||||||||||||||||||
| TLS | UT2 | |||||||||||||||||||
| TON | VF1 | |||||||||||||||||||
| TTO | X01 | |||||||||||||||||||
| TUN | X02 | |||||||||||||||||||
| TUR | X06 | |||||||||||||||||||
| TUV | X98 | |||||||||||||||||||
| TWN | Y01 | |||||||||||||||||||
| TZA | Y02 | |||||||||||||||||||
| UGA | Z01 | |||||||||||||||||||
| UKR | Z02 | |||||||||||||||||||
| UMI | ||||||||||||||||||||
| URY | ||||||||||||||||||||
| USA | ||||||||||||||||||||
| UZB | ||||||||||||||||||||
| VAT | ||||||||||||||||||||
| VCT | ||||||||||||||||||||
| VEN | ||||||||||||||||||||
| VGB | ||||||||||||||||||||
| VIR | ||||||||||||||||||||
| VNM | ||||||||||||||||||||
| VUT | ||||||||||||||||||||
| WLF | ||||||||||||||||||||
| WSM | ||||||||||||||||||||
| YEM | ||||||||||||||||||||
| ZAF | ||||||||||||||||||||
| ZMB | ||||||||||||||||||||
| ZWE |
Sheet 6: Trial Data Element Specs
| Trial elements Order | Trial data element | Required for original submission | Required for amendment | Required for update | Valid Values | Comments |
| 1 | Unique Trial Identifier | Yes | Yes | Yes | ||
| 2 | Submission Type | Yes | Yes | Yes | O, A, U | O - original submission (including the first submission to CTRP); A - amendment submission to the already published trial in CTRP; U - update to the CTRP trial. Amendment submission can be accepted only if the trial processing status is 'Abstraction Verified Response' or 'Abstraction Verified No Response'. Update can be submitted for trials that have been accepted or have processing status other than 'Submitted' and 'Rejected'. See Processing Status Transition tab for information about trial processing statuses |
| 3 | NCI Trial Identifier | Yes | Yes | This element is applicable to amendment submission and update to the CTRP trials only. This is the trial identifier assigned by the CTRP. Amendment can only be accepted for trials that have 'Abstraction Verified Response' or 'Abstraction Verified No Response' processing status in CTRP. Update can be submitted for trials that have 'Accepted' status and above. | ||
| 4 | Amendment Number | This element is applicable to amendment submission only. Use amendment number that is recorded in user's system. | ||||
| 5 | Amendment Date | Yes | This element is applicable to amendment submission only. Use date of amendment as documented in the amended protocol document . Format mm/dd/yyyy. | |||
| 6 | Lead Organization Trial Identifier | Yes | Yes | AS IS in the protocol document & assigned by the lead organization (unique in the lead organization system) | ||
| 7 | NCT | Unique identifier assigned to the published trials in PRS (ClinicalTrials.gov) | ||||
| 8 | Other Trial Identifier | If more than one exists, provide them in one column separated with semicolon (;) | ||||
| 9 | Title | Yes | Yes | Max 4000 characters | Title from the protocol document | |
| 10 | Trial Type | Yes | Yes | Yes | Interventional, Observational | Currently only Interventional trials are accepted |
| 11 | Primary Purpose | Yes | Yes | Yes | Treatment, Prevention, Supportive Care, Screening, Diagnostic, Health Service Research, Basic Science, Other | |
| 12 | [Primary Purpose] Additional Qualifier | Yes if Primary Purpose is 'Other' | Yes if Primary Purpose is 'Other' | Yes if Primary Purpose is 'Other' | Other | Use value 'Other' if Primary Purpose value is 'Other' (this applies to interventional trials only) |
| 13 | [Primary Purpose] Other Text | Yes if Primary Purpose is 'Other' | Yes if Primary Purpose is 'Other' | Yes if Primary Purpose is 'Other' | Provide description if Primary Purpose is 'Other' (col 13) | |
