Other Transactions Application Form Instructions
Attachment 20B_Other Transaction_ASSIST user instruction guide.docx
PHS Applications and Pre-award Related Reporting (OD)
Other Transactions Application Form Instructions
OMB: 0925-0001
Instruction Guide for OTA Submissions

Use these instructions, together with the forms and information found in the funding opportunity announcement, to complete your application. The research opportunity announcement (ROA) will include specific instruction and forms needed for your application submission. Remember that the ROA instructions always supersede these application instructions.
Prepare to Apply
NIH typically makes awards to organizations, not individuals. At the time of submission, the Program Director/Principal Investigator (PD/PI) and their organization must be registered at eRA Commons. In addition to the PD/PI, an individual with the role of Signing Official (SO) is needed. If an application is awarded, additional registrations (e.g. System Award Management) will be required.
OTA applications must be submitted using NIH’s ASSIST web-based application submission system. Users can access ASSIST directly or through eRA Commons. To complete the application, users must have access to a browser, a pdf generator, and Adobe Reader software.
Log into ASSIST using eRA Commons credentials (username and password)
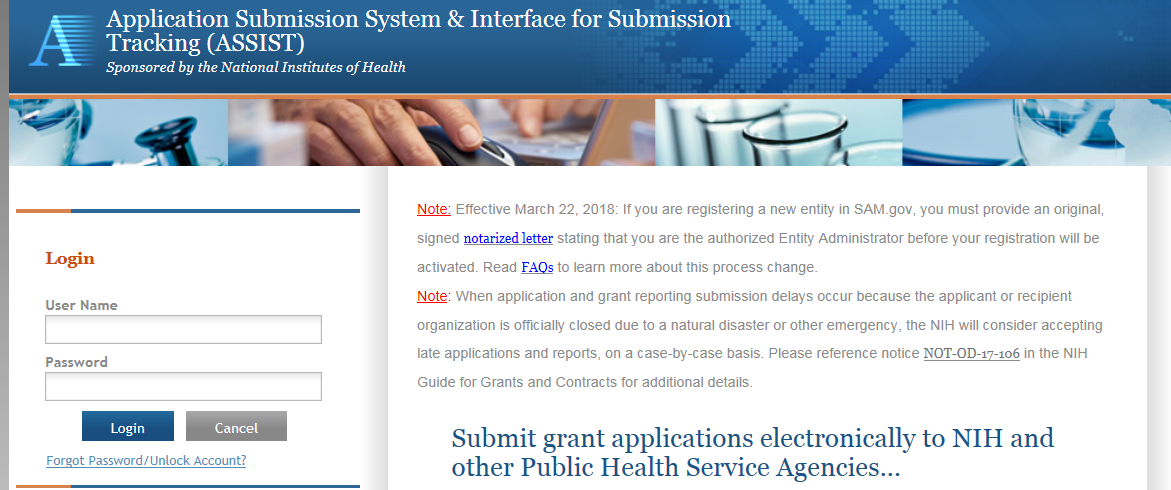
Initiating the Application
On the ASSIST Welcome screen, enter the OTA ROA number in the Funding Opportunity Announcement # field and then click ‘Go’.
