Supporting Statement Part B 6.28.22
Supporting Statement Part B 6.28.22.docx
Online Submission Form for Supplemental Evidence and Data for Systematic Reviews for the Evidence-based Practice Center Program
OMB: 0935-0231
SUPPORTING STATEMENT
Part B
Online Submission Form for Supplemental Evidence and Data for Systematic Reviews for the Evidence-based Practice Center Program
Version: June 28, 2022
Agency of Healthcare Research and Quality (AHRQ)
Table of Contents
B. Collections of Information Employing Statistical Methods 3
B1. The Potential Respondent Universe and any Sampling or other Respondent Selection Methods to be Used……………………………………………………………………….. 3
B2. The Procedures for the Collection of Information 3
B3. Methods to Maximize Response Rates and to Deal with Issues of Non-response…… 3
B4. Tests of Procedures or Methods to be Undertaken…………………………………… 3
Appendixes
Appendix A. Website Portal for Submission of Supplemental Evidence and Data for Systematic
Reviews
Appendix B. Opportunity to Submit Scientific Information E-mail
Appendix C. Federal Register Notice
B. Collections of Information Employing Statistical Methods
B1. The Potential Respondent Universe and any Sampling or other Respondent Selection Methods to be Used
AHRQ contacts industry stakeholders such as investigators, pharmaceutical and device manufacturers, app developers, and other non-governmental institutions and professional associations through the Effective Health Care Program (EHC) listserv and Evidence-based Practice Center listserv for the purposes of supplementing evidence and data (SEADS) collected from published and grey literature searches. 189,923 people are included on EHC listserv, and 129,326 are subscribed to the EPC listserv. Subscription to the listserves are voluntary. In some cases, we also post a note about the opportunity to submit SEADS through a Federal Register notice.
B2. The Procedures for the Collection of Information
The online submission form (OSF; see Attachment A) is set up to retrieve this information without creating a heavy burden on the responder. However, other than the OSF, stakeholders are able to respond to the request via email.
The OSF was developed to provide stakeholders with flexibility in how they respond to the request. At a minimum, respondents are requested to input their name along with the information packet.
Each SEADS submission portal is a single unique event reflecting the topic chosen by AHRQ and its partners that is not repeated unless it is deemed ready for an update at an undetermined future date.
B3. Methods to Maximize Response Rates and to Deal with Issues of Non-response
The information collection process is initiated with an email notification of the opportunity to submit SEADS via the EHC and EPC listservs (see Attachment B). In cases where AHRQ determines that a more general notice to the public is necessary, such as when there are not specific manufacturers of products under review, AHRQ may issue a Federal Register notice (see Attachment C) of the opportunity to submit SEADS.
B4. Tests of Procedures or Methods to be Undertaken
This approach has been used by AHRQ for over 5 years. Tests have been done to ensure that the OSF or user interface does not cause any confusion. AHRQ consults with the EHC webteam to ensure that best practices are used for the OSF and webpage. AHRQ regularly reviews SEADS submissions and any reports of issues with the EHC webteam to identify issues and ensure smooth functioning.