| 14 | Phase | Yes | Yes | Yes | Early Phase I, I, I/II, II, II/III, III, IV, NA, | |
| 15 | Pilot Trial? | Yes, No | Will be recorded only if Phase value is NA. Default: No | |||
| 16 | [Sponsor] Organization PO-ID | Yes | Yes | |||
| 17 | Responsible Party | PI, Sponsor, Sponsor Investigator | ||||
| 18 | [Responsible Party] Investigator Person PO-ID | Yes if ‘Responsible Party’ is PI or Sponsor Investigator | Yes if ‘Responsible Party’ is PI or Sponsor Investigator | Yes if ‘Responsible Party’ is PI or Sponsor Investigator | ||
| 19 | [Responsible Party] Title | Yes if ‘Responsible Party’ is PI or Sponsor Investigator | Yes if ‘Responsible Party’ is PI or Sponsor Investigator | Yes if ‘Responsible Party’ is PI or Sponsor Investigator | ||
| 20 | [Responsible Party] Affilliation Organization PO-ID | Yes if ‘Responsible Party’ is PI or Sponsor Investigator | Yes if ‘Responsible Party’ is PI or Sponsor Investigator | Yes if ‘Responsible Party’ is PI or Sponsor Investigator | ||
| 21 | [Lead Organization] Organization PO-ID | Yes | Yes | |||
| 22 | [Principal Investigator] Person PO-ID | Yes | Yes | |||
| 23 | Data Table 4 Funding Category | Yes | Yes | Yes | National, Externally Peer-Reviewed, Institutional | |
| 24 | [Data Table 4 Funding Sponsor/Source] Organization PO-ID | Yes | Yes | Yes | ||
| 25 | Program Code | Data Table 4 element, no LOV exists, codes are specific to cancer centers | ||||
| 26 | [NIH Grant] Funding Mechanism | Yes: if NIH grant exists | Yes: if NIH grant exists | Yes: if NIH grant exists | Refer Funding Mechanism in Valid Values worksheet. | If more than one grant is recorded provide this value for all grants separated by semicolon (;) |
| 27 | [NIH Grant] Institute Code | Yes: if NIH grant exists | Yes: if NIH grant exists | Yes: if NIH grant exists | Refer Institute Code in Valid Values worksheet. | If more than one grant is recorded provide this value for all grants separated by semicolon (;) |
| 28 | [NIH Grant] Serial Number | Yes: if NIH grant exists | Yes: if NIH grant exists | Yes: if NIH grant exists | format: 5 or 6 digits | If more than one grant is recorded provide this value for all grants separated by semicolon (;) |
| 29 | [NIH Grant] NCI Division/Program Code | Yes: if NIH grant exists | Yes: if NIH grant exists | Yes: if NIH grant exists | Refer NCI Division/Program Code in Valid Values worksheet. Specify only the code. | Defaults to N/A if not specified. If more than one grant is recorded provide this value for all grants separated by semicolon (;) |
| 30 | Current Trial Status | Yes | Yes | Yes | In Review, Approved, Active, Closed to Accrual, Closed to Accrual and Intervention , Temporarily Closed to Accrual, Temporarily Closed to Accrual and Intervention, Complete, Administratively Complete are applicable to original submission, amendment and update. Withdrawn status is only applicable to Update functionality. | 1) Trials with current trial status 'Withdrawn' are not accepted for the original submission. 2) Submission of amendment or update to existing study with Completed, Administratively Completed, Withdrawn and Disapproved current trial status are not accepted. 3) Please use 'In Review' status at submission of pre-IRB approved study. |
