8 NCI CTRP Aggregate Screenshot
The Clinical Trials Reporting Program (CTRP) Database (NCI)
Attach_3e_NCI CTRP Aggregate Accrual Screenshots
Accrual Updates
OMB: 0925-0600
⚠️ Notice: This form may be outdated. More recent filings and information on OMB 0925-0600 can be found here:
Document [docx]
Download: docx | pdf
NCI CTRP Attachment 3e
NCI CTRP Aggregate Accrual Portal Workflow and Screen Shots
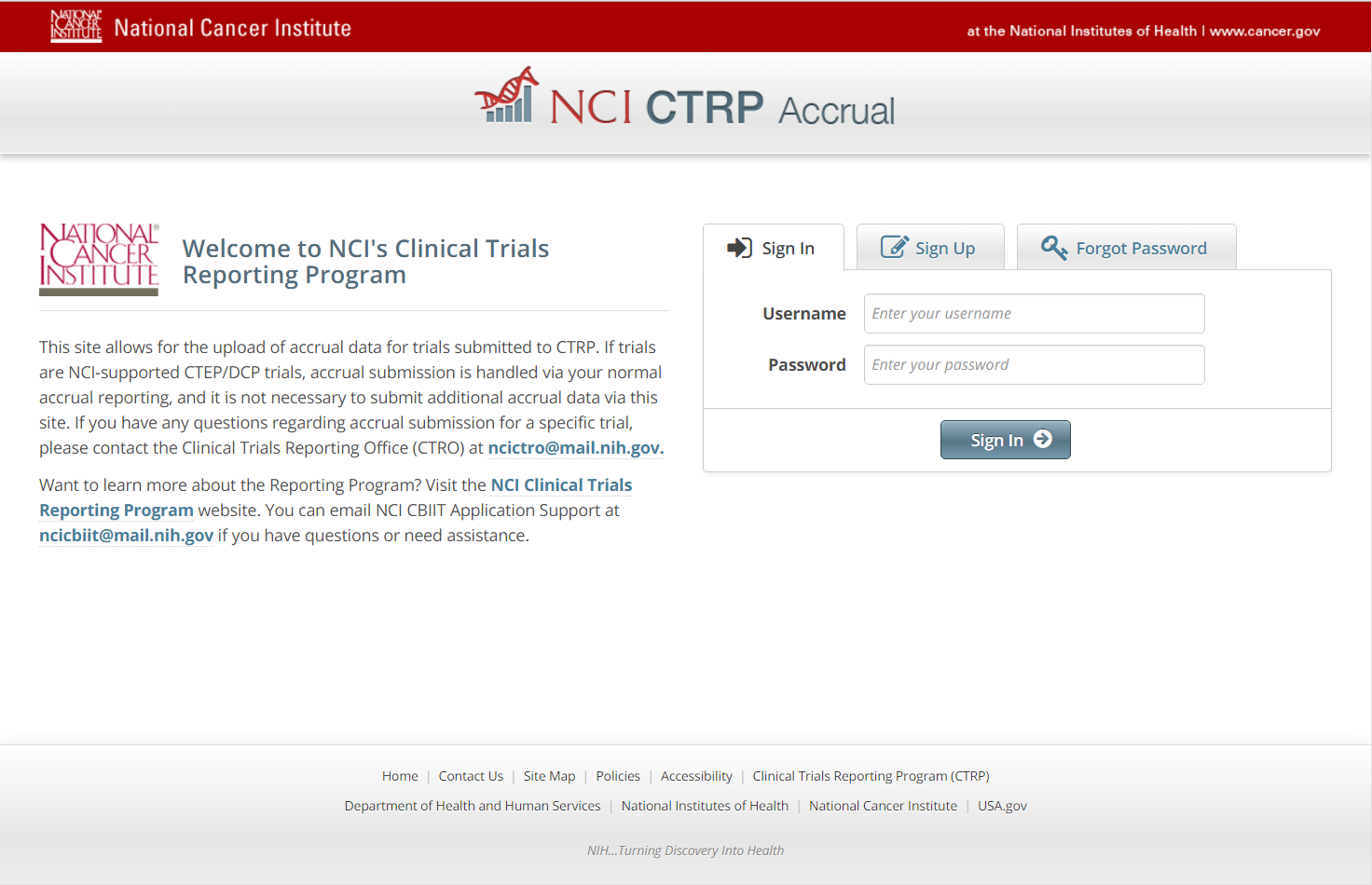
Step 1: User accesses the NCI Clinical Trials Reporting Program Accrual website at http://trials.nci.nih.gov/accrual – see screenshot, page 2
Step 2: User enters “Username” and “Password” – see screenshot, page 2
Step 3: User reviews NCI Clinical Trials Reporting Program Accrual burden statement – see screenshot, page 3
Step 4: User selects to “Submit Aggregate Study Subject Accrual Information” and submits aggregate accrual information on a registered trial – see screenshot, pages 4 - 5
CTRP Accrual Burden
Statement
Submit Aggregate Study
Subject Accrual Information




| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | David Loose |
| File Modified | 0000-00-00 |
| File Created | 2021-01-23 |
© 2026 OMB.report | Privacy Policy