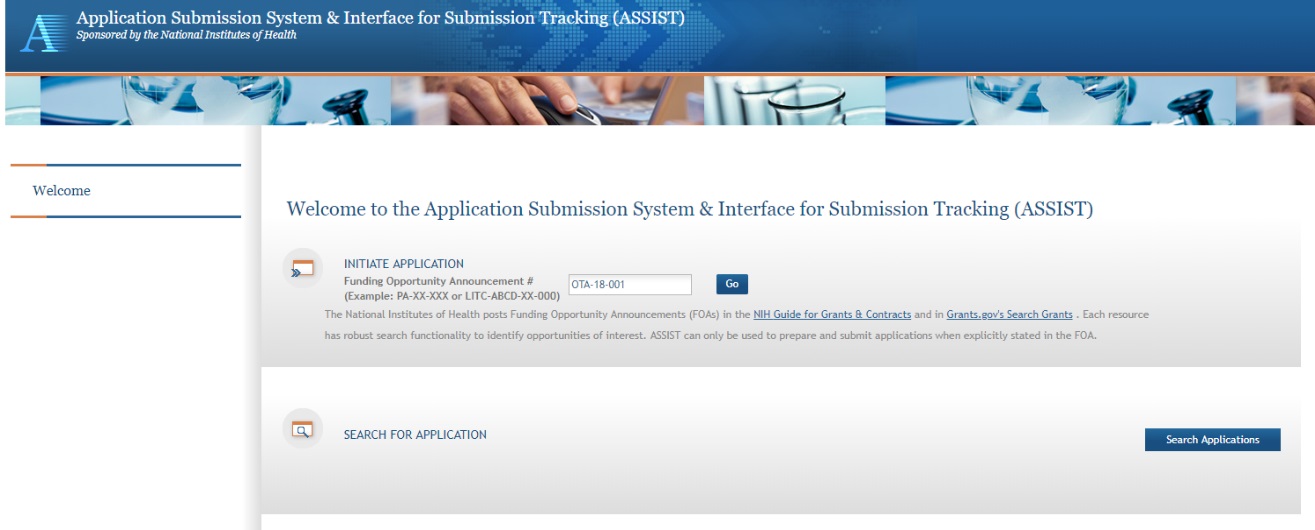
The Initiate Application screen contains several required elements: At a minimum the Application Project Title must be entered, and the Lead Applicant Organization must be selected from the drop-down menu.
The Contact PD/PI fields may be pre-filled from Commons Username using the button or entered manually. These fields will be available to edit in the application once it has been initiated.
Once required fields have been satisfied, press the “Initiate Application” button to create an application record.
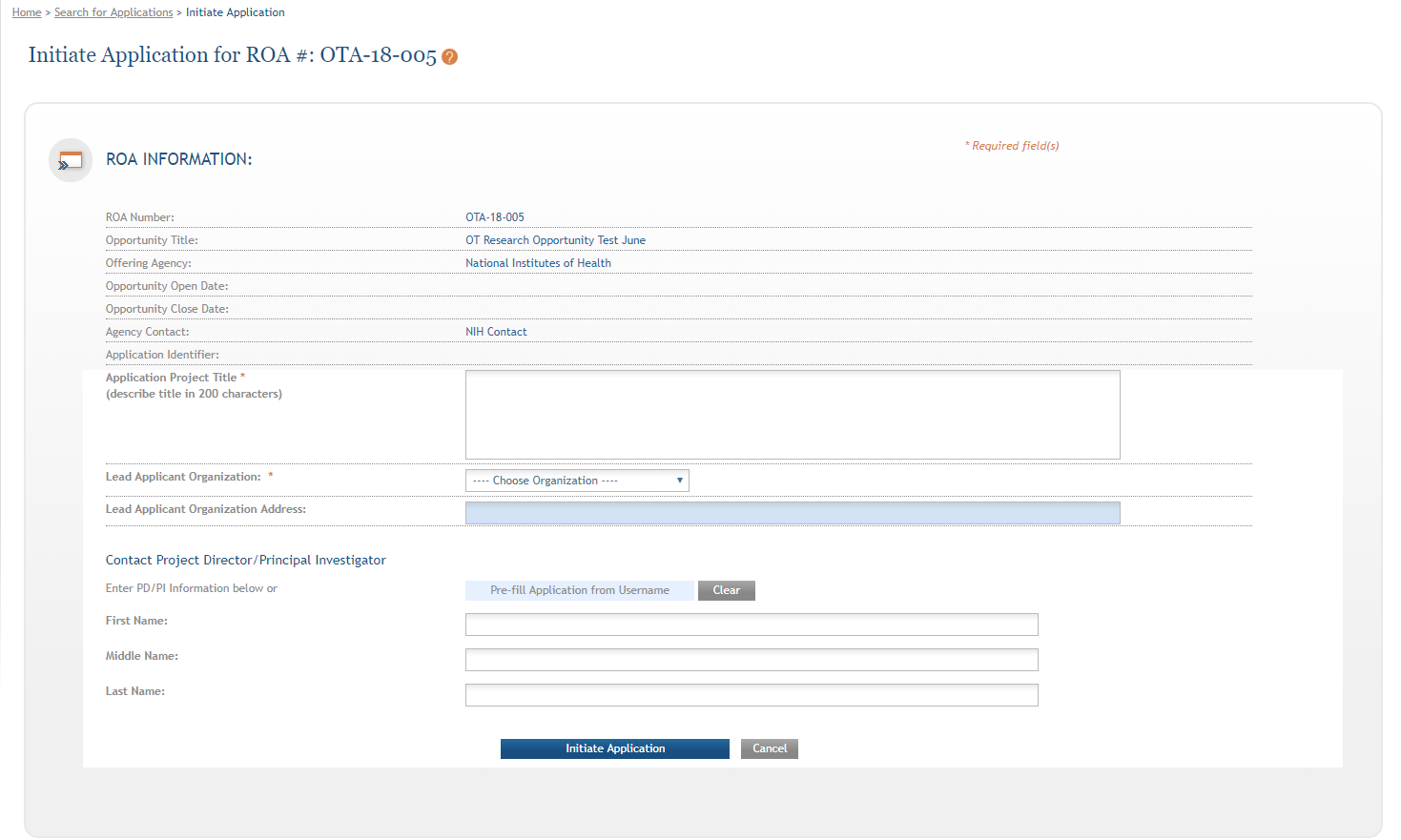
Navigating the Application
Each application in ASSIST receives a unique Application Identifier at creation. This value is displayed on the Application Information Summary page and can be used as a search key on the Search for Applications screen if returning to work on the application at a later time.

Actions are available on the left-hand side of the screen. The Summary screen and any constituent forms in the application are loaded as tabs to the right of the action menu.
Navigate to the OTA Core form by clicking the grey tab for the form. The currently active tab will be highlighted in blue.
Completing the Application Form
All fields marked by an asterisk (*) are required.