| 31 | Why Study Stopped? | Yes if Current Trial Status is Withdrawn, Temporarily Closed to Accrual, Temporarily Closed to Accrual and Intervention or Administratively Complete | Yes if Current Trial Status is Withdrawn, Temporarily Closed to Accrual, Temporarily Closed to Accrual and Intervention or Administratively Complete | Yes if Current Trial Status is Withdrawn, Temporarily Closed to Accrual, Temporarily Closed to Accrual and Intervention or Administratively Complete | Mandatory if Current Trial Status is Withdrawn, Temporarily Closed to Accrual, Temporarily Closed to Accrual and Intervention or Administratively Complete | |
| 32 | Current Trial Status Date | Yes | Yes | Yes | Date when the status has came in effect. Format: mm/dd/yyyy | |
| 33 | Study Start Date | Yes | Yes | Yes | Date that enrollment to the protocol begins. Format: mm/dd/yyyy | |
| 34 | Study Start Date Type | Yes | Yes | Yes | Actual, Anticipated | Only current/past date (in respect to batch upload date) is accepted for actual type and only future date is accepted for anticipated type. 'Anticipated' type is valid for 'In Review' and 'Approved' and 'Withdrawn' current trial status only. 'Actual' type is valid for any other current trial status besides 'In Review', 'Approved' and 'Withdrawn'. For more information check State-Dates tab in this file. |
| 35 | Primary Completion Date | Yes | Yes | Yes | Date that the final subject was examined or received an intervention for the purposes of final collection of data for the primary outcome, whether the clinical trial concluded according to the prespecified protocol or was terminated. Format: mm/dd/yyyy | |
| 36 | Primary Completion Date Type | Yes | Yes | Yes | Actual, Anticipated | Only current/past date (in respect to batch upload date) is accepted for actual type and only future date is accepted for anticipated type. 'Actual' type is valid for 'Administratively Complete' or 'Complete' current trial statuses only. 'Anticipated' type is valid for any other current trial status besides 'Administratively Complete' or 'Complete'. For more information check State-Dates tab in this file. |
| 37 | Study Completion Date | |||||
| 38 | Study Completion Date Type | |||||
| 39 | IND/IDE Type | Yes: if IND/IDE trial | Yes: if IND/IDE trial | Yes: if IND/IDE trial | IND, IDE | If more than one IND/IDE is recorded provide this value for all IND/IDE separated by semicolon (;). |
| 40 | IND/IDE Number | Yes: if IND/IDE trial | Yes: if IND/IDE trial | Yes: if IND/IDE trial | If more than one IND/IDE is recorded provide this value for all IND/IDE separated by semicolon (;) | |
| 41 | IND/IDE Grantor | Yes: if IND/IDE trial | Yes: if IND/IDE trial | Yes: if IND/IDE trial | CDER, CBER, CDRH | If more than one IND/IDE is recorded provide this value for all IND/IDE separated by semicolon (;) |
| 42 | IND/IDE Holder Type | Yes: if IND/IDE trial | Yes: if IND/IDE trial | Yes: if IND/IDE trial | Investigator, Organization, Industry, NIH, NCI | If more than one IND/IDE is recorded provide this value for all IND/IDE separated by semicolon (;) |
| 43 | [IND/IDE] NIH Institution | Yes If IND/IDE trial AND (IND/IDE Holder Type) = NIH | Yes If IND/IDE trial AND (IND/IDE Holder Type) = NIH | Yes If IND/IDE trial AND (IND/IDE Holder Type) = NIH | Refer NIH Institution in Valid Values worksheet. | If more than one IND/IDE is recorded provide this value for all IND/IDE separated by semicolon (;). If NIH institution is not applicable to a single IND/IDE, provide NA as replacement for the value |
| 44 | [IND/IDE] NCI Division /Program | Yes if IND/IDE trial AND If (IND/IDE Holder Type) = NCI | Yes if IND/IDE trial AND If (IND/IDE Holder Type) = NCI | Yes if IND/IDE trial AND If (IND/IDE Holder Type) = NCI | Refer NCI Division/Program Code in Valid Values worksheet. | If more than one IND/IDE is recorded provide this value for all IND/IDE separated by semicolon (;). If NCI division/program is not applicable to a single IND/IDE, provide NA as replacement for the value |