Attachment A -- Website Portal for Submission of Supplemental Evidence and Data for Systematic Reviews
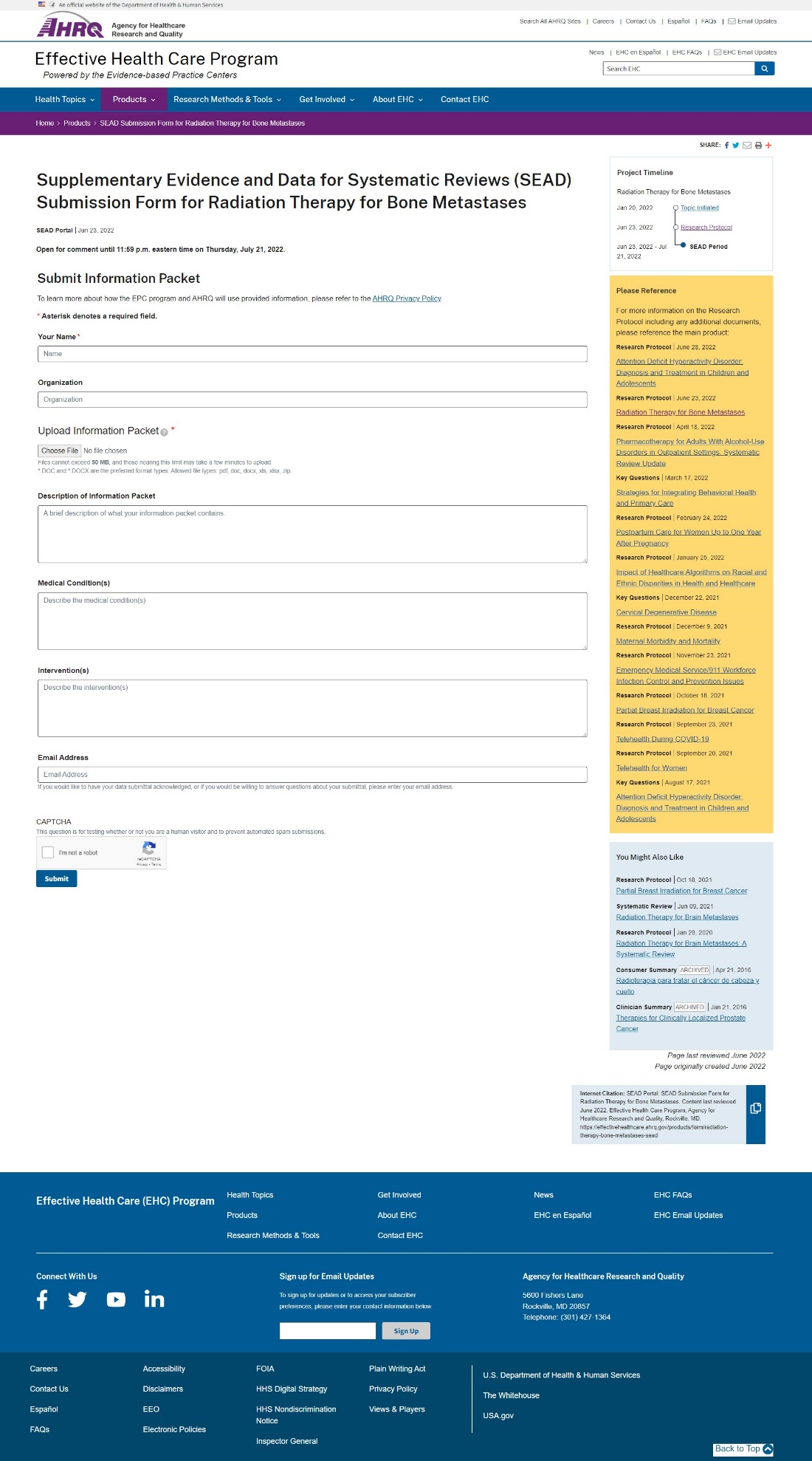
Attachment B -- Opportunity to Submit Scientific Information E-mail
From: Agency for Healthcare Research and Quality (AHRQ) <[email protected]> Sent: Thursday, March 10, 2022 5:23 PM To: Benns, Jenae (AHRQ/CEPI) <[email protected]> Subject: EHC Program Update: Protocol on Postpartum Management of Women who Experience Hypertensive Disorders of Pregnancy Now Available
|
|
|
|
Attachment C -- Federal Register Notice
DEPARTMENT OF HEALTH AND HUMAN SERVICES
Agency for Healthcare Research and Quality
Supplemental
Evidence and Data Request on Pharmacotherapy for Adults with
Alcohol-Use Disorders in Outpatient Settings: Systematic Review
Update
AGENCY: Agency for Healthcare Research and Quality (AHRQ), HHS.
ACTION:
Request for Supplemental Evidence and Data Submissions
SUMMARY:
The Agency for Healthcare Research and Quality (AHRQ) is seeking
scientific information submissions from the public. Scientific
information is being solicited to inform our review on
Pharmacotherapy
for Adults with Alcohol-Use Disorders in Outpatient Settings:
Systematic Review Update,
which
is currently being conducted by the AHRQ’s Evidence-based
Practice Centers (EPC) Program. Access to published and unpublished
pertinent scientific information will improve the quality of this
review.
DATES: Submission Deadline on or before [INSERT DATE 30 DAYS AFTER DATE OF PUBLICATION IN THE FEDERAL REGISTER].
ADDRESSES:
E-mail submissions: [email protected]
Print submissions:
Mailing Address:
Center for Evidence and Practice Improvement
Agency for Healthcare Research and Quality
ATTN: EPC SEADs Coordinator
5600 Fishers Lane
Mail Stop 06E53A
Rockville, MD 20857
Shipping Address (FedEx, UPS, etc.):
Center for Evidence and Practice Improvement
Agency for Healthcare Research and Quality
ATTN: EPC SEADs Coordinator
5600 Fishers Lane
Mail Stop 06E77D
Rockville, MD 20857
FOR FURTHER INFORMATION CONTACT:
Jenae Benns, Telephone: 301-427-1496 or Email: [email protected].
SUPPLEMENTARY INFORMATION:
The Agency for Healthcare Research and Quality has commissioned the Evidence-based Practice Centers (EPC) Program to complete a review of the evidence for Pharmacotherapy for Adults with Alcohol-Use Disorders in Outpatient Settings: Systematic Review Update. AHRQ is conducting this systematic review pursuant to Section 902 of the Public Health Service Act, 42 U.S.C. 299a.
The
EPC Program is dedicated to identifying as many studies as possible
that are relevant to the questions for each of its reviews. In order
to do so, we are supplementing the usual manual and electronic
database searches of the literature by requesting information from
the public (e.g., details of studies conducted). We are looking for
studies that report on Pharmacotherapy for Adults with Alcohol-Use
Disorders in Outpatient Settings: Systematic Review Update,
including those that describe adverse events. The entire research
protocol is available online at:
https://effectivehealthcare.ahrq.gov/products/alcohol-misuse-drug-therapy/protocol
This is to notify the public that the EPC Program would find the following information on Pharmacotherapy for Adults with Alcohol-Use Disorders in Outpatient Settings: Systematic Review Update helpful:
A list of completed studies that your organization has sponsored for this indication. In the list, please indicate whether results are available on ClinicalTrials.gov along with the ClinicalTrials.gov trial number.