All attachments should be in the format of a PDF file.
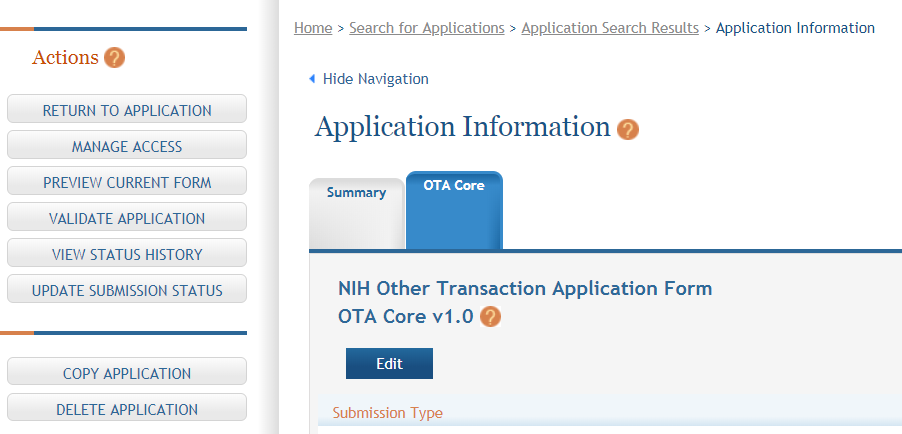
Click on “Edit” button to begin data entry. In the “edit” mode, data entry by other users is blocked until the lock-holder either releases the lock or it expires.
At the bottom of the form there are several saving options:
![]()
Save and Keep Lock: saves data and restricts data entry access to current user
Save and Release Lock: saves data and releases form to other users
Cancel and Release Lock: does not save data and releases form to other users
Submission Type: If the application is being resubmitted after correcting errors/warning, check “Corrected Submission” and enter prior submission tracking number as it appears in the footer of the prior submission.

Applicant Information

Organization Name: This field is required. Enter the name of the organization for the SO
Contact and Address fields: Enter the field data for the Applicant Organization as each label indicates. The Organization Name, Street 1, City, and Country are required fields. The State and ZIP/Postal Code fields will become required upon Country selection of United States. Note that ZIP/Postal Code must be entered in ZIP+4 (nine-digit postal code) format. Province is enabled for all non-US countries and required for Canada.