| 45 | [IND/IDE] Availability of Expanded Access Expanded Access? | Yes if IND/IDE trial | Yes if IND/IDE trial | Yes if IND/IDE trial | Yes, No, Unknown | If more than one IND/IDE is recorded provide this value for all IND/IDE separated by semicolon (;). |
| 46 | [IND/IDE] Expanded Access Record | If (Has Expanded Access?) = Yes | If (Has Expanded Access?) = Yes | If (Has Expanded Access?) = Yes | If more than one IND/IDE is recorded provide this value for all IND/IDE separated by semicolon (;). If expanded access is not applicable to a single IND/IDE, provide NA as replacement for the value | |
| 47 | Studies a US FDA regulated Drug Product | Yes, No, | ||||
| 48 | Studies a US FDA regulated Device Product | Yes, No, | ||||
| 49 | Unapproved/Uncleared Device | Yes, No, | ||||
| 50 | Pediatric Post-Market Survelliance | Yes, No, | ||||
| 51 | Product Exported from the US | Yes, No, | ||||
| 52 | FDA Regulatory Information Indicator | Yes, No | ||||
| 53 | Section 801 Indicator | Yes if FDA Regulatory Information Indicator is 'Yes' | Yes if FDA Regulatory Information Indicator is 'Yes' | Yes if FDA Regulatory Information Indicator is 'Yes' | Yes, No | |
| 54 | Data Monitoring Committee Appointed Indicator | Yes, No | ||||
| 55 | Protocol Document File Name | Yes | Yes | 1) Include file extension. 2) If you have at least two files with the same name, rename files (ex. prefix unique trial identifier to document name). 3) Submit amended protocol for amendment submission. | ||
| 56 | IRB Approval Document File Name | Yes | Yes | 1) Include file extension. 2) if you have at least two files with the same name, rename files (ex. prefix unique trial identifier to document name). 3) Submit dummy file if IRB approval is not required with the statement 'IRB' approval is not required'. 4) Submit dummy file with the following info: name of Review Board (address, phone, email) and Board Affiliation name in case of pre-IRB approved studies submission. 5) One IRB Approval is only needed. | ||
| 57 | Participating Sites Document File Name | 1) Include file extension. 2) f you have at least two files with the same name, rename files (ex. prefix unique trial identifier to document name). 3) Requited if case of multi-site trial and if the participation sites information is not included in the protocol document. 4) If participating site changes (recruitment status, program code) or collaborator's info change occur, submit this document for amendment or update | ||||
| 58 | Informed Consent Document File Name | 1) Requited if is not included in the protocol document.2) Include file extension. 3) f you have at least two files with the same name, rename files (ex. prefix unique trial identifier to document name). | ||||
| 59 | Other Trial Related Document File Name | 1) Include file extension. 2) f you have at least two files with the same name, rename files (ex. prefix unique trial identifier to document name). | ||||
| 60 | Change Memo Document Name | Yes | 1) This element is applicable to the amendment only and includes the changes that occurred in the protocol document due to amendment. 2) Include file extension. 3) f you have at least two files with the same name, rename files (ex. prefix unique trial identifier to document name). | |||
