SUDORS Proposed Changes - Screen Shots
Att F1 SUDORS_screen_Shots_Changes.docx
State Unintentional Drug Overdose Reporting System (SUDORS)
SUDORS Proposed Changes - Screen Shots
OMB: 0920-1128
F1 SUDORS Screen Shots Changes
SUDORS Tab: Case Classification and Drug Overdose/Poisoning Sections
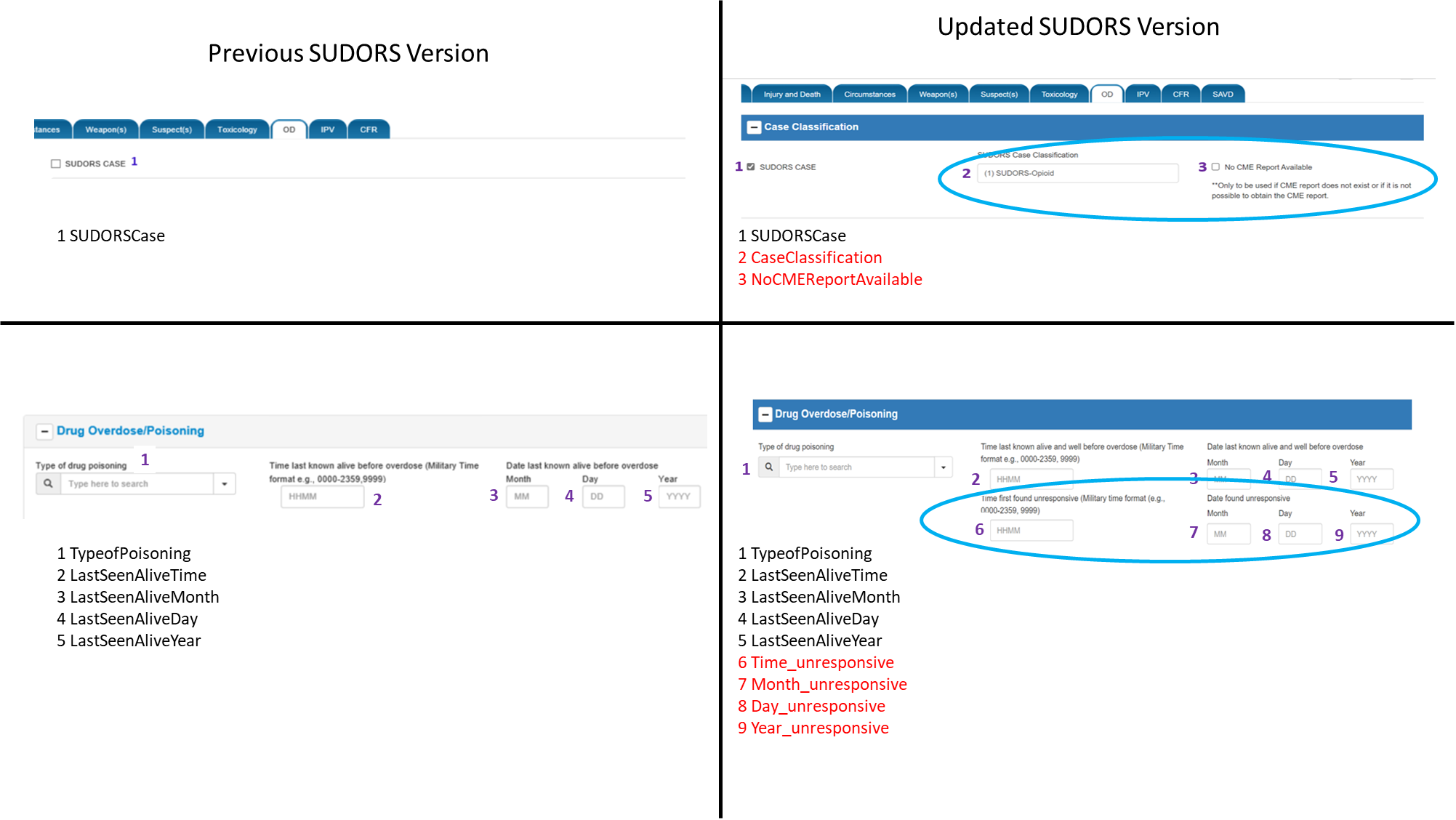
Changes to Case Classification and Drug Overdose/Poisoning sections:
Case Classification section created and SUDORSCase (#1) field moved into it
2 additional data elements added to Case Classification section (#2 and #3) to further classify cases by opioid/non-opioid involvement and to indicate that no coroner/medical examiner report was available