Employer Identification (EIN) or (TIN): Enter either the organization’s Taxpayer Identification Number (TIN) or Employer Identification Number (EIN) as assigned by the Internal Revenue Service. If your organization is not in the United States, enter 44-4444444. Your EIN may be 12 digits, and if this is the case, enter all 12 digits.
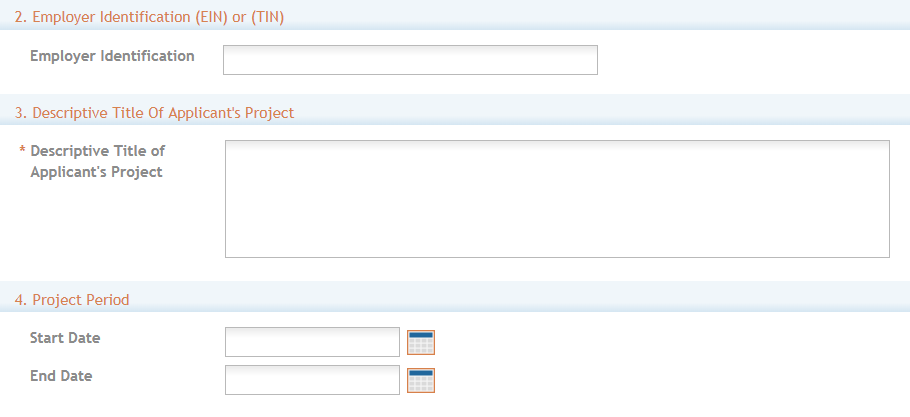
3. Descriptive Title of Applicant’s Project: This field is required. The descriptive title is limited to 200 characters, including spaces and punctuation.
4. Project Period: Enter the proposed start date of the project.
The Start Date is an estimate. The project period should not exceed what is allowed in the ROA.
The End Date is an estimate and must occur in the future of the Start Date.
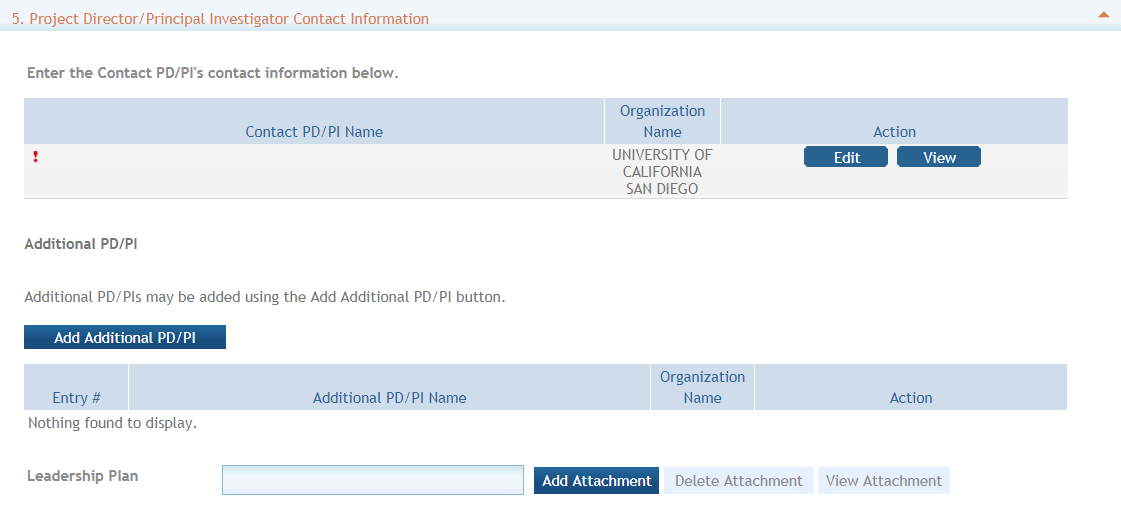
5. Project Director/Principal Investigator (PD/PI) Contact Information
The PD/PI is the individual responsible for the overall scientific and technical direction of the project.
In the eRA Commons profile, the person listed here must be affiliated with the applicant organization entered in “1. Applicant Information”.