| 61 | Protocol Highlight Document Name | 1) This element is applicable to the amendment only and includes the protocol document with highlighted changes from the previous version. 2) Include file extension. 3) f you have at least two files with the same name, rename files (ex. prefix unique trial identifier to document name). |
Sheet 7: NIH & NCI Values
| NOTE: These are the valid values for the data elements. Although they are presented in vertical format, there is no correlation between the columns. | ||||||
| Funding Mechanism | Institute Code | NCI Division/Program Code | NIH Institution | |||
| B01 | AA | CCR | NEI-National Eye Institute | |||
| B08 | AE | CTEP | NHLBI-National Heart, Lung, and Blood Institute | |||
| B09 | AF | CIP | NHGRI-National Human Genome Research Institute | |||
| C06 | AG | CDP | NIA-National Institute on Aging | |||
| DP1 | AI | CCT/CTB | NIAAA-National Institute on Alcohol Abuse and Alcoholism | |||
| DP2 | AM | DCB | NIAID-National Institute of Allergy and Infectious Diseases | |||
| DP3 | AO | DCCPS | NIAMS-National Institute of Arthritis and Musculoskeletal and Skin Diseases | |||
| D43 | AR | DCEG | NIBIB-National Institute of Biomedical Imaging and Bioengineering | |||
| D71 | AT | DTP | NICHD-Eunice Kennedy Shriver National Institute of Child Health and Human Development | |||
| E11 | BC | DCP | NIDCD-National Institute on Deafness and Other Communication Disorders | |||
| F05 | BX | DEA | NIDCR-National Institute of Dental and Craniofacial Research | |||
| F30 | CA | OD | NIDDK-National Institute of Diabetes and Digestive and Kidney Diseases | |||
| F31 | CB | OSB/SPOREs | NIDA-National Institute on Drug Abuse | |||
| F32 | CD | TRP | NIEHS-National Institute of Environmental Health Sciences | |||
| F33 | CE | RRP | NIGMS-National Institute of General Medical Sciences | |||
| F34 | CH | N/A | NIMH-National Institute of Mental Health | |||
| F37 | CI | NINDS-National Institute of Neurological Disorders and Stroke | ||||
| F38 | CK | NINR-National Institute of Nursing Research | ||||
| G07 | CL | NLM-National Library of Medicine | ||||
| G08 | CM | CIT-Center for Information Technology | ||||
| G11 | CN | CSR-Center for Scientific Review | ||||
| G12 | CO | FIC-John E. Fogarty International Center for Advanced Study in the Health Sciences | ||||
| G13 | CP | NCCAM-National Center for Complementary and Alternative Medicine | ||||
| G20 | CR | NCMHD-National Center on Minority Health and Health Disparities | ||||
| G94 | CT | NCRR-National Center for Research Resources (NCRR | ||||
| HD4 | CU | CC-NIH Clinical Center | ||||
| HR! | CX | OD-Office of the Director | ||||
| DA | ||||||
| H13 | DC | |||||
| H23 | DD | |||||
| H25 | DE | |||||
| H28 | DK | |||||
| H50 | DP | |||||
| H57 | EB | |||||
| H62 | EH | |||||
| H64 | EM | |||||
| H75 | EP | |||||
| H79 | ES | |||||
| I01 | EY | |||||
| KD1 | FD | |||||
| KL1 | GD | |||||
| KL2 | GH | |||||
| K01 | GM | |||||
| K02 | GW | |||||
| K05 | HB | |||||
| K06 | HC | |||||
| K07 | HD | |||||
| K08 | HG | |||||
| K12 | HI | |||||
| K14 | HK | |||||
| K18 | HL | |||||
| K21 | HM | |||||
| K22 | HO | |||||
| K23 | HP | |||||
| K24 | HR | |||||
| K25 | HS | |||||
| K26 | HV | |||||
| K30 | HX | |||||
| K99 | HY | |||||
| L30 | IP | |||||
| L32 | JT | |||||
| L40 | LM | |||||
| L50 | MD | |||||
| L60 | MH | |||||
| M01 | MN | |||||
| N01 | NB | |||||
| N02 | NH | |||||
| N03 | NR | |||||
| N43 | NS | |||||
| N44 | NU | |||||
| PL1 | OA | |||||
| PN1 | OC | |||||
| PN2 | OD | |||||
| P01 | OF | |||||
| P20 | OH | |||||
| P30 | OL | |||||
| P40 | OR | |||||
| P41 | PC | |||||
| P42 | PH | |||||
| P50 | PR | |||||
| P51 | PS | |||||
| P60 | RD | |||||
| P76 | RX | |||||