For completed studies that do not have results on ClinicalTrials.gov, a summary, including the following elements: study number, study period, design, methodology, indication and diagnosis, proper use instructions, inclusion and exclusion criteria, primary and secondary outcomes, baseline characteristics, number of patients screened /eligible /enrolled /lost to follow-up /withdrawn /analyzed, effectiveness/efficacy, and safety results.
A list of ongoing studies that your organization has sponsored for this indication. In the list, please provide the ClinicalTrials.gov trial number or, if the trial is not registered, the protocol for the study including a study number, the study period, design, methodology, indication and diagnosis, proper use instructions, inclusion and exclusion criteria, and primary and secondary outcomes.
Description of whether the above studies constitute ALL Phase II and above clinical trials sponsored by your organization for this indication and an index outlining the relevant information in each submitted file.
Your contribution is very beneficial to the Program. Materials submitted must be publicly available or able to be made public. Materials that are considered confidential; marketing materials; study types not included in the review; or information on indications not included in the review cannot be used by the EPC Program. This is a voluntary request for information, and all costs for complying with this request must be borne by the submitter.
The
draft of this review will be posted on AHRQ’s EPC Program
website and available for public comment for
a period of 4 weeks. If you would like to be notified when the
draft is posted, please sign up for the e-mail list at:
https://www.effectivehealthcare.ahrq.gov/email-updates.
The systematic review will answer the questions below. This information is provided as background and AHRQ is not requesting that the public provide answers to these questions.
Key
Questions (KQ)
KQ 1a: Which medications are efficacious for improving consumption outcomes for adults with alcohol-use disorders in outpatient settings?
KQ 1b: How do medications for adults with alcohol-use disorders compare for improving consumption outcomes in outpatient settings?
KQ 2a: Which medications are efficacious for improving health outcomes (including functioning and quality-of-life outcomes) for adults with alcohol-use disorders in outpatient settings?
KQ 2b: How do medications for adults with alcohol-use disorders compare for improving health outcomes (including functioning and quality-of-life outcomes) in outpatient settings?
KQ 3a: What adverse effects are associated with medications for adults with alcohol-use disorders in outpatient settings?
KQ 3b: How do medications for adults with alcohol-use disorders compare for adverse effects in outpatient settings?
KQ 4: Are medications for treating adults with alcohol-use disorders effective in primary care settings?
KQ 5: Are any of the medications more or less effective than other medications for older adults, younger adults, smokers, or those with co-occurring disorders?
PICOTS (Populations, Interventions, Comparators, Outcomes, Timing, and Setting)
Population(s)
Adults (age 18 years or older) with alcohol-use disorders
Interventions
Pharmacotherapy for relapse prevention. This includes:
Medications approved by FDA for treating alcohol dependence:
acamprosate
disulfiram
naltrexone (oral or injectable)
Certain medications in use off label that are available in the United States:
baclofen
gabapentin
ondansetron
topiramate
prazosin
varenicline
Studies evaluating pharmacotherapy that used co-interventions with other treatments for AUDs (e.g., behavioral counseling, cognitive behavioral therapy, motivational enhancement therapy, psychosocial treatments, or self-help such as 12-step programs [e.g., Alcoholics Anonymous]) will be eligible for inclusion, as long as they meet other inclusion/exclusion criteria.
This review will not include pharmacotherapy for alcohol withdrawal.
Comparators
Studies must compare one of the medications listed above with placebo or another eligible medication.
Outcomes
Consumption outcomes
abstinence/any drinking
rates of continuous abstinence
percentage of days abstinent
time to first drink/lapse
time to heavy drinking/relapse
reduction in alcohol consumption
number of heavy drinking days
percentage of subjects with no heavy drinking days
number of drinking days
drinks per drinking day
drinks per week
Health outcomes
accidents
injuries
quality of life
function
mortality
Adverse effects of intervention(s)
withdrawals due to adverse events
nausea/vomiting
diarrhea
anorexia
palpitations
headache
dizziness
cognitive dysfunction
taste abnormalities
paresthesias (numbness, tingling)
metabolic acidosis
glaucoma
vision changes
suicidal ideation
insomnia
anxiety
rash
tiredness
weakness
constipation
Timing
Studies with at least 12 weeks of planned pharmacologic treatment and followup from the time of medication initiation
Setting
Outpatient healthcare settings; KQ 4 applies to primary care settings only (i.e., internal medicine, family medicine, obstetrics/gynecology, or college and university health clinics)
Dated:
Marquita Cullom,
Associate Director.
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | McKenna, Ryan T (Portland) |
| File Modified | 0000-00-00 |
| File Created | 2022-10-21 |
© 2026 OMB.report | Privacy Policy