4 additional data elements added to Drug Overdose/Poisoning section to capture time/date the decedent was found unresponsive after the overdose
SUDORS Tab: Substance Use/Misuse and Treatment History Section
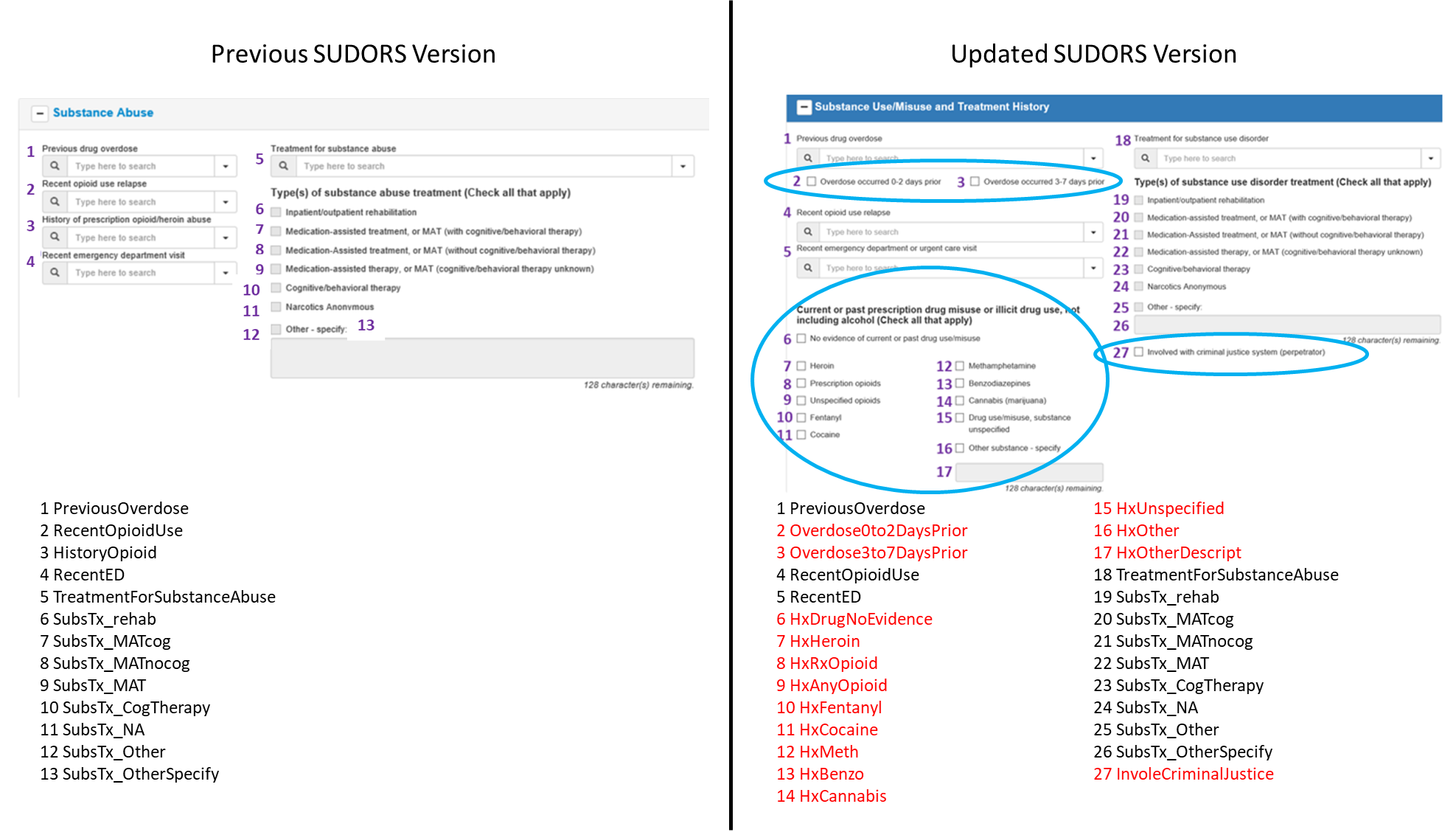
Changes to Substance Use/Misuse and Treatment History section:
Section label updated (previously, “Substance Abuse” section)
2 data elements added to capture more specific information about timing of previous nonfatal overdose (#2, #2)
1 data element capturing heroin/prescription opioid use history replaced by 12 data elements to capture more broad drug use/misuse history (#6-#17)