| RC1 | SC | |||||
| RC2 | SF | |||||
| RC3 | SH | |||||
| RC4 | SM | |||||
| RL1 | SP | |||||
| RL2 | SU | |||||
| RL5 | TI | |||||
| RL9 | TP | |||||
| RS1 | TS | |||||
| R00 | WH | |||||
| R01 | RC | |||||
| R03 | RG | |||||
| R04 | RM | |||||
| R06 | RR | |||||
| R08 | TW | |||||
| R13 | WT | |||||
| R15 | VA | |||||
| R17 | WC | |||||
| R18 | ||||||
| R21 | ||||||
| R24 | ||||||
| R25 | ||||||
| R30 | ||||||
| R33 | ||||||
| R34 | ||||||
| R36 | ||||||
| R37 | ||||||
| R41 | ||||||
| R42 | ||||||
| R43 | ||||||
| R44 | ||||||
| R49 | ||||||
| R55 | ||||||
| R56 | ||||||
| R90 | ||||||
| SC1 | ||||||
| SC2 | ||||||
| SC3 | ||||||
| S06 | ||||||
| S10 | ||||||
| S11 | ||||||
| S21 | ||||||
| S22 | ||||||
| TL1 | ||||||
| TU2 | ||||||
| T01 | ||||||
| T02 | ||||||
| T03 | ||||||
| T06 | ||||||
| T09 | ||||||
| T14 | ||||||
| T15 | ||||||
| T32 | ||||||
| T34 | ||||||
| T35 | ||||||
| T36 | ||||||
| T37 | ||||||
| T42 | ||||||
| T90 | ||||||
| UA1 | ||||||
| UC1 | ||||||
| UC2 | ||||||
| UC3 | ||||||
| UC6 | ||||||
| UC7 | ||||||
| UD1 | ||||||
| UE1 | ||||||
| UE2 | ||||||
| UH1 | ||||||
| UH2 | ||||||
| UH3 | ||||||
| UL1 | ||||||
| UR1 | ||||||
| UR3 | ||||||
| UR6 | ||||||
| UR8 | ||||||
| US3 | ||||||
| US4 | ||||||
| UT1 | ||||||
| UT2 | ||||||
| U01 | ||||||
| U09 | ||||||
| U10 | ||||||
| U11 | ||||||
| U13 | ||||||
| U14 | ||||||
| U17 | ||||||
| U18 | ||||||
| U19 | ||||||
| U1A | ||||||
| U1Q | ||||||
| U1S | ||||||
| U1T | ||||||
| U1V | ||||||
| U21 | ||||||
| U22 | ||||||
| U23 | ||||||
| U24 | ||||||
| U27 | ||||||
| U2G | ||||||
| U2R | ||||||
| U30 | ||||||
| U32 | ||||||
| U34 | ||||||
| U36 | ||||||
| U38 | ||||||
| U41 | ||||||
| U42 | ||||||
| U43 | ||||||
| U44 | ||||||
| U45 | ||||||
| U47 | ||||||
| U48 | ||||||
| U49 | ||||||
| U50 | ||||||
| U51 | ||||||
| U52 | ||||||
| U53 | ||||||
| U54 | ||||||
| U55 | ||||||
| U56 | ||||||
| U57 | ||||||
| U58 | ||||||
| U59 | ||||||
| U60 | ||||||
| U61 | ||||||
| U62 | ||||||
| U65 | ||||||
| U66 | ||||||
| U75 | ||||||
| U79 | ||||||
| U81 | ||||||
| U82 | ||||||
| U83 | ||||||
| U84 | ||||||
| U87 | ||||||
| U88 | ||||||
| U90 | ||||||
| VF1 | ||||||
| X01 | ||||||
| X02 | ||||||
| X06 | ||||||
| X98 | ||||||
| Y01 | ||||||
| Y02 | ||||||
| Z01 | ||||||
| Z02 |
Sheet 8: NCI Code Definitions
| NCI Division/Program Code | Definition |
| CCR | Center for Cancer Research |
| CCT/CTB | Center for Cancer Training / Cancer Training Branch |
| CTEP | Cancer Therapy Evaluation Program |
| DCB | Division of Cancer Biology |
| DCCPS | Division of Cancer Control and Population Sciences |
| DCEG | Division of Cancer Epidemiology and Genetics |
| DTP | Developmental Therapeutics Program |
| DCP | Division of Cancer Prevention |
| DEA | Division of Extramural Activities |
| OD | Office of the Director, NCI, NIH |
| OSB/SPOREs | Organ Systems Branch (OSB) /Specialized Programs of Research Excellence (SPOREs) |
| CIP | Cancer Imaging Program |
| CDP | Cancer Diagnosis Program |
| TRP | Translational Research Program |
| RRP | Radiation Research Program |
| N/A | Not applicable |
Sheet 9: 3-letter country code
| Country | 3-letter code |
| Afghanistan | AFG |
| Aland Islands | ALA |
| Albania | ALB |
| Algeria | DZA |
| American Samoa | ASM |
| Andorra | AND |
| Angola | AGO |
| Anguilla | AIA |
| Antarctica | ATA |
| Antigua And Barbuda | ATG |
| Argentina | ARG |
| Armenia | ARM |
| Aruba | ABW |
| Australia | AUS |
| Austria | AUT |
| Azerbaijan | AZE |
| Bahamas | BHS |
| Bahrain | BHR |
| Bangladesh | BGD |
| Barbados | BRB |
| Belarus | BLR |