If submitting an application with multiple PD/PIs, the main or primary PD/PI should be entered in the first or top section as the Contact PD/PI. The “Add Additional PD/PI” button may be used to add other PD/PIs. Following data entry, the user may “Edit” or “View” the PD/PI entries; Additional PD/PI entries can also be individually removed from the application.
If the ROA requires a leadership plan for a multi-PD/PI application, provide the rationale for choosing a multiple PD/PI approach. The governance and organizational structure of the leadership team and the research project should be described, including communication plans, processes for making decisions on scientific direction, and procedures for resolving conflicts. The roles and administrative, technical, and scientific responsibilities for the project or program should be delineated for the PD/PIs and other collaborators.
If budget allocation is planned, the distribution of resources to specific components of the project or the individual PD/PIs should be delineated in the Multiple PD/PI Leadership Plan. In the event of an award, the requested allocations may be reflected in a footnote on the Notice of Award.
The attachment should be attached as a PDF file.
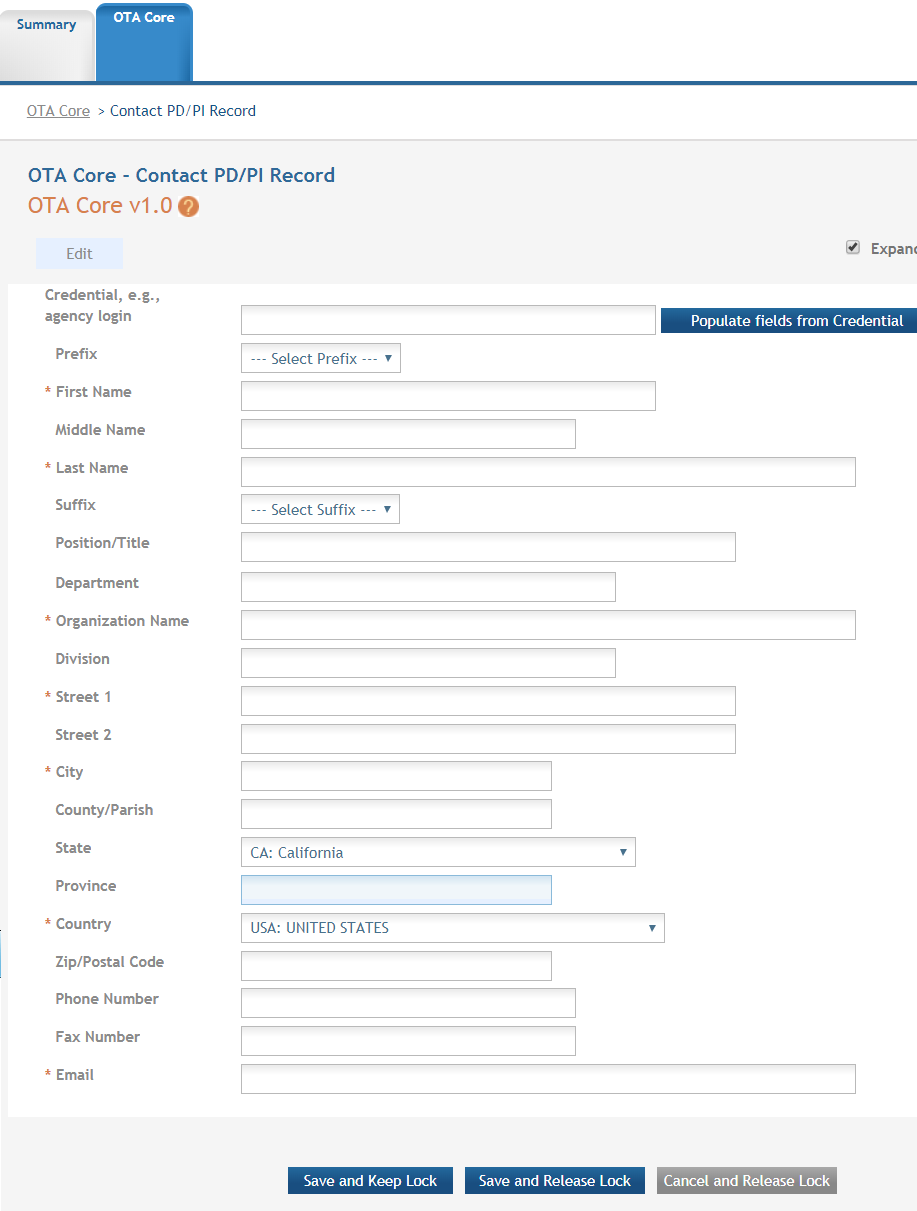
Credential, e.g. agency login: enter the eRA Commons user name for the PD/PI. This field is not required to save the form, however the Commons user Identification is for the Contact PD/PI at the time of submission.