1 data element added to capture information about decedent’s involvement with the criminal justice system prior to the overdose (#27)
Scene Indications of Drug Use Section
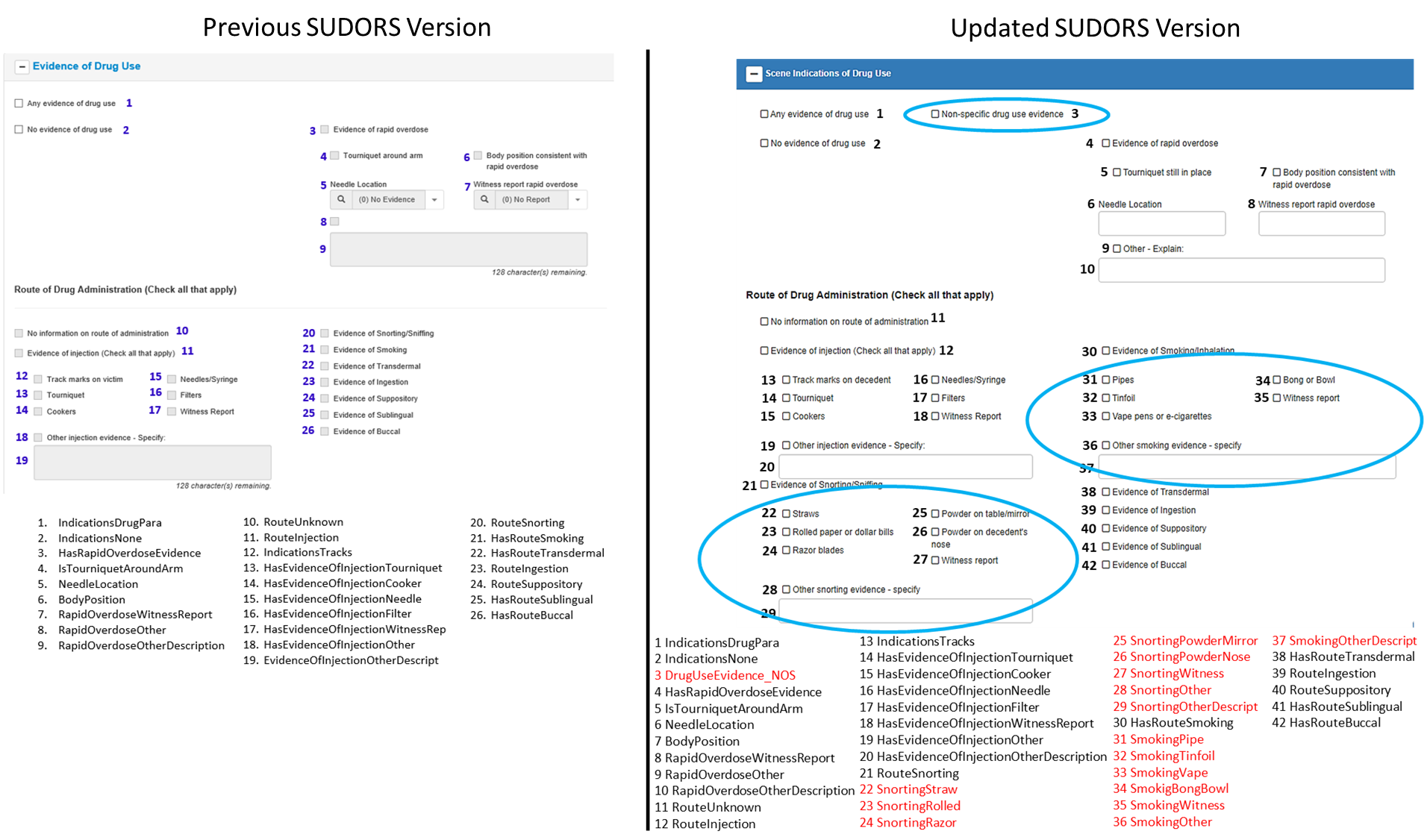
Changes to Scene Indications of Drug Use section:
Section label updated (previously, “Evidence of Drug Use” section)
1 data element added to capture non-specific drug use evidence (#3)
8 data elements added to capture details about supporting evidence of snorting/sniffing for route of drug administration (#22-#29)
7 data elements added to capture details about supporting evidence of smoking for route of drug administration (#31-#37)