| Belgium | BEL |
| Belize | BLZ |
| Benin | BEN |
| Bermuda | BMU |
| Bhutan | BTN |
| Bolivia | BOL |
| Bosnia And Herzegowina | BIH |
| Botswana | BWA |
| Bouvet Island | BVT |
| Brazil | BRA |
| British Indian Ocean Territory | IOT |
| Brunei Darussalam | BRN |
| Bulgaria | BGR |
| Burkina Faso | BFA |
| Burundi | BDI |
| Cambodia | KHM |
| Cameroon | CMR |
| Canada | CAN |
| Cape Verde | CPV |
| Cayman Islands | CYM |
| Central African Republic | CAF |
| Chad | TCD |
| Chile | CHL |
| China | CHN |
| Christmas Island | CXR |
| Cocos (Keeling) Islands | CCK |
| Colombia | COL |
| Comoros | COM |
| Congo | COG |
| Congo, Democratic Republic of th | COD |
| Cook Islands | COK |
| Costa Rica | CRI |
| Cote D Ivoire | CIV |
| Croatia | HRV |
| Cuba | CUB |
| Cyprus | CYP |
| Czech Republic | CZE |
| Denmark | DNK |
| Djibouti | DJI |
| Dominica | DMA |
| Dominican Republic | DOM |
| Ecuador | ECU |
| Egypt | EGY |
| El Salvador | SLV |
| Equatorial Guinea | GNQ |
| Eritrea | ERI |
| Estonia | EST |
| Ethiopia | ETH |
| Falkland Islands (Malvinas) | FLK |
| Faroe Islands | FRO |
| Fiji | FJI |
| Finland | FIN |
| France | FRA |
| French Guiana | GUF |
| French Polynesia | PYF |
| French Southern Territories | ATF |
| Gabon | GAB |
| Gambia | GMB |
| Georgia | GEO |
| Germany | DEU |
| Ghana | GHA |
| Gibraltar | GIB |
| Greece | GRC |
| Greenland | GRL |
| Grenada | GRD |
| Guadeloupe | GLP |
| Guam | GUM |
| Guatemala | GTM |
| Guernsey | GGY |
| Guinea | GIN |
| Guinea-Bissau | GNB |
| Guyana | GUY |
| Haiti | HTI |
| Heard And Mc Donald Islands | HMD |
| Holy See (Vatican City State) | VAT |
| Honduras | HND |
| Hong Kong | HKG |
| Hungary | HUN |
| Iceland | ISL |
| India | IND |
| Indonesia | IDN |
| Iran (Islamic Republic Of) | IRN |
| Iraq | IRQ |
| Ireland | IRL |
| Isle of Man | IMN |
| Israel | ISR |
| Italy | ITA |
| Jamaica | JAM |
| Japan | JPN |
| Jersey | JEY |
| Jordan | JOR |
| Kazakhstan | KAZ |
| Kenya | KEN |
| Kiribati | KIR |
| Korea, Democratic Peoples Republic of | PRK |
| Korea, Republic of | KOR |
| Kuwait | KWT |
| Kyrgyzstan | KGZ |
| Lao Peoples Democratic Republic | LAO |
| Latvia | LVA |
| Lebanon | LBN |
| Lesotho | LSO |
| Liberia | LBR |
| Libyan Arab Jamahiriya | LBY |
| Liechtenstein | LIE |
| Lithuania | LTU |
| Luxembourg | LUX |
| Macau | MAC |
| Macedonia | MKD |
| Madagascar | MDG |
| Malawi | MWI |
| Malaysia | MYS |
| Maldives | MDV |
| Mali | MLI |
| Malta | MLT |
| Marshall Islands | MHL |
| Martinique | MTQ |
| Mauritania | MRT |
| Mauritius | MUS |
| Mayotte | MYT |
| Mexico | MEX |
| Micronesia, Federated States of | FSM |
| Moldova, Republic of | MDA |
| Monaco | MCO |
| Mongolia | MNG |
| Montenegro | MNE |
| Montserrat | MSR |
| Morocco | MAR |
| Mozambique | MOZ |
| Myanmar | MMR |
| Namibia | NAM |
| Nauru | NRU |
| Nepal | NPL |
| Netherlands | NLD |
| Netherlands Antilles | ANT |
| New Caledonia | NCL |
| New Zealand | NZL |
| Nicaragua | NIC |
| Niger | NER |
| Nigeria | NGA |
| Niue | NIU |
| Norfolk Island | NFK |
| Northern Mariana Islands | MNP |
| Norway | NOR |
| Oman | OMN |
| Pakistan | PAK |
| Palau | PLW |
| Palestinian Territory | PSE |
| Panama | PAN |
| Papua New Guinea | PNG |
| Paraguay | PRY |
| Peru | PER |
| Philippines | PHL |
| Pitcairn | PCN |
| Poland | POL |
| Portugal | PRT |
| Puerto Rico | PRI |
| Qatar | QAT |
| Reunion | REU |
| Romania | ROU |
| Russian Federation | RUS |
| Rwanda | RWA |
| Saint Barthelemy | BLM |
| Saint Helena | SHN |
| Saint Kitts And Nevis | KNA |
| Saint Lucia | LCA |
| Saint Martin (French Part) | MAF |