Complete the personal information for the PD/PI.
Contact and Address fields: Enter the field data for the PD/PI as each label indicates. The Organization Name, Street 1, City, and Country are required fields. The State and ZIP/Postal Code fields will become required upon Country selection of United States. Note that ZIP/Postal Code must be entered in ZIP+4 (nine-digit postal code) format. Province is enabled for all non-US countries and required for Canada.
Following data entry, Save changes. To return to the main form use ‘Save and Release Lock’ or click the ‘OTA Core’ breadcrumb under the blue ‘OTA Core’ form tab.
6. Business Official Contact Information
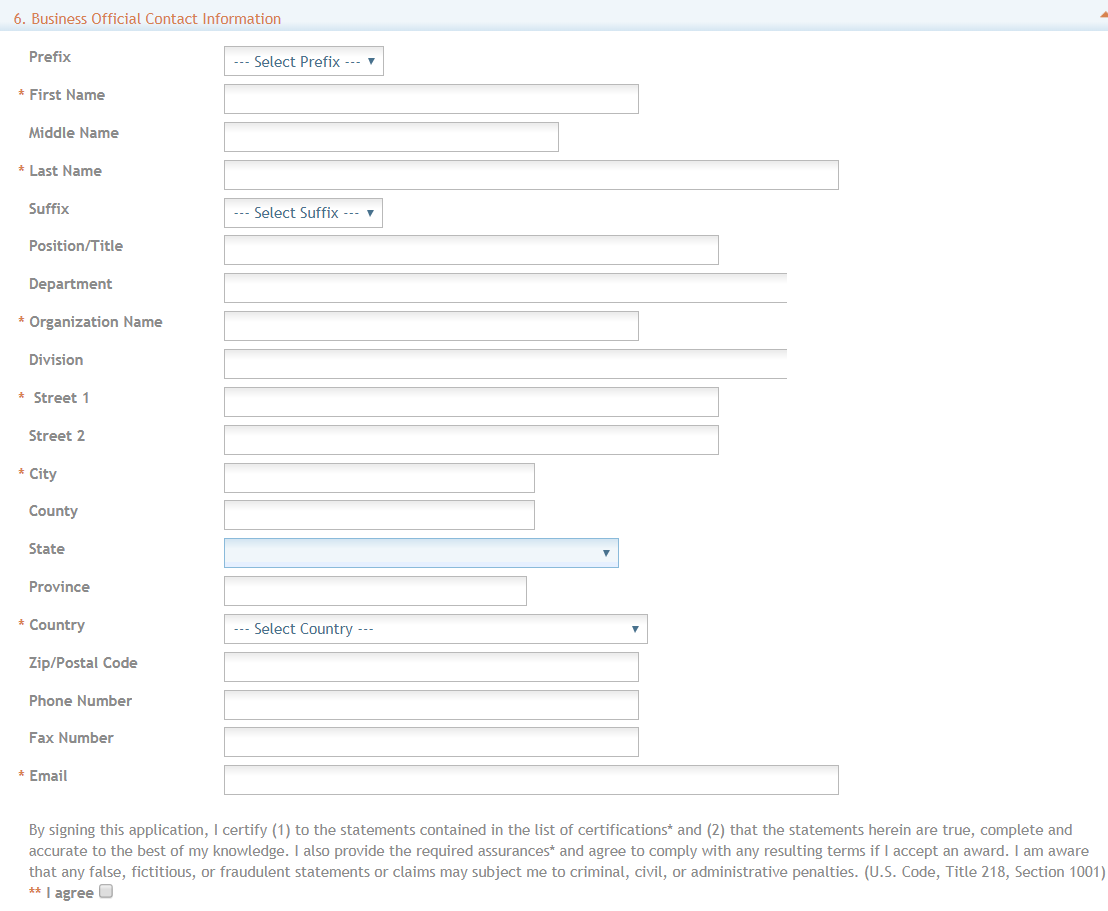
Complete the information for the Business Official Contact.