Illicit or Prescription Drugs Section
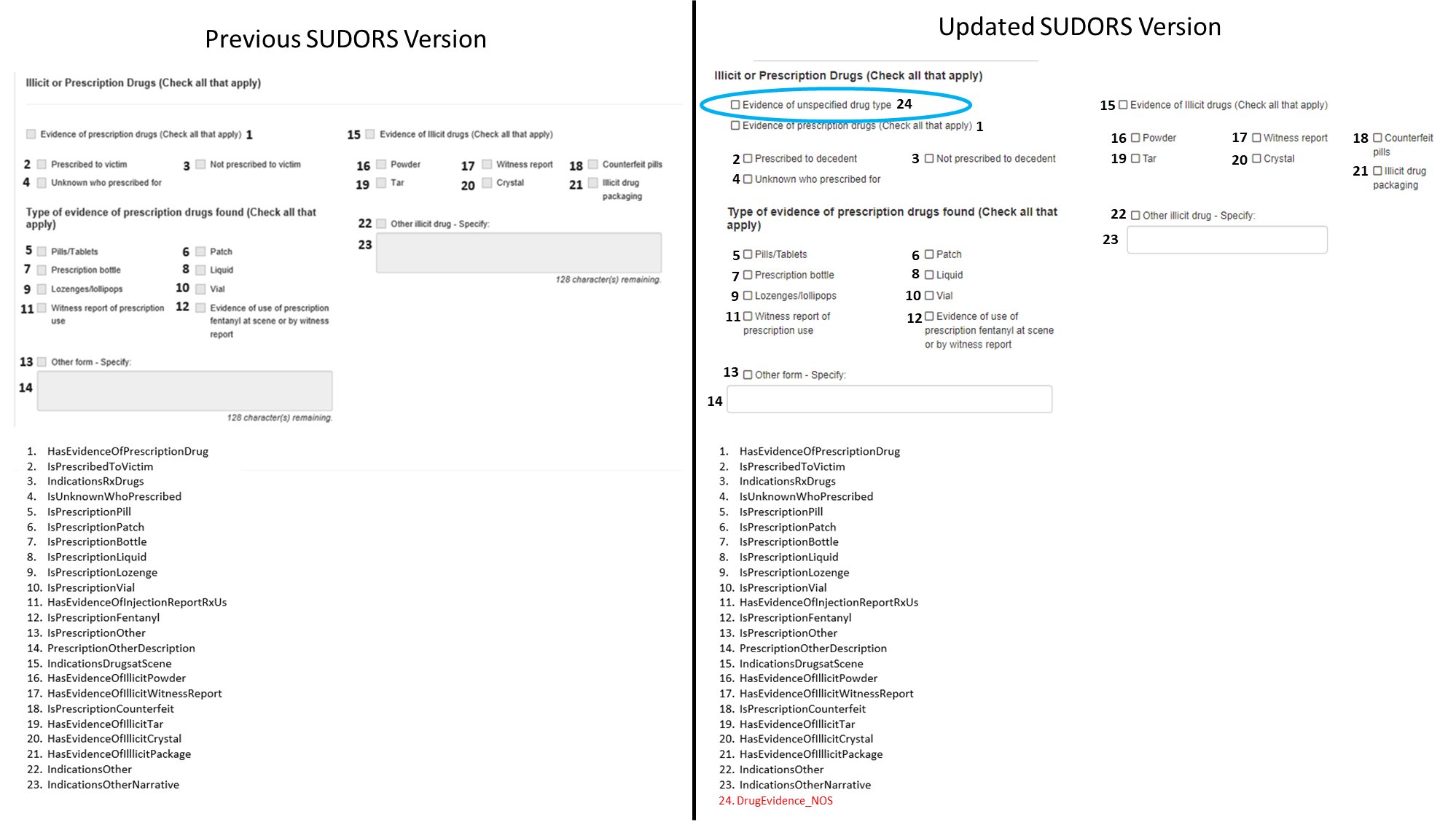
Changes to Illicit or Prescription Drugs section:
1 data element added to capture evidence of unspecified drug type (#24)
Response to Drug Overdose Section
Changes to Response to Drug Overdose section:
None
Response to Drug Overdose Section, continued

Changes to Response to Drug Overdose section:
None
Response to Drug Overdose Section, continued, and Medical History Section

Changes to Response to Drug Overdose section:
None
Changes to Medical History section:
None
Prescription Information Section