| Saint Pierre and Miquelon | SPM |
| Saint Vincent And The Grenadines | VCT |
| Samoa | WSM |
| San Marino | SMR |
| Sao Tome And Principe | STP |
| Saudi Arabia | SAU |
| Senegal | SEN |
| Serbia | SRB |
| Seychelles | SYC |
| Sierra Leone | SLE |
| Singapore | SGP |
| Slovakia (Slovak Republic) | SVK |
| Slovenia | SVN |
| Solomon Islands | SLB |
| Somalia | SOM |
| South Africa | ZAF |
| South Georgia And The South Sandwich Islands | SGS |
| Spain | ESP |
| Sri Lanka | LKA |
| Sudan | SDN |
| Suriname | SUR |
| Svalbard And Jan Mayen Islands | SJM |
| Swaziland | SWZ |
| Sweden | SWE |
| Switzerland | CHE |
| Syrian Arab Republic | SYR |
| Taiwan | TWN |
| Tajikistan | TJK |
| Tanzania | TZA |
| Thailand | THA |
| Timor-Leste | TLS |
| Togo | TGO |
| Tokelau | TKL |
| Tonga | TON |
| Trinidad And Tobago | TTO |
| Tunisia | TUN |
| Turkey | TUR |
| Turkmenistan | TKM |
| Turks And Caicos Islands | TCA |
| Tuvalu | TUV |
| Uganda | UGA |
| Ukraine | UKR |
| United Arab Emirates | ARE |
| United Kingdom | GBR |
| United States | USA |
| United States Minor Outlying Islands | UMI |
| Uruguay | URY |
| Uzbekistan | UZB |
| Vanuatu | VUT |
| Venezuela | VEN |
| Viet Nam | VNM |
| Virgin Islands (British) | VGB |
| Virgin Islands (U.S.) | VIR |
| Wallis And Futuna Islands | WLF |
| Western Sahara | ESH |
| Yemen | YEM |
| Zambia | ZMB |
| Zimbabwe | ZWE |
Sheet 10: 2-letter state_province
| Country | Country 3-letter code | State/Province | 2-3 letter state/province code | Old values |
| UNITED STATES | USA | |||
| Alabama | AL | |||
| Alaska | AK | |||
| Arizona | AZ | |||
| Arkansas | AR | |||
| California | CA | |||
| Colorado | CO | |||
| Connecticut | CT | |||
| Delaware | DE | |||
| Florida | FL | |||
| Georgia | GA | |||
| Hawaii | HI | |||
| Idaho | ID | |||
| Illinois | IL | |||
| Indiana | IN | |||
| Iowa | IA | |||
| Kansas | KS | |||
| Kentucky | KY | |||
| Louisiana | LA | |||
| Maine | ME | |||
| Maryland | MD | |||
| Massachusetts | MA | |||
| Michigan | MI | |||
| Minnesota | MN | |||
| Mississippi | MS | |||
| Missouri | MO | |||
| Montana | MT | |||
| Nebraska | NE | |||
| Nevada | NV | |||
| New Hampshire | NH | |||
| New Jersey | NJ | |||
| New Mexico | NM | |||
| New York | NY | |||
| North Carolina | NC | |||
| North Dakota | ND | |||
| Ohio | OH | |||
| Oklahoma | OK | |||
| Oregon | OR | |||
| Pennsylvania | PA | |||
| Rhode Island | RI | |||
| South Carolina | SC | |||
| South Dakota | SD | |||
| Tennessee | TN | |||
| Texas | TX | |||
| Utah | UT | |||
| Vermont | VT | |||
| Virginia | VA | |||
| Washington | WA | |||
| West Virginia | WV | |||
| Wisconsin | WI | |||
| Wyoming | WY | |||
| CANADA | CAN | |||
| Alberta | AB | |||
| British Columbia | BC | |||
| Manitoba | MB | |||
| New Brunswick | NB | |||
| Newfoundland and Labrador | NL | |||
| Northwest Territories | NT | |||
| Nova Scotia | NS | |||
| Nunavut | NU | |||
| Ontario | ON | |||
| Prince Edward Island | PE | |||
| Quebec | QC | |||
| Saskatchewan | SK | |||
| Yukon | YT | |||
| AUSTRALIA | AUT | |||
| Australian Capital Territory | ACT | |||
| New South Wales | NSW | |||
| Northern Territory | NT | |||
| Queensland | QLD | |||
| South Australia | SA | |||
| Tasmania | TAS | |||
| Victoria | VIC | |||
| Western Australia | WA |
Sheet 11: Trial Status Date Diagram
| Trial Start, Primary Completion, and Completion Dates | |||||||||||||||
|
|||||||||||||||
| File Type | application/vnd.openxmlformats-officedocument.spreadsheetml.sheet |
| File Modified | 0000-00-00 |
| File Created | 0000-00-00 |
© 2026 OMB.report | Privacy Policy