Contact and Address fields: Enter the field data for the Business Official as each label indicates. The Organization Name, Street 1, City, Country, and Email are required fields. The State and ZIP/Postal Code fields will become required upon Country selection of United States. Note that ZIP/Postal Code must be entered in ZIP+4 (nine-digit postal code) format. Province is enabled for all non-US countries and required for Canada.
Assurances: The applicant organization is responsible for verifying its eligibility and the accuracy, validity, and conformity with the most current institutional guidelines of all the administrative, fiscal, and scientific information in the application, including the Facilities and Administrative rate. Deliberate withholding, falsification, or misrepresentation of information could result in administrative actions, such as withdrawal of an application, suspension and/or termination of an award, debarment of individuals, as well as possible criminal and/or civil penalties. The signer further certifies that the applicant organization will be accountable both for the appropriate use of any funds awarded and for the performance of the grant-supported project or activities resulting from this application. The grantee institution may be liable for the reimbursement of funds associated with any inappropriate or fraudulent conduct of the project activity.
Check “I agree” to provide the required certifications and assurances.
7. Estimated Project Funding: Enter the total federal funds, including Direct Costs and F&A (Indirect Costs) requested for the entire project period.

8.
Human
Subjects:
Enter whether studies involving human subjects are anticipated. If
yes, indicate if the project is exempt from federal regulations and
check the appropriate exemption number, if applicable.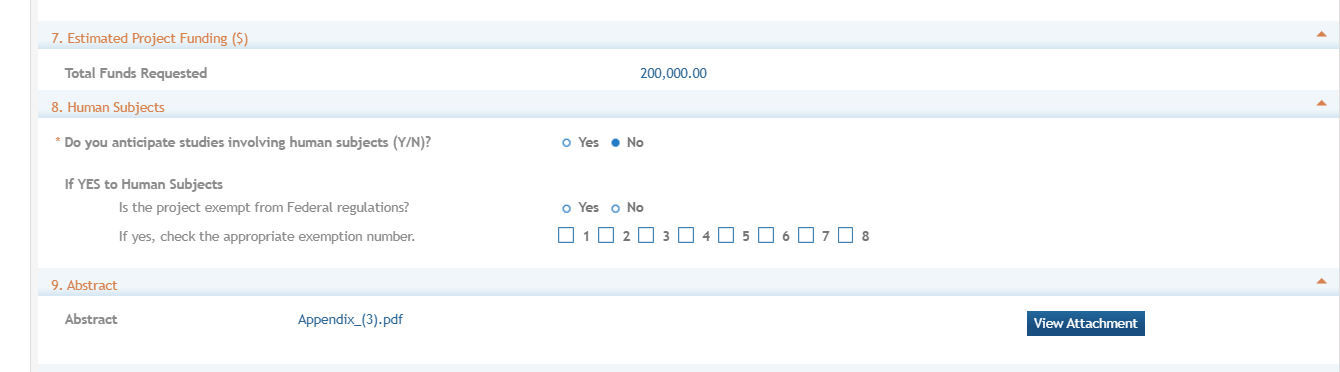
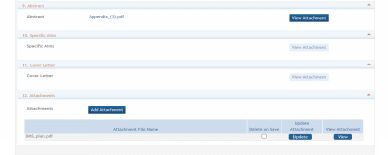
9. Abstract: Attach PDF file in accordance with the ROA and/or specific instructions using the “add attachment” button. The attachment should be in the format of a PDF file. To delete an uploaded attachment, check ‘Delete on Save’ and the file will be removed upon next save. Individual attachments can be replaced or updated by clicking the ‘Update’ button of the corresponding row.