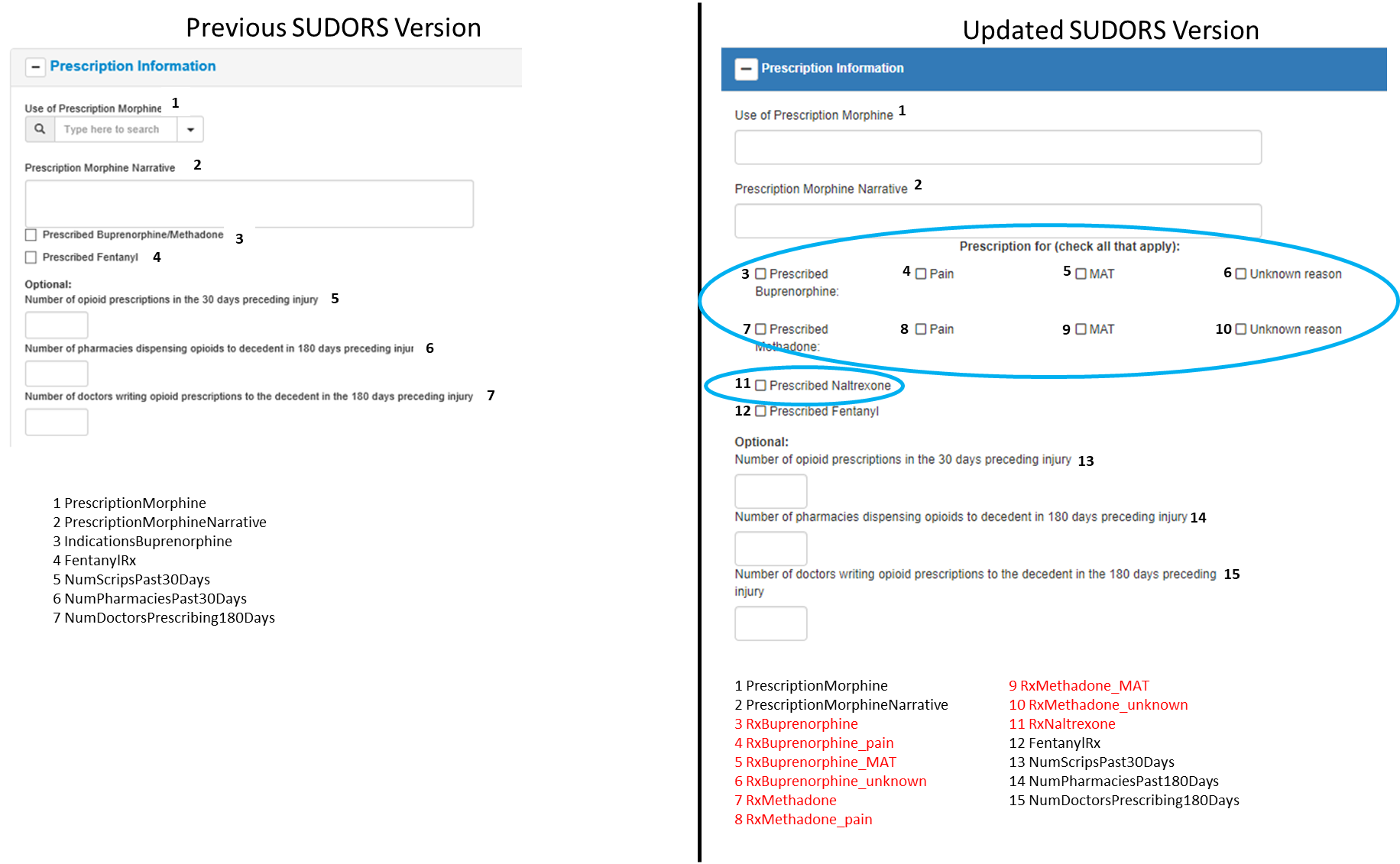
Changes to Prescription Information section:
1 data element broken into 2 separate elements (previous #3 to updated #3, #7) to capture information separately about prescriptions of buprenorphine and methadone, and expanded with 6 additional data elements to capture reason(s) for prescription(s) (#4-#6, #8-#10)
1 data element added to capture information about prescription of naltrexone (#11)
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | ODonnell, Julie K. (CDC/DDNID/NCIPC/DOP) |
| File Modified | 0000-00-00 |
| File Created | 2022-09-15 |
© 2026 OMB.report | Privacy Policy