10. Specific Aims: Attach PDF file in accordance with the ROA and/or specific instructions using the “add attachment” button. The attachment should be in the format of a PDF file. To delete an uploaded attachment, check ‘Delete on Save’ and the file will be removed upon next save. Individual attachments can be replaced or updated by clicking the ‘Update’ button of the corresponding row.
11. Cover Letter: The cover letter is for internal use only. It should be included any special considerations or explanatory details regarding the submission of the application. The letter should include the application title. The attachment should be in the format of a PDF file. To delete an uploaded attachment, check ‘Delete on Save’ and the file will be removed upon next save. Individual attachments can be replaced or updated by clicking the ‘Update’ button of the corresponding row.
12. Attachments: Attach PDF file in accordance with the ROA and/or specific instructions using the “add attachment” button. The attachment should be in the format of a PDF file. If multiple attachments are added, each should have a unique file name. To delete an uploaded attachment, check ‘Delete on Save’ and the file will be removed upon next save. Individual attachments can be replaced or updated by clicking the ‘Update’ button of the corresponding row.
Submitting your Application
 Validating
your application: Select
“Validate Application” from the left-hand panel to check
your application for business errors and warning. Errors must be
corrected prior to submission. Warnings will not stop prevent your
application from being submitted.
Validating
your application: Select
“Validate Application” from the left-hand panel to check
your application for business errors and warning. Errors must be
corrected prior to submission. Warnings will not stop prevent your
application from being submitted.
Preview Application: presents the PDF version of the application. This is a view of the assembled image is nearly identical to that the reviewers/evaluators will assess.
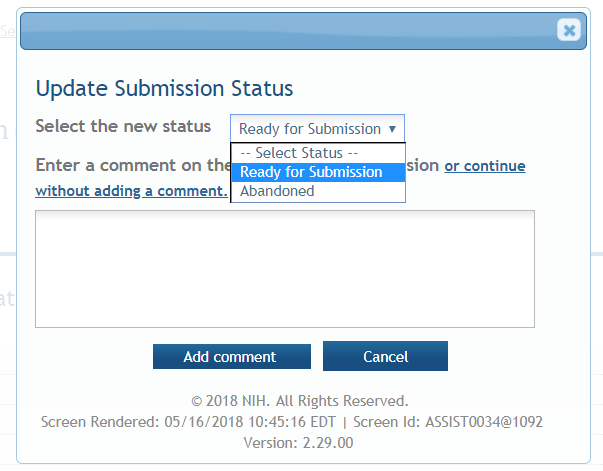
Update Submission Status: Prior to submission, the status must be updated to “Ready for Submission” which will also perform additional validations against business errors prior to changing the status. Click “Add Comment” or “continue without adding a comment” to continue.
Submission
Once
the application has been placed in “Ready for Submission”
status, the Signing Official will be able to click “Submit
Application”. After a confirmation that the SO does wish to
submit the application, it will be sent to NIH for processing. The
status can be viewed and updated by clicking the “View
Submission Status Details” hyperlink next to the application
status.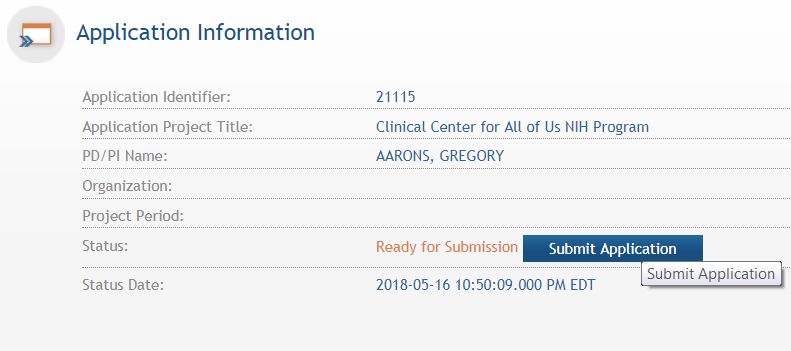

Follow the status of your submission in eRA Commons

| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | Roman, Laurie (NIH/OD) [E] |
| File Modified | 0000-00-00 |
| File Created | 2023-08-28 |
© 2026 OMB.report | Privacy Policy